Abstract
Polymer-wrapped single-walled carbon nanotubes (SWNTs) are a potential method for obtaining high-purity semiconducting (sc) SWNT solutions. Conjugated polymers (CPs) can selectively sort sc-SWNTs with different chiralities, and the structure of the polymer side chains influences this sorting capability. While extensive research has been conducted on modifying the physical, optical, and electrical properties of CPs through side-chain modifications, the impact of these modifications on the sorting efficiency of sc-SWNTs remains underexplored. This study investigates the introduction of various conjugated side chains into naphthalene diimide-based CPs to create a biaxially extended conjugation pattern. The CP with a branched conjugated side chain (P3) exhibits reduced aggregation, resulting in improved wrapping ability and the formation of larger bundles of high-purity sc-SWNTs. Grazing incidence X-ray diffraction analysis confirms that the potential interaction between sc-SWNTs and CPs occurs through π–π stacking. The field-effect transistor device fabricated with P3/sc-SWNTs demonstrates exceptional performance, with a significantly enhanced hole mobility of 4.72 cm2 V–1 s–1 and high endurance/bias stability. These findings suggest that biaxially extended side-chain modification is a promising strategy for improving the sorting efficiency and performance of sc-SWNTs by using CPs. This achievement can facilitate the development of more efficient and stable electronic devices.
Keywords: biaxial conjugation, conjugated polymers, single-walled carbon nanotubes, sorting, field-effect transistors
Introduction
Conjugated polymers (CPs) have been widely applied to organic optoelectronic devices because of their malleability, self–assembly properties, solution processability, large-area flexibility, eco-friendliness, and excellent compatibility with stretchable electronics.1−3 Organic field-effect transistors (FETs) can be diversely functionalized and further improved owing to the ability to perform structural modifications of organic materials.4−6 Recent studies have demonstrated that side-chain modifications such as biaxial conjugated extension on CPs could modify their backbone coplanarity, chain packing patterns, and optical absorptions.7−9 In addition, the biaxial conjugated extension can improve the charge-transport performance and mobility–stretchability of CPs.10−14 However, the application of the biaxial conjugated extension in N-type CPs is undeveloped. Recently, naphthalene diimide (NDI)-based CPs have been intensively investigated.15,16 Therefore, improving the performance of NDI-based CPs using side-chain engineering is of great importance and research interest.17
The research interest in single-walled carbon nanotubes (SWNTs) persists due to their remarkable mechanical and electrical properties, positioning them as promising candidates for various advanced applications. SWNTs are being explored for integration into FETs, thermoelectric devices, biosensors, and solar cells, which could serve as active layers and significantly enhance the device’s performance.18 Nevertheless, commercially available SWNTs contain one-third metallic SWNTs (m-SWNTs), two-thirds semiconducting SWNTs (sc-SWNTs), some amorphous carbon, and catalysts for the rest. In this case, the purification and sorting of sc-SWNTs become crucial for fabricating high-performance devices. Based on previous literature, the sc-SWNTs could be sorted by using the following approaches: (i) density gradient ultracentrifugation,19,20 (ii) gel agarose chromatography,21 and (iii) noncovalent bond selective wrapping of sc-SWNTs with DNA22 and CPs.23−25 From a scale-up point of view, noncovalent bond selective wrapping is preferable. From an economic point of view, the last approach above, wrapping by DNA, is too expensive to apply, so CPs are the potential choice. With regard to the backbone engineering of CP, Lei et al. developed fluorene-based polyazomethine as a removable and recyclable CP for highly selective and high-yield dispersion and release of low-cost sc-SWNTs.23 Hwang et al. used a series of diketopyrrolopyrrole (DPP)-based CPs for effectively enriching sc-SWNTs of the high-pressure carbon monoxide (HiPco) SWNTs and obtaining high-quality sc-SWNT solutions without impurities due to the dispersibility from the slightly kinked backbone.24
With regard to the side-chain modification of CPs, Gomulya et al. investigated the side-chain length effect of polyfluorene in sc-SWNT sorting. They found that CPs with long alkyl side chains can wrap sc-SWNTs with large diameters and chirality because of their different configurations, with the CP backbone perpendicular (T) or parallel (P) to the tube surface. This is the first research on side-chain modification of CPs in sc-SWNT sorting.25 Later, Wang et al. used a polyfluorene-based alternative copolymer modified with a benzophenone group for wrapping and achieving higher yield than the reference CP of poly(9,9-dioctylfluorene) via a solution process.26 Then, they exposed the CP/sc-SWNT film to UV irradiation to pattern the film through photolithography. Recently, Ye and Talsma et al. used NDI-based CPs with low bandgap and polar/nonpolar side chains for wrapping, and they found that adjusting side chains could influence dispersion at a certain level.27,28 Ouyang et al. reported a backbone strategy of polyfluorene with C–C or C=C linkages for wrapping different chiralities of sc-SWNTs. They found that, through multicycle conjugated polymer extraction processes, the single chirality sc-SWNTs could be enriched and show better purity, yield, and selectivity. This can certainly improve the performance of thin-film transistors.29 Luo et al. showed another backbone strategy using pentiptycene polymers containing metal-chelating groups for immortalizing metal selectors on sc-SWNT chemiresistors. They can serve as breath biomarkers, improving the life of patients suffering from chronic kidney disease.30 Previously, we developed a series of NDI-based CPs with different donors to sort sc-SWNTs for advanced phototransistor memory.31 The result indicated that the CP’s coplanarity and aggregation play an important role in the wrapping of sc-SWNTs. In addition, the design concept of extractor and enhancer with a synergistic effect is also practical in sc-SWNT sorting.32 Nonetheless, no research has systematically investigated the influence of side-chain modification, especially the biaxially extended conjugations of CPs on sc-SWNT sorting. The conjugated side chain in biaxially extended conjugations of CPs can enlarge the side chain domain to potentially optimize the wrapping patterns on sc-SWNTs.
There is limited research on the relationships between side-chain modification of CPs and sc-SWNT sorting thus far, and there is no study on the influence of biaxially extended conjugation in NDI-based CPs. Therefore, in this study, we modified NDI-based n-type CPs for wrapping sc-SWNTs with conjugated side chains. By introducing conjugated side chains into the CPs, we explored the relationship between side-chain modifications and wrapping of sc-SWNTs. Accordingly, the conventional CP with a donor of bithiophene (2T) and an alkyl side chain is named P1. The CPs with conjugated dibenzoyl imide moieties and linear or branched alkyl side chains are named P2 and P3. These NDI-based CPs showed good selectivity to plasma discharge SWNTs (PD-SWNTs). The sorting efficiency of CP/sc-SWNT solutions was evaluated using ultraviolet–visible–near-infrared (UV–vis–NIR) and Raman spectroscopies. The morphology of the CP/sc-SWNT films was probed using atomic force microscopy (AFM) and grazing incidence X-ray diffraction (GIXD) techniques. We surprisingly discovered that the noncovalent bond interactions between CP and sc-SWNT, which is a significant way for wrapping sc-SWNTs, might be influenced by the size and branches of the conjugated side chains in CPs. The experimental results are consistent with the calculated density functional theory (DFT) and molecular dynamics (MD) simulations. Furthermore, we found that the device of P3/sc-SWNTs demonstrated better electrical performance: the mobility of the device increased to 4.72 cm2 V–1 s–1 of P3-wrapped sc-SWNTs, which is significantly higher than that of 2.21 cm2 V–1 s–1 for P1-wrapped sc-SWNTs. In addition, the device of P3/sc-SWNTs owns a higher endurance stability. Therefore, the NDI-based CPs with biaxially extended side chains enhance the wrapping selectivity of sc-SWNTs to improve the FET device performance.
Results and Discussion
Synthesis, Optical, and Electrochemical Characterizations of the Polymers
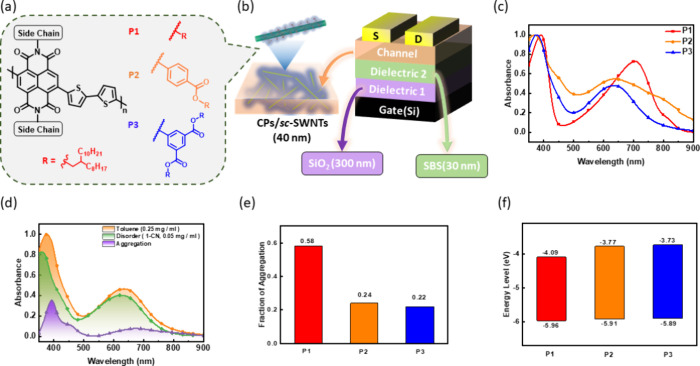
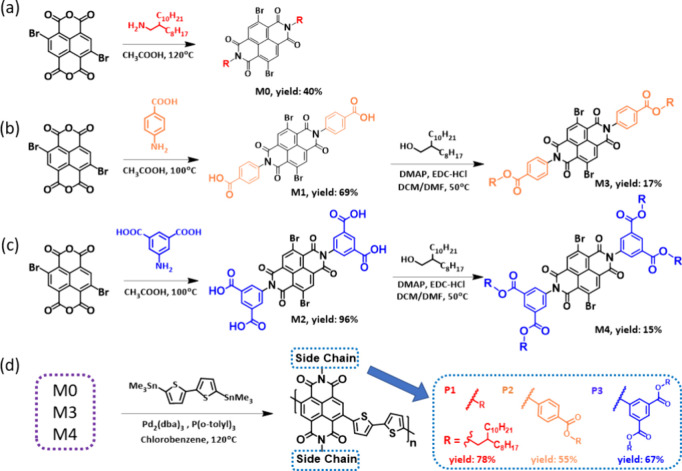
Wrapping sc-SWNTs via CPs through sorting processes has been broadly considered a high-efficiency way for fabricating sc-SWNTs-based FET devices. Modified CPs with conjugated side chains enlarge the polymers’ size and modify their characteristics. Several properties, such as physical, optical, electrochemical, and aggregation behavior, have changed by extending the molecule’s size. Figure 1a displays a series of NDI-based CPs synthesized in this study; their synthetic routes, chemical structures, and monomers are shown in Scheme 1. M1 was synthesized with a benzoic acid side group by imidization from brominated naphthalene diamide (Br–NDA–Br) and alkylated with octyl-dodecane by Steglich esterification for obtaining M3.33M2 was synthesized with 5-amino isophthalic acid and Br–NDA–Br by imidization, and M4 was obtained by alkylation with octyl-dodecane. The phenyl group modified NDI-based monomers were polymerized through Pd(0)-catalyzed Migita–Kosugi–Stille coupling polymerization with bithiophene.34 More detailed steps for synthesis are presented in the Experimental Section. Figure S1 demonstrates the gel permeation chromatography (GPC) profiles with THF as an eluent. The monomers’ 1H and 13C NMR spectra are shown in Figures S2–S9 (Supporting Information), and the polymers’ 1H and 13C NMR spectra are shown in Figures S10–S15 (Supporting Information). The chemical shifts and integral areas correspond to each chemical structure. Regarding the thermal properties, the decomposition temperature (Td) and glass transition temperature (Tg) measured by the thermogravimetric analyzer (TGA) and differential scanning calorimetry (DSC) are shown in Figure S16 (Supporting Information). The Tg of P3 can be observed at 170 °C because it contains more alkyl side chains, making the polymer softer than those of P1 and P2. As summarized in Table S1 (Supporting Information), the Td values of these CPs are high and at 436, 312, and 358 °C, respectively, representing their high thermal stabilities.
Figure 1.
(a) Chemical structures of the reported NDI-based CPs. (b) Device structure with a semiconducting channel composed of CP/sc-SWNT. (c) UV–vis absorption spectra of the pure CP solutions. (d) Fractions of aggregate and disorder in the UV–vis absorption spectra of P3 solution. (e) Fractions of the aggregate evaluated based on the UV–vis absorption spectra of CP solutions in toluene and in 1-chloronaphthalene. (f) Frontier energy levels of the CPs studied.
Scheme 1. Synthetic Routes for the Naphthalene Diimide Monomers with the (a) Alkyl Groups, (b) Alkyl Benzoate Groups, (c) Di-Alkyl Benzoate Groups, and (d) Polymerization of CPs with Branched and Conjugated Side Chain Moieties.
The CPs were applied to wrap and sort the sc-SWNTs selectively. The composite was used as the semiconducting channel for FET devices, including the thickness of the dielectric and channel layers (Figure 1b). Table S2 shows the device information, including the layer thickness and dielectric properties. Different sizes of side chains might induce intrachain charge transfer (ICT) related to phenyl groups, chain coplanarities, and steric hindrances, primarily owing to the long alkyl chains. Figure 1c reveals the UV–vis–NIR absorption spectrum of the CPs studied. The (0:1) peaks of P1, P2, and P3 are at 705, 637, and 635 nm, respectively. In addition, P1’s π–π* peak is at 391 nm, while P2 and P3’s peaks blue shift to 368 and 375 nm. It might be caused by reducing the density of electrons and backbone aggregations. Figure 1d represents P3’s aggregation behavior, and the rest of P1 and P2 is shown in Figure S17 (Supporting Information). Note that the polymer solutions in toluene were prepared at a concentration of 0.25 mg mL–1, the same concentration in the sorting solution discussed in the following sections; the disordered state was the polymer solution prepared in 1-chloronaphthalene at a concentration of 0.05 mg mL–1. The calculation is detailed in the Experimental Section. The calculated aggregation fraction of the CPs is shown in Figure 1e. Accordingly, the aggregation fractions are 0.58, 0.24, and 0.22 for P1, P2, and P3, respectively. P1 had the highest value, while P2 and P3 had values lower than those of P1. It might be attributed to side chain modifications and the hindrance of the long/branched alkyl chain. Compared with P1, CPs with phenyl side groups are more sterically hindered in the solvent, resulting in a relatively lower aggregation fraction. Furthermore, the lower aggregation fraction of P3 than P2 is because the branched side chains dilute the contents and interactions of main chains so that P3 would be more dispersed in the solvent.35 To summarize, conjugated side-chain extension with such phenyl groups has a particular intrinsic impact on the aggregation properties of the polymers. This outcome can also have a significant influence on the sorting processes.
Figure 1f and Table S1 (Supporting Information) present the energy level of each polymer calculated by UV–vis–NIR spectroscopy. The lowest unoccupied molecular orbital (LUMO) level was calculated from the cyclic voltammetry profiles (CV, Figure S18, Supporting Information): LUMO = −e·(Ere – E1/2(ferrocene) + 4.8), where LUMO is the lowest unoccupied molecular orbital, e is the elementary charge, and Ere represents the reduction potential in the CV sweeping. The calculation of the energy bandgap is calculated by Eg = 1240/λonset, where λonset is the onset wavelength. The calculation of HOMO is HOMO = LUMO – Eg, where HOMO is the highest occupied molecular orbital. As P2 and P3 blue-shift to 635 nm in the (0–1) peak, the LUMO decreased to −3.77 and −3.73 eV, respectively. The conjugated side-chain extension with phenyl groups makes an increase in the energy levels. It might be attributed to the improved structural rigidity by the biaxial extension of the benzene rings. The conjugated side groups may disrupt the delocalization of the electrons on NDI acceptors along the backbone, thus enabling increased LUMO levels.36 From the above calculations, P2 and P3 are polymers with a higher electron-donating ability that might possess higher ICT intrinsically.37 However, the branched alkyl side chains of P3 hinder its aggregation, thereby showing a more blue-shifted ICT band.
Polymer/sc-SWNT Sorting Properties
The CPs and PD-SWNTs were sonicated in toluene with a bar sonicator to sort the sc-SWNTs. After sonication, the mixed solution with sufficiently dispersed CPs entangled with sc-SWNTs was centrifugated to remove impurities, like amorphous carbon and m-SWNTs. The UV–vis–NIR absorption characterization was conducted to gain more insight into the sorting solutions, and the results are displayed in Figure 2a. P3 obtained more apparent signals of sc-SWNTs (in a range of about 800 to 1600 nm) than P2 and P1. Several crucial parameters were defined to calculate the efficiency of wrapping sc-SWNTs. Selectivity (ϕ) means the purity of sc-SWNTs, defined as the integral areas of S22 peak absorbance divided by the sum of the integral of S22 peak absorbance and the baseline absorbance: ϕ = AS22/(AS22 + Abaseline),38 where AS22 is the integral of the S22 peak and Abaseline is the integral of the baseline; yield means the number of sc-SWNTs (targeted SWNTs). The yield was calculated by using Beer’s law to evaluate the UV–vis–NIR absorbance: λ = ε bc, where ε is the absorption coefficient of sc-SWNTs in toluene, b means the optical path length, and c implies the concentration of the tested solution. After c was obtained, the yield could be determined: Yield = (Csc-SWNTs × V)/(2WSWNTs/3), where Csc-SWNTs means the concentration of sc-SWNTs in the sorting solution, V implies the volume of the sorting solvent, and WSWNTs means the total weight of SWNTs weighted for the sorting process. The sorting parameters are summarized in Table S3 (Supporting Information). The absorbances of NDI-based CPs were deconvoluted and subtracted from the absorption spectra of CP/sc-SWNT solutions. The ϕ values of P1, P2, and P3 are 0.33, 0.43, and 0.59, respectively (Figure S19, Supporting Information). From the literature, a high ϕ value means higher purities of sc-SWNTs. With a ϕ value >0.33–0.40, the corresponding purity is higher than 99%.39,40 Thus, the purity of sc-SWNTs sorted by P1 is approximately 99%, and P2 and P3 have purities >99%. The calculated yields are 34.0%, 2.3%, and 23.3%, respectively. As can be seen, P2 and P3 have higher purities than P1; P2 has a poor yield due to its poor solubility in toluene, and P3 has the highest selectivity and sufficient yield for device fabrication.
Figure 2.
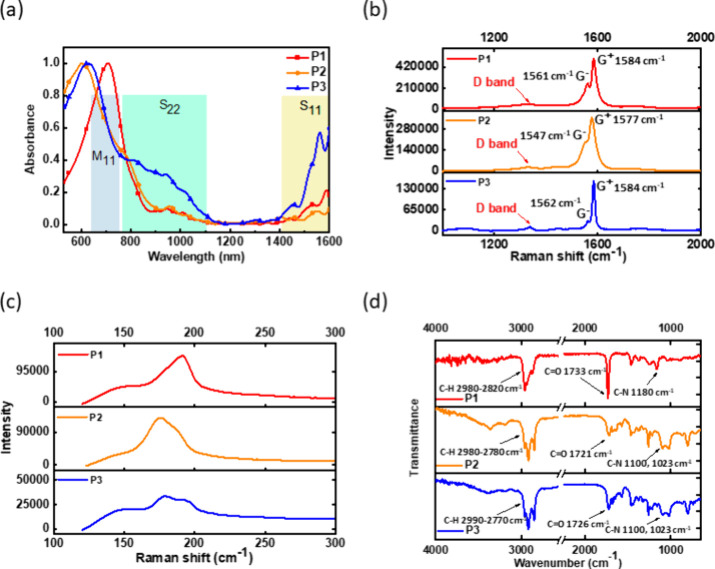
(a) UV–vis absorption spectra of the CP/sc-SWNT sorting solutions. Raman spectra of the drop-cast CP/sc-SWNT films with an excitation wavelength of 633 nm at the bands spanning the range of (b) 1000 to 2000 cm–1 and (c) 100 to 300 cm–1. (d) FT–IR spectra of the CP/s-SWNT films.
Furthermore, to further evaluate the properties of sc-SWNTs, Raman spectroscopy with 633 nm laser excitation was used to measure the drop-cast film from the sorting solutions. Figure 2b,c displays the Raman spectra’s high-wavenumber and low-wavenumber regions, respectively. As can be seen, the G-band shows the SWNT’s features, composed of two main parts in Figure 2b. The G+ band, about 1590 cm–1, represents carbon atoms vibrating along the SWNT axis, while the G– band, about 1570 cm–1, represents carbon atoms vibrating along the circumferential axis of the SWNT. The D-band refers to a specific characteristic associated with structural defects and impurities in SWNTs. Hence, the G- and D-band ratios can be used to evaluate the structural integrity and purity of SWNT. Due to this, the G/D ratios of reported polymers/sc-SWNTs are 9, 15, and 13, meaning that as the side chain modifications are conducted, the defect ratio decreases relatively. Jorio et al. reported the series of Raman spectroscopy of SWNTs, including raw SWNTs and sc-SWNTs.41 It is worth noting that the peak in the D-band of P3 is sharper, in other words, narrower than that of P1. This disparity indicates that, after the wrapping process, sc-SWNTs wrapped by P3 have more uncomplicated defects than those of P1. The broader peak of the D-band might indicate more defects, leading to impurities (such as amorphous carbon) or damage in the structure of sc-SWNTs. The G+/G– ratio of reported polymers is about 1.95, 1.93, and 5.30, respectively, indicating P3 performs better in the ability to bundle longer and less-defect sc-SWNTs. Su et al. report the Raman spectrum of raw SWNTs containing numerous defects and metallic components. The corresponding G+/G– and G/D ratios are approximately 0.94 and 9.2.42 The significantly higher ratios achieved by P3 indicate the efficacy of the conjugated side chain in improving the sorting efficiency of sc-SWNTs. In addition, in the low-wavenumber region (Figure 2c), the radial-breathing mode (RBM) is about 160 to 210 cm–1, referring to a specific vibration mode, typically attributed to characterizing the diameter distribution of SWNTs. For medium-diameter SWNTs, the diameter can be calculated by wRBM = A/dt + B, where wRBM is the width of PD-SWNTs, A = 234 cm–1, and B = 10 cm–1.43 By combining the above equation and data plot, the diameter of PD-SWNTs could be approximately 1.27 nm. The carrier scattering processes are reduced for sc-SWNTs in the diameter range of 1.5–2.0 nm because the phonon energy is comparable to the room-temperature thermal energy.25 Therefore, the developed CP/sc-SWNTs have potential in FET device applications, which will be further investigated in a subsequent section.
With regard to the existence of CPs on sc-SWNTs, Fourier–transform infrared (FT–IR) spectroscopy was applied with the drop-cast film mentioned in the last section. In the spectra displayed in Figure 2d, several characteristic peaks were observed: the peaks at 1100 to 1180 cm–1 represent the stretching of C–N in imide groups; the peaks at 1730 cm–1 represent the stretching of C=O in tertiary amide groups and ester groups; the peaks at 1650 to 2000 cm–1 represent the bending of C–H in aromatic compounds; and the peaks at 2800 to 3000 cm–1 represent the stretching of C–H in side-chain alkanes. Figure S20 shows the FT–IR spectra of the raw SWNT and the pure polymers of P1, P2, and P3. Notably, compared to the polymer powder, the peaks at 3000 to 3500 cm–1 refer to the occurrence of hydrogen bonding caused by the water accidentally mixing in the film during the device fabrication process. The peaks above provide insights into the compositions and structures of CPs in the composites.
Theoretical Calculations of the Polymer/sc-SWNTs
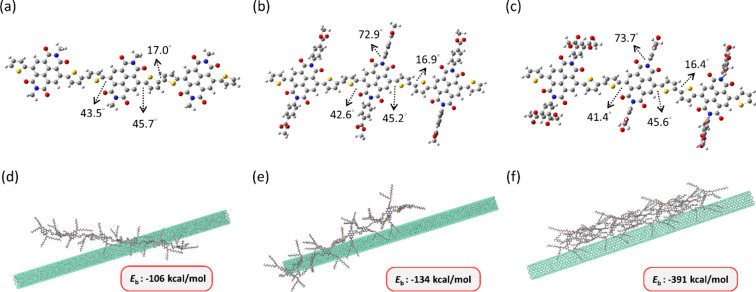
DFT and MD simulations were conducted to further understand how the coplanarity of CPs after side chain modification with different conjugated side groups would impact the whole structure and sorting processes. The DFT calculation was used to optimize the geometry and conformation of the CPs studied, providing a thorough analysis of bond length, bond angles, dihedral angles, etc. In particular, DFT could optimize the dihedral conformers of the CP backbones, elucidating the intrinsic coplanarity of CPs. To simplify the simulations, three NDI and two 2T monomers were used for the calculation and methyl groups were used for replacing long alkyl side chains. As can be seen in Figure 3a–c, P3 has the smallest dihedral angle of 41.4° between the NDI acceptor and the 2T donor units, while P1 and P2 have slightly larger angles of 43.5° and 42.6°, respectively. The dihedral angles in the donor unit are similar at 17.0°, 16.9°, and 16.4° for P1, P2, and P3, respectively. This indicates that the increase in alkyl side chains can undoubtedly have an impact on the coplanarity on the backbone with the same donor–acceptor combination. With regard to the side chains, the phenyl groups in P2 and P3 rotate to 72.9° and 73.7° in conjunction with the NDI moiety due to their steric hindrances. This outcome reveals that the aggregation behaviors are related to the steric hindrances of conjugated side chains and backbone coplanarity. P3 with side-chain branching leads to a more rotated side group and coplanar backbone than P1 and P2, thereby giving rise to its low aggregation fraction; also, it implies that a larger plane for covering the SWNTs and more branched side chains for bundling SWNTs are beneficial for enhancing the purity of sc-SWNTs.
Figure 3.
(a–c) Optimized molecular structures of CPs simulated from DFT calculations and (d–f) MD simulated conformation and binding energy of CP/sc-SWNT for (a, d) P1, (b, e) P2, and (c, f) P3.
The MD simulation can simulate the complex interaction within CPs/sc-SWNTs and more realistically analyze the absorption energies. Figure 3d–f shows the polymer conformation of a CP series of separate donor–acceptor combinations with ten repeat monomers interacting with a 19.6 nm long (9, 9) armchair SWNT using MD simulations. From the simulation snapshots, we can observe how the CPs attach to the SWNTs via π–π interactions. P2 and P3 manifest attachment abilities higher than those of P1. Particularly, we observed that P1 adapts more likely to the P configuration, where the polymer backbone is perpendicular to the SWNT’s surface; in comparison, P3 is becoming more like the T configuration, as the polymer backbone is parallel to the SWNT’s surface. This concept was first proposed by Gomulya et al.25 T configuration can give side chains sufficient space for bundling around the whole SWNT. As the size of the side chains increases, the binding energy (Eb) of CPs/SWNTs becomes more negative at −106, −134, and −391 kcal mol–1 for P1, P2, and P3, respectively. The more negative Eb indicates a better interaction between CP and SWNT. These results suggest that the rise in coplanarities of backbones and side-chain size helps to wrap sc-SWNTs;44 furthermore, the conjugated side groups and the side-chain branching could reduce aggregation ability and disperse well in the solvent for the sorting process. The above simulations can explain why the ϕ value of P2 and P3 improve significantly compared to P1 and present their superior selectivity. These results are also compatible with DFT calculations.
Morphological Characterization of the Polymer/sc-SWNTs Films
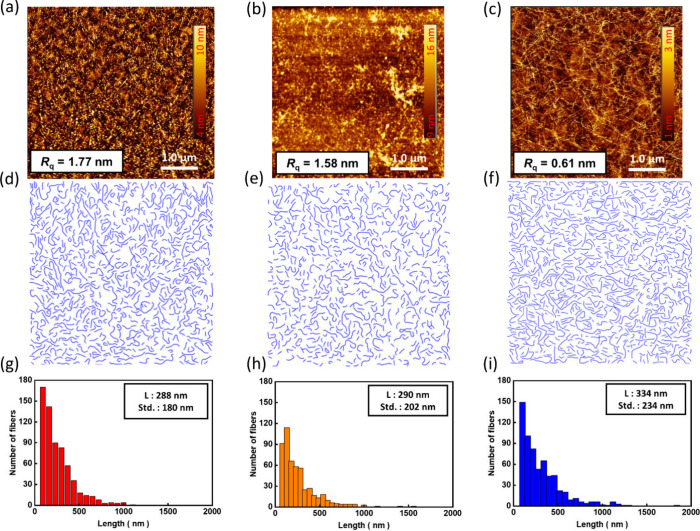
After the molecular interactions were investigated, the morphology of polymers/sc-SWNTs films is further characterized. With the CP/sc-SWNTs solutions, the materials were densely grown onto a hydrophilic substrate, dextran, by sinking the wafer with the sorting solvent. Next, poly(methyl methacrylate) (PMMA) was stuck to the wafer for transferring the CP/sc-SWNTs films to a silicon wafer with bilayered thin films of SiO2 and cross-linked poly(styrene–butadiene–styrene) (SBS) rubber.45 More detailed experimental steps are shown in the Experimental Section. The AFM technique was used, and the topographies are presented in Figure 4a–c. As can be seen, the film of P1/sc-SWNTs shows several nanotubes lying on the substrate, and some black dots refer to the aggregation of P1 (Figure 4a). Figure 4b displays the film of P2/sc-SWNTs, where barely a few nanotubes can be seen. This is due to the poor solubility of the polymer in toluene, leading to the lowest yield of all; the film of P3/sc-SWNTs is shown in Figure 4c, and many nanotubes can be observed clearly in the figure, showing the same outcome compatible with simulations. Then, the relative roughness (Rg) of P1, P2, and P3 becomes smoother for 1.77, 1.58, and 0.61 nm, respectively, representing the nanotubes growing more uniformly on the substrate. To realize the morphology of grown CP/sc-SWNTs, the mapping technique extracted from the GTFiber program developed by Persson et al. was applied to display the details of nanotubes in Figure 4d–f,46 and the corresponding statics of nanotube lengths are shown in Figure 4g–i. The screening parameters of topographic mapping are presented in Figure S21 (Supporting Information). Compared to P1 and P2, P3 shows more nanotubes, indicating that the wrapping ability of P3 is better. From the length statics, the film of P3/sc-SWNTs shows longer nanotubes than that of P1/sc-SWNTs. Through the side-chain modification and biaxial extension of the backbone, P3 owns the specialties of longer sc-SWNTs with better percolation on the surface. This result strongly relates to the binding energy and coplanarity of CPs, providing better wrapping ability of the sc-SWNTs and is compatible with the trend of the G+/G– ratio.44 The extension of phenyl groups provides a better interaction covering the circumferential axis; thus, the backbone can extend for bundling with sc-SWNTs.
Figure 4.
(a–c) AFM topographies, (d–f) nanotube morphology mapping of the AFM image, and (g–i) the statistical length distribution, indicating the average SWNT length and standard deviations of CP/sc-SWNT comprising (a, d, g) P1, (b, e, h) P2, and (c, f, i) P3.
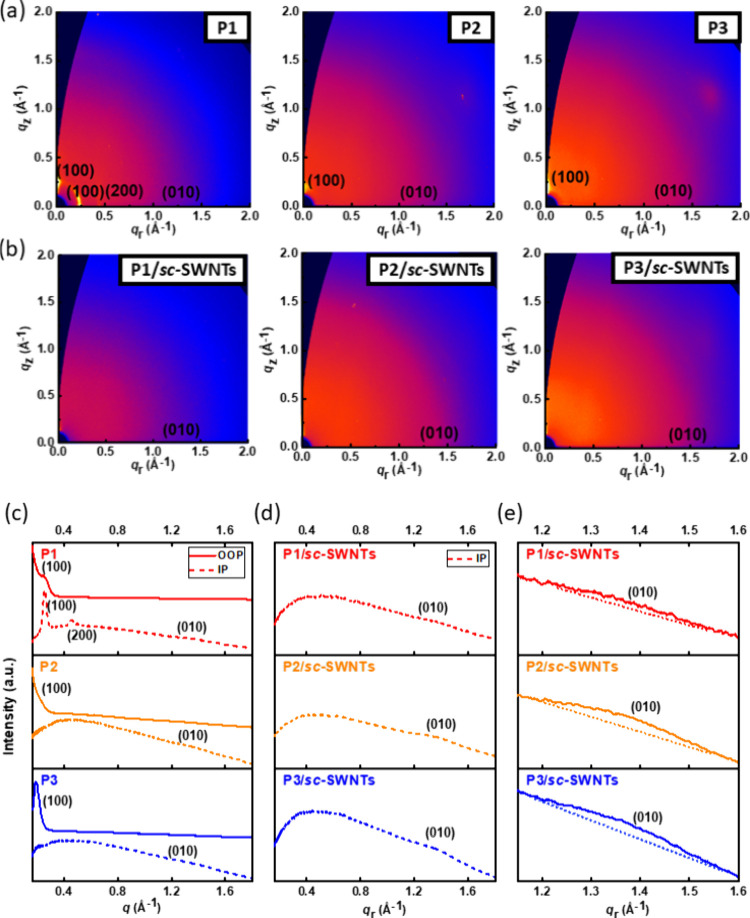
GIXD techniques were applied to gain insight into the crystallographic parameters, molecular packing, and orientation of the CP and CP/sc-SWNT films. Figure 5a shows the 2D-GIXD patterns of each polymer film; Figure 5b shows the measured 2D-GIXD patterns of CPs/sc-SWNTs films, and their corresponding out-of-plane (OOP) and in-plane (IP) 1D-scanning profiles are displayed in Figures 5c,d and S22, respectively. The relevant crystallographic parameters are summarized in Table S3 (Supporting Information) for pristine CPs and in Table S4 (Supporting Information) for CP/sc-SWNTs. From Figure 5a,c, along the qz- and qr-axis in the profiles, P1 has more obvious (n00) diffraction peaks than P2 and P3. This indicates that the crystallinities are reduced via conjugated side-chain modifications and the face-on orientations are inhibited. The values of lamellar stacking distance (d100) of each CP are 25.1, 31.9, and 34.3 Å for P1, P2, and P3, respectively. This reveals that introducing conjugated side chains would influence the alignment of the polymers. After the sorting processes, the lamellar stacking peaks along the qz-axis (out-of-plane direction, OOP) are barely seen, but the π–π stacking peaks along the qr-axis in the in-plane profile (IP, Figure 5d) increase. This result shows that, after bundling sc-SWNTs, polymers have weaker lamellar stacking in comparison to pure polymer films. This might be attributed to the polymer chains arranged more closely, forming an alignment parallel to the surface of the nanotubes. In more detail, these profiles show the morphology of CPs/sc-SWNTs existing in only π–π stacking along the radial orientation between CPs, owing to the side chains bundling with sc-SWNTs. In contrast, lamellar stacking around SWNTs or between CPs does not appear. According to the calculation of IP (010) diffractions in Figure 5d, the π–π stacking distances (d010) are 4.65, 4.61, and 4.59 Å for P1, P2, and P3, respectively. The CPs with conjugated side chains show narrower π–π stacking than P1. This result coincides with the simulated conformations, indicating that P3 exhibited better backbone coplanarity. In addition, a good π–π stacking between CPs can warrant a compact packing of CP/sc-SWNTs in the solid state because of the low steric hindrance between CPs, which is conducive to charge transport in FET devices. Next, the values of paracrystalline disorder (g010) are 16.9%, 17.5%, and 17.6%, respectively (Table S4, Supporting Information), indicating a similar mechanism for CPs wrapping around SWNTs.47 These results benefit the interaction of CPs/sc-SWNTs and are favorable for FET device applications.48
Figure 5.
(a, b) 2D GIXD patterns and (c–e) the extracted 1D GIXD line-cutting profiles of (a, c) the spin-coated CP films and (b, d, e) the CP/sc-SWNT films. Note that the 1D GIXD profiles were integrated along the OOP/IP directions for the pristine polymer films and the IP direction for the CP/sc-SWNT films, and the dotted line inside (e) indicates the baseline of π–π stacking diffraction spanning the range of 1.2 to 1.5 Å–1.
FET Device Characterization of Polymer/sc-SWNTs
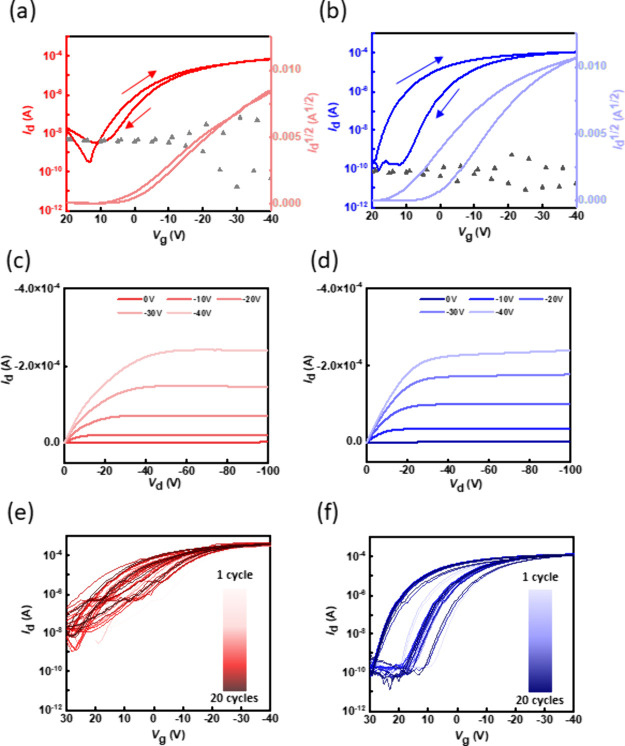
After realizing the relationships and morphologies inside CPs/sc-SWNTs, the FET device performance of CP/sc-SWNTs was finally characterized. With the CP/sc-SWNTs films transferred on a bilayered thin film of SiO2 and cross-linked poly(styrene–butadiene–styrene) (SBS) rubber, the bilayered thin films can serve as the gate dielectrics in FET device operations. Then, the device was thermally deposited with top-contact gold electrodes to form a FET device. The bottom-gate/top-contact (BG/TC) device architecture is illustrated in Figure 1b. The fabrication procedure of the device is detailed in the Experimental Section. At the same time, the yield of P2 is seriously affected by the solubility, leading to poor ability to fabricate an FET device. Figure 6a,b presents the transfer curves of P1 and P3, showing typical p-type characteristic curves managed by the channel of sc-SWNTs. The corresponding hole mobility (μh), threshold voltage (Vth), and on–off current ratio (Ion/Ioff) are summarized in Table S5 (Supporting Information). The device parameters were averaged among five devices per batch and from two batches. Transfer characteristics were recorded by sweeping the gate voltage (Vg) from 20 to −40 V, as can be seen in Figures 6a,b and S23; the maximum drain current can reach as high as 10–4–10–3 A. Accordingly, the μh values of P1 are 0.48 ± 0.03 cm2 V–1 s–1 at Vd = −10 V and 2.21 ± 0.12 cm2 V–1 s–1 at Vd = −100 V; those for P3 are 0.99 ± 0.08 cm2 V–1 s–1 at Vd = −10 V and 4.72 ± 0.58 cm2 V–1 s–1 at Vd = −100 V, indicating that, through side-chain modification, the mobility of P3/sc-SWNTs as the channel layer gets significantly increased. This result is compatible with the findings discussed in the morphology section, attributed to higher purity, stronger binding energy, and longer sc-SWNTs. Through calculation, the corresponding maximum transconductance (gm) values of P1 and P3 are 4 and 7 nS/μm. This indicates that, compared to P1, P3 has a smaller change in Vg, leading to a significant change in Id, making the device more efficient in amplifying signals.49 In addition, the relatively low gm can be attributed to the low capacitance of these devices fabricated based on 300 nm-thick SiO2 wafers. With regard to the device hysteresis and Vth values, Dallaire et al. reported that using an octyltrichlorosilane (OTS) modified device process and appropriate wavelength of light exposure to the device would lower the Vth for about 4 V and the dark currents, reducing the activation energy required for activating the device.50 The OTS dielectric layer would provide relatively low interfacial charge traps due to the hydrophobic characteristics, leading to better electric properties. While using dextran and SBS for fabricating devices in the film-transfer process in this work, we could grow more CPs/sc-SWNTs for our device. In this work, the AFM topographies show significantly the whole scene of sc-SWNTs. Compared to the reported system,31 the μh values of the device with pure sc-SWNTs (5.3 cm2 V–1 s–1) are about five times higher than the devices in this study. This disparity shows the different charge affinities between sc-SWNTs and these n-type CPs. While P3 has a more considerable hysteresis of about 8 V, it might be the combinatory result of adsorbed water and oxygen present at the interface between the channel and dielectric layers caused by the film-transfer process in fabricating the device.51−53 In addition, Srimani et al. applied an advanced sc-SWNT purification approach by adding silica gel particles after the first centrifugation, which can further lower the dark current of the device.54 Notably, P3 has a better Ion/Ioff ratio because the dark current of P3 is lower than that of P1 (Figure 6a,b). The dark current is dependent on charge traps and sc-SWNT packing densities.50P1 has higher SWNT densities (higher yield) and trap densities than P3, leading to a relatively higher dark current caused by the higher m-SWNTs and amorphous carbon contents, as seen in the broad background in Figure S19a. The quantized trap density is further discussed in the subsequent section.
Figure 6.
(a, b) Transfer characteristic curves, (c, d) output characteristic curves, and (e, f) endurance tests by applying 20 consecutive transfer sweeps of the devices comprising (a, c, e) P1/sc-SWNT and (b, d, f) P3/sc-SWNT. Note that the gray scattered dots represent the gate current measured, and the transfer curve was forwardly swept from 20 to −40 V at Vd = −10 V for p-type operation.
As for the device comprising P2/sc-SWNTs, Figure S24 (Supporting Information) displays the corresponding transfer and output curve of the device. The low yield of P2/sc-SWNT gives rise to its poor device performance. Figure 6c,d displays P1 and P3’s output curves, showing that drain currents can be scaled when Vg is given. With the same Vg, P3/sc-SWNT turns to on-state faster at a smaller Vd than P1/sc-SWNT. This electrical property enables the device to switch faster, as it can quickly respond to the saturation state with lower voltages. In addition, the saturated on-state currents of P3/sc-SWNT at different Vg are also higher than those of P1/sc-SWNT. Although the general mobility of the device with commercial SWNTs is still about an order higher than that in this study, the sorting processes with specific CPs can greatly help wrap the consistent length of sc-SWNTs without impurities and different chiralities taken from commercial SWNTs,55,56 and the scalability of CP/sc-SWNT has potential.
Regarding pure CPs for the channel layers (Figure S25, Supporting Information), the typical n-type characteristic curves and the electron mobility (μe) are low enough to be neglected in conjunction with sc-SWNT. The device data are summarized in Table S6 (Supporting Information). Nevertheless, to preclude the contribution of electron transport, n-type operations with Vg swept from −20 to 40 V were conducted again to check whether the CPs formed a conductive channel. The transfer characteristics of P1/sc-SWNTs and P3/sc-SWNTs are demonstrated in Figure S26 (Supporting Information). As seen in the profiles, the drain currents in the positive Vg range are 1,000 times lower than those in the negative Vg range. Therefore, μe is much smaller than μh, indicating that the n-type channel does not constitute the conduction.
With regard to operational stability, Figure 6e,f displays consecutive sweeping profiles. Although the mobilities were the same, the off current of P1/sc-SWNTs increased after 20 cycles, and |Vth| became higher. It reveals its instability in electrical endurances. However, P3/sc-SWNTs maintained almost the same trend in transfer characteristics as the first cycle after sweeping 20 times, indicating better endurance and stability. For quantifying this instability, we further applied the following equation: Ntr = Ci·[qS/kBT·ln(10) – 1]/q, where q is the elementary charge, S is the subthreshold swing (V/decade), Ntr is the maximum interfacial trap density estimated from S, kB is Boltzmann’s constant, and Ci is the capacitance of the bilayered gate dielectrics (8.61 nF cm–2). The calculated results are summarized in Table S5 (Supporting Information). Compared to P3, P1 has a higher Ntr, indicating a more significant number of interfacial hole traps, which is relatively detrimental to the device operation.57 Next, a bias-stress test was conducted to observe their long-term stability. The time-dependent stability of the drain current was recorded under constant bias stress of Vg = −10 V and Vd = −1 V for over 9000 s (Figure S27, Supporting Information). The corresponding transfer curves at Vd = −1 V are displayed in Figure S28 (Supporting Information) to confirm the current levels observed in the bias stress test. As seen in the profiles, the drain current of P1 is not as stable as that of P3, although P3 still has an increasing current.58 These results can be attributed to the poor aggregation of P3 and the better ability of bundle sc-SWNTs to fill up the interface between them. Although the gating effect on the sc-SWNTs channel is not negligible, it can be further improved by testing the device in a nitrogen atmosphere.
Conclusion
In conclusion, side-chain modified CPs with biaxially extended conjugations were synthesized and applied for wrapping sc-SWNTs. With modifications, the increasing steric hindrances sufficiently disperse CPs well in the toluene. The alkyl-phenyl groups as side chains not only enlarge the side chain pattern but also rotate the side chain moieties proximal to the backbones, so the CP’s aggregation behavior decreases. The aggregation behaviors are related to the steric hindrances of conjugated side chains and the backbone coplanarity. The conjugated side groups and the side-chain branching could reduce aggregation ability and disperse well in the solvent for the sorting process. The phenyl groups provide sufficient spaces for the alkyl chains, making them available to bundle sc-SWNTs. As the size of the side chains increases, the interaction between CP/sc-SWNTs is enhanced. The simulated result is compatible with the experimental findings. The high performance of P3/SWNTs is attributed to the higher purity, stronger binding energy, longer sc-SWNTs, and narrower π–π spacing between the CPs. Accordingly, the device composed of P3/sc-SWNTs exhibited better device performance with higher μh of 4.72 cm2 V–1 s–1 than that of 2.21 cm2 V–1 s–1 for P1/sc-SWNTs. In addition, P3/sc-SWNTs showed better sweeping endurance stability than did P1/sc-SWNTs. In comparison, the performance of P2/sc-SWNTs is confined due to their poor solubility. In summary, side-chain modifications with biaxially extended conjugation are conducive to improving the sorting efficiency of NDI-based CPs with respect to sc-SWNTs. The purer sc-SWNTs give rise to advanced FET device performance. The underlying structure–performance relationship of biaxially extended CPs deserves further investigation.
Experimental Section
Materials
Naphthalene-1,4,5,8-tetracarboxylic dianhydride (>97%), dibromoisocyanuric acid (>97%), 4-aminobenzoic acid (>99%), 5-aminoisophthalic acid (>94%), acetic acid glacial (>99.7%), 2-octyl-1-dodecanol (ODOH, >97%), 4-(dimethylamino)pyridine (DMAP, >99%), N1-((ethylimino)methylene)-N3,N3-dimethylpropane-1,3-diamine hydrochloride (EDC-HCl, >95%), tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3, >98%), 5,5′-bis(trimethylstannyl)-2,2′-bithiophene (2T, >97%), tri(o-tolyl)phosphine (P(o-tolyl)3, >98%), anhydrous chlorobenzene (CB, >99.8%), 2-bromothiophene (>98%), 2-(tributylstannyl)thiophene (>97%), dextran, poly(methyl methacrylate) (PMMA), pentaerythritol tetrakis(3-mecraptopropionate) (>95%), phenylbis(2,4,6-trimethylbenzoyl) (>97%), and polystyrene-block-polybutadiene (30% styrene, SBS) were purchased from Sigma-Aldrich, Tokyo Chemical Industry Co., Thermo Fisher Scientific, Acro Organics B.V.B.A., DUKSAN, Alfa Aesar, Ultra Fine Chemical Technology Corp., and Luminescence Technology Corp. Plasma discharge-SWNTs (PD-SWNTs) were ordered from Yuang Hong Inc. and NanoIntegris Inc. The above chemicals were used as received without further purification. 2,6-Dibromonaphthalene-1,4,5,8-tetracarboxylic dianhydride (Br-NDA-Br) and 4,9-dibromo-2,7-bis(2-octyldodecyl)benzo[lmn][3,8]-phenanthroline-1,3,6,8(2H,7H)-tetraone (Br-NDI-Br) were synthesized following the reported method.59
Synthesis of 4,4′-(4,9-Dibromo-1,3,6,8-tetraoxo-1,3,6,8-tetrahydrobenzo[lmn][3,8]phenanthroline-2,7-diyl)dibenzoic Acid (M1)
Br–NDA–Br (320 mg, 0.75 mmol), 4-aminobenzoic acid (309 mg, 2.25 mmol), and glacial acetic acid (5 mL) were placed in a 50 mL flask. The solution was then stirred and refluxed under N2 at 100 °C overnight. After the reaction, the solution was poured into water and dried; subsequently, it was dissolved in methanol and precipitated in dichloromethane to afford M1 as a dark red solid (345 mg, 69%). 1H NMR (500 MHz, DMSO-d6, δ ppm, 25 °C, Figure S2): 8.78 (s, 2H), 8.13 (t, J = 16 Hz, 4H), 7.6 (d, J = 8 Hz, 4H). 13C NMR (100 MHz, DMSO-d6, δ ppm, 25 °C, Figure S3): 119.45, 122.24, 124.83, 125.89, 129.99, 130.91, 134.50, 136.07, 138.52, 142.79, 160.86, 164.54, 166.83. Anal. Calcd for C28H12Br2N2O8 (%): C, 50.6; H, 1.8; N, 4.2. Found (%): C, 48.8; H, 1.9; N, 3.9.
Synthesis of 5,5′-(4,9-Dibromo-1,3,6,8-tetraoxo-1,3,6,8-tetrahydrobenzo[lmn][3,8]phenanthroline-2,7-diyl)diisophthalic Acid (M2)
Br–NDA–Br (320 mg, 0.75 mmol), 5-aminoisophthalic acid (406 mg, 2.25 mmol), and acetic acid glacial (5 mL) were placed in a 50 mL flask. The solution was then stirred and refluxed under N2 at 100 °C overnight. After the reaction, the solution was poured into water and dried; subsequently, it was dissolved in methanol and precipitated in dichloromethane to afford M2 as a dark red solid (542 mg, 96%). 1H NMR (500 MHz, DMSO-d6, δ ppm, 25 °C, Figure S4): 8.78 (s, 2H), 8.59 (t, J = 3.5 Hz, 2H), 8.32 (d, J = 1.5 Hz, 4H). 13C NMR (100 MHz, DMSO-d6, δ ppm, 25 °C, Figure S5): 124.29, 126.24, 128.05, 131.62, 132.29, 134.22, 136.38, 139.86, 161.06, 165.93, 168.77, 169.55. Anal. Calcd for C30H12Br2N2O12 (%): C, 47.9; H, 1.6; N, 3.7. Found (%): C, 48.3; H, 3.0; N, 5.0.
Synthesis of Bis(2-octyldodecyl)4,4′-(4,9-dibromo-1,3,6,8-tetraoxo-1,3,6,8-tetrahydrobenzo[lmn][3,8]phenanthroline-2,7-diyl)dibenzoate (M3)
ODOH (664 mg, 2.2 mmol), DCM (4.2 mL), DMF(0.8 mL), DMAP (61 mg, 0.5 mmol), EDC-HCl (480 mg, 2.5 mmol), and M1 (664 mg, 1 mmol) were placed in a 50 mL flask. The solution was stirred under N2 at 50 °C overnight. The solution was rotary-evaporated and extracted with dichloromethane and water for a slurry-like crude product. Then, the mixture was rinsed with methanol to afford M3 as a light red solid (210 mg, 17%). 1H NMR (500 MHz, CDCl3, δ ppm, 25 °C, Figure S6): 9.08 (s, 2H), 8.26 (d, J = 8.5 Hz, 4H), 7.42 (d, J = 8.5 Hz, 4H), 4.29 (d, J = 5 Hz, 4H), 1.81 (q, J = 23.5, 12 Hz, 2H), 1.55 (s, 4H),1.34–1.22 (br, 66H), 0.91–0.85 (br, 6H). 13C NMR (100 MHz, CDCl3, δ ppm, 25 °C, Figure S7): 14.10, 22.67, 26.79, 29.34, 29.57, 29.66, 29.98, 31.90, 124.47, 125.64, 128.27, 128.66, 129.25, 130.94, 131.65, 138.18, 139.64, 160.61, 160.68, 165.71. Anal. Calcd for C68H92Br2N2O8 (%): C, 62.2; H, 7.0; N, 2.1. Found (%): C, 66.3; H, 7.0; N, 3.5.
Synthesis of Tetrakis(2-octyldodecyl)5,5′-(4,9-dibromo-1,3,6,8-tetraoxo-1,3,6,8-tetrahydrobenzo[lmn][3,8]phenanthroline-2,7-diyl)diisophthalate (M4)
ODOH (1090.3 mg, 3.65 mmol), DCM (4.5 mL), DMF(1.0 mL), DMAP (61 mg, 0.5 mmol), EDC-HCl (796 mg, 4.15 mmol), and M2 (626.5 mg, 0.83 mmol) were placed in a 50 mL flask. Then, the solution was stirred and refluxed under N2 at 50 °C overnight. The solution was rotary-evaporated and extracted with dichloromethane and water to afford a slurry-like mixture. The mixture was washed with methanol to afford M4 as a light red solid (225 mg, 14.5%). 1H NMR (500 MHz, CDCl3, δ ppm, 25 °C, Figure S8): 8.90 (s, 2H), 8.82 (t, J = 3.0 Hz, 2H), 8.19 (d, J = 2.0 Hz, 4H), 4.29 (d, J = 5.5 Hz, 8H), 1.80 (q, J = 24.0, 12.0 Hz, 8H), 1.55 (s, 8H), 1.33–1.19 (br, 128H), 0.90–0.83 (br, 12H). 13C NMR (100 MHz, CDCl3, δ ppm, 25 °C, Figure S9):14.09, 22.67, 26.89, 29.33, 30.93, 31.90, 37.43, 40.54, 65.77, 68.49, 122.67, 126.25, 127.67, 131.33, 133.90, 134.67, 136.59, 141.16, 160.32, 160.71, 164.80. Anal. Calcd for C110H172Br2N2O12 (%): C, 69.0; H, 9.0; N, 1.5. Found (%): C, 70.3; H, 8.3; N, 3.3.
General Procedure for Migita–Kosugi–Stille Coupling Polymerization
The synthetic route for CPs is shown in Scheme 1. For a typical synthesis of P1, Br–NDI–Br (705 mg, 0.64 mmol), 2T (316 mg, 0.64 mmol), and CB (6.4 mL) were placed into a 50 mL two-necked flask. To this solution, N2 was bubbled in for 30 min. Pd2(dba)3 (58.8 mg, 0.064 mmol) and P(o-tolyl)3 (195.6 mg, 0.64 mmol) were added to the solution, and the solution was stirred and refluxed under N2 at 120 °C for 24 h. After the reaction, 2-bromothiophene (19 μL) and 2-(tributylstannyl)thiophene (61 μL) were individually added to end-cap the reaction at 120 °C for 2 h. After the reaction was cooled to room temperature, the crude polymer was precipitated in methanol and suction-filtered for a dark blue solid product. Then, the product was further purified by Soxhlet extraction using acetone and n-hexanes and recovered by chloroform (24 h for each). After rotary-evaporating chloroform, the polymer was redissolved in chloroform for saturation and precipitated in methanol again to afford a dark blue solid (550 mg, 78.2%; the 1H and 13C NMR spectra are shown in Figures S10 and S11).
P2.M3 (197 mg, 0.16 mmol), 2T (78.7 mg, 0.16 mmol), Pd2(dba)3 (8.8 mg, 0.016 mmol), P(o-tolyl)3 (14.6 mg, 0.05 mmol), CB (8 mL), 2-bromothiophene (4.7 μL), and 2-(tributylstannyl)thiophene (10.2 μL). Dark green solid (100 mg, 55%; the 1H and 13C NMR spectra are shown in Figures S12 and S13).
P3.M4 (200 mg, 0.11 mmol), 2T (52.6 mg, 0.11 mmol), Pd2(dba)3 (8.6 mg, 0.01 mmol), P(o-tolyl)3 (9.8 mg, 0.03 mmol), CB (5.2 mL), 2-bromothiophene (3.1 μL), and 2-(tributylstannyl)thiophene (12.3 μL). Dark green solid (130 mg, 67%; the 1H and 13C NMR spectra are shown in Figures S14 and S15).
Sorting Procedure of sc-SWNTs
The n-type CPs of 5 mg were dissolved in toluene (20 mL), and the solution was fully dissolved using the ultrasonic cleaner DC300H (DELTA Ultrasonic Co., Ltd.). Then, PD-SWNTs (10 mg) were added with a weight ratio of CP/SWNTs = 1:2. The mixture was then sonicated with 40% amplitude for 30 min by using a VCX750 (Sonic & Materials, INC.), and isopropanol was used for an ice bath to maintain −60 °C. Next, the sorting solutions were centrifuged at 12 000 rpm (relative centrifugal force, RCF = 27 300g) and 25 °C for 1 h on a FL3012 (FANLINYL). Finally, the sorting solutions with enriched sc-SWNTs were extracted without the remaining impurities in the SWNTs. Note that, when sorting SWNTs with P2, the solution was sonicated at 20 °C because P2 would precipitate at low temperature, resulting in a poor yield.
Fabrication of FET Devices
Through sequential bath sonication, the Si/SiO2 wafers with an oxide thickness of 300 nm and size of 1.5 × 1.5 cm were cleaned via toluene, isopropanol, acetone, deionized water, and ethanol. Wafers were dried with N2 and cleaned in an oxygen plasma for 5 min. To fabricate the FET with a BG/TC configuration, an aqueous dextran solution (30 mg mL–1) was spin-coated on a wafer at 1000 rpm for 60 s. After spin-coating, the wafer was dried at 140 °C for 10 min to remove the remaining water. Then, the wafer was soaked in a diluted CP/sc-SWNT sorting solution (mother liquid/fresh toluene = 2:1 vol/vol) for 3 days. After the process, the wafer was rinsed with more than 20 mL of toluene 4 times to remove excess unbound polymer and solution;60 then, the wafer was spin-coated with PMMA (40 mg mL–1 in toluene) at 1000 rpm for 60 s. SBS, phenylbis(2,4,6-trimethylbenzoyl), and pentaerythritol tetrakis(3-mercaptopropionate) had a weight ratio of 25:1:1 in toluene (solid content: 1.6 wt %). The solution was spin-coated on another piece of silicon wafer with a 300 nm-thick SiO2 layer, and then, the film was heated at 120 °C for 10 min and photo-cross-linked to serve as a polymer dielectric. For the film peeling process, the bilayered PMMA/sc-SWNT film was stuck with tape and the film was peeled off from the wafer by sinking it into a water bath. For the film-transfer process, the film was transferred to the SBS-coated wafer with a covered drop of water and we waited until the water evaporated. Subsequently, the film was cut off, and a 40 nm-thick Au layer was thermally deposited with a channel length (L) and width (W) of 100 and 2000 μm, respectively, through a mask to obtain the top-contact electrode.
Characterization
The chemical structure,
thermal, and
electrochemical characterization approaches are described in the Supporting Information. UV–vis–NIR
absorption spectra were recorded using Jasco V-770 scanning from 200
to 1600 nm. To calculate the degree of aggregation of reported CPs
in toluene, the CPs were dissolved in toluene and 1-chloronaphthalene
at 0.25 and 0.05 mg mL–1, respectively, and the
latter could represent the disorder fraction. The aggregation fraction
of CPs in toluene could be obtained by comparing the optical absorption
of two samples, and the calculation was based on the reported method.61 Raman and FT–IR spectra of the CPs/sc-SWNTs films drop-cast on the glass substrate were measured
with an excitation wavelength of 633 nm by a UniDRON (CL Technology
Co., Ltd.) and a Nicolet 6700 (Thermo Scientific) spectrometer. The
morphology of the CPs/sc-SWNTs films was probed by
an AFM100plus (Hitachi), and the film thickness was measured using
an Alpha-Step D-300 (KLA). Grazing incidence X-ray diffraction (GIXD)
profiles of the CPs and CPs/sc-SWNT films were determined
on beamlines 13A1 at the National Synchrotron Radiation Research Center
(NSRRC), Taiwan, with an X-ray wavelength of 1.027 Å and an incident
angle at 0.12°. For the calculation of paracrystalline disorder
(g), 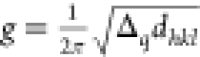 , where Δq is the full width at half the maximum of a diffraction peak
and dhkl is the interplanar
separation
along the crystallographic direction.62 All FET performance was documented using a Keithley 4200-SCS semiconductor
parameter analyzer (Keithley Instruments, Inc.). Note that the testing
was performed under ambient conditions. The capacitance of gate dielectric
was determined as follows:
, where Δq is the full width at half the maximum of a diffraction peak
and dhkl is the interplanar
separation
along the crystallographic direction.62 All FET performance was documented using a Keithley 4200-SCS semiconductor
parameter analyzer (Keithley Instruments, Inc.). Note that the testing
was performed under ambient conditions. The capacitance of gate dielectric
was determined as follows: 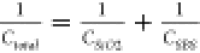 , where Ctotal is the areal capacitance of the SiO2 and
SBS bilayered
dielectrics,63CSiO2 is the areal capacitance of SiO2, and CSBS is the capacitance of SBS. The hole mobility (μh) and threshold voltage (Vth)
were calculated following the slope or extrapolation of the square
root of drain-to-source current (Ids1/2) versus gate voltage (Vg) in
the saturation region of the transfer curves:
, where Ctotal is the areal capacitance of the SiO2 and
SBS bilayered
dielectrics,63CSiO2 is the areal capacitance of SiO2, and CSBS is the capacitance of SBS. The hole mobility (μh) and threshold voltage (Vth)
were calculated following the slope or extrapolation of the square
root of drain-to-source current (Ids1/2) versus gate voltage (Vg) in
the saturation region of the transfer curves: 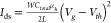 , where W and L are the width and
length of the channel electrodes. The transconductance
(gm) is a key parameter in FETs. It measures
the device’s ability to convert a change in Vg to a change in Id, and the
relationship is defined as gm = dId/dVg. For the saturation
region,
, where W and L are the width and
length of the channel electrodes. The transconductance
(gm) is a key parameter in FETs. It measures
the device’s ability to convert a change in Vg to a change in Id, and the
relationship is defined as gm = dId/dVg. For the saturation
region, 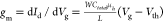 .
.
Simulation
For the DFT calculations, the Avogadro software was applied to minimize the energy of chemical structures of the polymers under the Merck molecular force field 94 static variant (MMF94s) force field.64 Then, the structure was applied for the Gaussian09 W program with the Becke, 3 parameters, Lee–Yang–Parr (B3LYP) method, and a 6-31G basis to obtain the optimizing ground state configurations of the chemical structures. Note that the polymer’s structure was represented using three repeating units, and the alkyl side-chain was replaced with a methyl group to simplify the simulation. The MD calculations followed the reported method to evaluate the interaction within CPs/sc-SWNTs.65 The simulations interacted with the armchair (9,9) SWNTs under vacuum, and the length was set as 19.67 nm (12 repeats). In the software framework of Material Studio, the COMPASSIII force field was used as an adsorption locator tool to simulate the attachment of CPs on the surface of SWNTs.66 Note that the CPs with ten repeating units were attached to the surface of SWNTs to simulate their interactions and binding energy by applying one cycle with two million steps.
Acknowledgments
The authors are thankful for the financial support from the National Science and Technology Council in Taiwan (NSTC 111-2222-E-006-020-MY2; 111-2628-E-224-001) and the Featured Area Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (113L9006). The authors also acknowledge the National Synchrotron Radiation Research Center (NSRRC) of Taiwan for the GIXD experiments in BL13A (TLS). The authors gratefully acknowledge the use of NMR (NMR005000), elemental analysis (EA000600), and the film thickness measurement (OTHER003600) of NSTC 112-2740-M-006-001 belonging to the Core Facility Center of National Cheng Kung University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.4c08981.
Thermal analysis of TGA and DSC profiles for the polymers; NMR spectra, GPC profiles, and electrochemical characteristics of the polymers; FET transfer/output characteristics of the reference devices; 1D GIXD profiles of the CP/sc-SWNT along the out-of-plane direction and the relevant crystallographic parameters of the films (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Liu D.; Mun J.; Chen G.; Schuster N. J.; Wang W.; Zheng Y.; Nikzad S.; Lai J. C.; Wu Y.; Zhong D.; et al. A Design Strategy for Intrinsically Stretchable High-Performance Polymer Semiconductors: Incorporating Conjugated Rigid Fused-Rings with Bulky Side Groups. J. Am. Chem. Soc. 2021, 143 (30), 11679–11689. 10.1021/jacs.1c04984. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Zhang S.; Tok J. B.; Bao Z. Molecular Design of Stretchable Polymer Semiconductors: Current Progress and Future Directions. J. Am. Chem. Soc. 2022, 144 (11), 4699–4715. 10.1021/jacs.2c00072. [DOI] [PubMed] [Google Scholar]
- Ding L.; Yu Z.-D.; Wang X.-Y.; Yao Z.-F.; Lu Y.; Yang C.-Y.; Wang J.-Y.; Pei J. Polymer Semiconductors: Synthesis, Processing, and Applications. Chem. Rev. 2023, 123 (12), 7421–7497. 10.1021/acs.chemrev.2c00696. [DOI] [PubMed] [Google Scholar]
- Kim M.; Ryu S. U.; Park S. A.; Choi K.; Kim T.; Chung D.; Park T. Donor–Acceptor-Conjugated Polymer for High-Performance Organic Field-Effect Transistors: A Progress Report. Adv. Funct. Mater. 2020, 30 (20), 1904545. 10.1002/adfm.201904545. [DOI] [Google Scholar]
- Ashizawa M.; Zheng Y.; Tran H.; Bao Z. Intrinsically Stretchable Conjugated Polymer Semiconductors in Field Effect Transistors. Prog. Polym. Sci. 2020, 100, 101181. 10.1016/j.progpolymsci.2019.101181. [DOI] [Google Scholar]
- Sung C. Y.; Lin C. Y.; Chueh C. C.; Lin Y. C.; Chen W. C. Investigating the Mobility-Compressibility Properties of Conjugated Polymers by the Contact Film Transfer Method with Prestrain. Macromol. Rapid Commun. 2024, 45 (1), e2300058 10.1002/marc.202300058. [DOI] [PubMed] [Google Scholar]
- Wu H. C.; Lai Y. C.; Chiu Y. C.; Lee W. Y.; Chen W. C. Syntheses of Biaxially Extended Octithiophene-Based Conjugated Copolymers for High-Open-Circuit-Voltage Photovoltaic-Cell Applications. Macromol. Chem. Phys. 2014, 215 (7), 638–647. 10.1002/macp.201300765. [DOI] [Google Scholar]
- Lu C.; Wu H. C.; Chiu Y. C.; Lee W. Y.; Chen W. C. Biaxially Extended Quaterthiophene– and Octithiophene–Vinylene Conjugated Polymers for High Performance Field Effect Transistors and Photovoltaic Cells. Macromolecules 2012, 45 (7), 3047–3056. 10.1021/ma300313y. [DOI] [Google Scholar]
- Tsai C.-H.; Su Y.-A.; Lin P.-C.; Shih C.-C.; Wu H.-C.; Chen W.-C.; Chueh C.-C. High-Performance Ternary Polymer Solar Cells Using Wide-Bandgap Biaxially Extended Octithiophene-Based Conjugated Polymers. J. Mater. Chem. C 2018, 6 (26), 6920–6928. 10.1039/C8TC01542B. [DOI] [Google Scholar]
- Chao P.-Y.; Wu H.-C.; Lu C.; Hong C.-W.; Chen W.-C. Biaxially Extended Conjugated Polymers with Thieno[3,2-b]thiophene Building Block for High Performance Field-Effect Transistor Applications. Macromolecules 2015, 48 (16), 5596–5604. 10.1021/acs.macromol.5b01243. [DOI] [Google Scholar]
- Huang Y.-W.; Lin Y.-C.; Wu Y.-S.; Wong Y.-T.; Kuo M.-Y.; Chen W.-C.; Chueh C.-C. Structure–Mobility Relationship of Benzodithiophene-Based Conjugated Polymers with Varied Biaxially Extended Conjugated Side Chains. Ind. Eng. Chem. Res. 2020, 59 (19), 9105–9115. 10.1021/acs.iecr.0c00738. [DOI] [Google Scholar]
- Huang Y.-W.; Lin Y.-C.; Yen H.-C.; Chen C.-K.; Lee W.-Y.; Chen W.-C.; Chueh C.-C. High Mobility Preservation of Near Amorphous Conjugated Polymers in the Stretched States Enabled by Biaxially-Extended Conjugated Side-Chain Design. Chem. Mater. 2020, 32 (17), 7370–7382. 10.1021/acs.chemmater.0c02258. [DOI] [Google Scholar]
- Lin Y.-C.; Huang Y.-W.; Wu Y.-S.; Li J.-S.; Yang Y.-F.; Chen W.-C.; Chueh C.-C. Improving Mobility–Stretchability Properties of Polythiophene Derivatives through Ester-Substituted, Biaxially Extended Conjugated Side Chains. ACS Appl. Polym. Mater. 2021, 3 (3), 1628–1637. 10.1021/acsapm.1c00006. [DOI] [Google Scholar]
- Yu P.-J.; Lin Y.-C.; Lin C.-Y.; Chen W.-C. Enhanced Mobility Preservation of Polythiophenes in Stretched States Utilizing Thienyl-Ester Conjugated Side Chain. Polymer 2023, 264, 125575. 10.1016/j.polymer.2022.125575. [DOI] [Google Scholar]
- Griggs S.; Marks A.; Bristow H.; McCulloch I. N-Type Organic Semiconducting Polymers: Stability Limitations, Design Considerations and Applications. J. Mater. Chem. C 2021, 9 (26), 8099–8128. 10.1039/D1TC02048J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C. R.; Frisbie C. D.; da Silva Filho D. A.; Brédas J.-L.; Ewbank P. C.; Mann K. R. Introduction to Organic Thin Film Transistors and Design of n-Channel Organic Semiconductors. Chem. Mater. 2004, 16 (23), 4436–4451. 10.1021/cm049391x. [DOI] [Google Scholar]
- Wang Y.; Gong Q.; Miao Q. Structured and Functionalized Organic Semiconductors for Chemical and Biological Sensors Based on Organic Field Effect Transistors. Mater. Chem. Front. 2020, 4 (12), 3505–3520. 10.1039/D0QM00202J. [DOI] [Google Scholar]
- Wang H.; Hsieh B.; Jimenez-Oses G.; Liu P.; Tassone C. J.; Diao Y.; Lei T.; Houk K. N.; Bao Z. Solvent Effects on Polymer Sorting of Carbon Nanotubes with Applications in Printed Electronics. Small 2015, 11 (1), 126–133. 10.1002/smll.201401890. [DOI] [PubMed] [Google Scholar]
- Wang J.; Lei T. Enrichment of High-Purity Large-Diameter Semiconducting Single-Walled Carbon Nanotubes. Nanoscale 2022, 14 (4), 1096–1106. 10.1039/D1NR06635H. [DOI] [PubMed] [Google Scholar]
- Janas D. Towards Monochiral Carbon Nanotubes: A Review of Progress in the Sorting of Single-Walled Carbon Nanotubes. Mater. Chem. Front. 2018, 2 (1), 36–63. 10.1039/C7QM00427C. [DOI] [Google Scholar]
- Yahya I.; Bonaccorso F.; Clowes S. K.; Ferrari A. C.; Silva S. R. P. Temperature Dependent Separation of Metallic and Semiconducting Carbon Nanotubes Using Gel Agarose Chromatography. Carbon 2015, 93, 574–594. 10.1016/j.carbon.2015.05.036. [DOI] [Google Scholar]
- Zheng M.; Jagota A.; Semke E. D.; Diner B. A.; McLean R. S.; Lustig S. R.; Richardson R. E.; Tassi N. G. DNA-Assisted Dispersion and Separation of Carbon Nanotubes. Nat. Mater. 2003, 2 (5), 338–342. 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- Lei T.; Chen X.; Pitner G.; Wong H. S.; Bao Z. Removable and Recyclable Conjugated Polymers for Highly Selective and High-Yield Dispersion and Release of Low-Cost Carbon Nanotubes. J. Am. Chem. Soc. 2016, 138 (3), 802–805. 10.1021/jacs.5b12797. [DOI] [PubMed] [Google Scholar]
- Hwang K.; Lim D.-H.; Lee M.-H.; Kim Y.-J.; Kim Y.-a.; Yang D.; Kim Y.; Kim D.-Y. Engineering the Structural Topology of Pyrene-Based Conjugated Polymers for the Selective Sorting of Semiconducting Single-Walled Carbon Nanotubes. Macromolecules 2021, 54 (13), 6061–6072. 10.1021/acs.macromol.1c00122. [DOI] [Google Scholar]
- Gomulya W.; Costanzo G. D.; de Carvalho E. J.; Bisri S. Z.; Derenskyi V.; Fritsch M.; Frohlich N.; Allard S.; Gordiichuk P.; Herrmann A.; et al. Semiconducting single-walled carbon nanotubes on demand by polymer wrapping. Adv. Mater. 2013, 25 (21), 2948–2956. 10.1002/adma.201300267. [DOI] [PubMed] [Google Scholar]
- Wang K.; Dong H.; Zhou D.; Ito Y.; Hu L.; Zhang Z.; Zhu X. Facile Fabrication of Semiconducting Single-Walled Carbon Nanotubes Patterns on Flexible Substrate Based on a Photoimmobilization Technique. ACS Appl. Mater. Interfaces 2020, 12 (7), 8722–8729. 10.1021/acsami.9b21142. [DOI] [PubMed] [Google Scholar]
- Ye G.; Talsma W.; Tran K.; Liu Y.; Dijkstra S.; Cao J.; Chen J.; Qu J.; Song J.; Loi M. A.; et al. Polar Side Chains Enhance Selection of Semiconducting Single-Walled Carbon Nanotubes by Polymer Wrapping. Macromolecules 2022, 55 (4), 1386–1397. 10.1021/acs.macromol.1c01842. [DOI] [Google Scholar]
- Talsma W.; Ye G.; Liu Y.; Duim H.; Dijkstra S.; Tran K.; Qu J.; Song J.; Chiechi R. C.; Loi M. A. Efficient Selective Sorting of Semiconducting Carbon Nanotubes Using Ultra-Narrow-Band-Gap Polymers. ACS Appl. Mater. Interfaces 2022, 14 (33), 38056–38066. 10.1021/acsami.2c07158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J.; Shin H.; Finnie P.; Ding J.; Guo C.; Li Z.; Chen Y.; Wei L.; Wu A. J.; Moisa S.; et al. Impact of Conjugated Polymer Characteristics on the Enrichment of Single-Chirality Single-Walled Carbon Nanotubes. ACS Appl. Polym. Mater. 2022, 4 (8), 6239–6254. 10.1021/acsapm.2c01022. [DOI] [Google Scholar]
- Luo S.-X. L.; Swager T. M. Wireless Detection of Trace Ammonia: A Chronic Kidney Disease Biomarker. ACS Nano 2024, 18 (1), 364–372. 10.1021/acsnano.3c07325. [DOI] [PubMed] [Google Scholar]
- Tung Y. H.; Su S. W.; Su E. J.; Jiang G. H.; Chen C. C.; Yu S. S.; Chiu C. C.; Shih C. C.; Lin Y. C. Semiconducting Carbon Nanotubes with Light-Driven Gating Behaviors in Phototransistor Memory Utilizing an N-Type Conjugated Polymer Sorting. Small Sci. 2024, 4 (4), 2300268. 10.1002/smsc.202300268. [DOI] [Google Scholar]
- Liu D.; Li P.; Yu X.; Gu J.; Han J.; Zhang S.; Li H.; Jin H.; Qiu S.; Li Q.; et al. A Mixed-Extractor Strategy for Efficient Sorting of Semiconducting Single-Walled Carbon Nanotubes. Adv. Mater. 2017, 29 (8), 1603565. 10.1002/adma.201603565. [DOI] [PubMed] [Google Scholar]
- Neises B.; Steglich W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem., Int. Ed. Engl. 1978, 17 (7), 522–524. 10.1002/anie.197805221. [DOI] [Google Scholar]
- Espinet P.; Echavarren A. M. The Mechanisms of the Stille Reaction. Angew. Chem., Int. Ed. 2004, 43 (36), 4704–4734. 10.1002/anie.200300638. [DOI] [PubMed] [Google Scholar]
- Fatemi S. M.; Foroutan M. Recent Developments Concerning the Dispersion of Carbon Nanotubes in Surfactant/Polymer Systems by MD Simulation. J. Nanostruct. Chem. 2016, 6 (1), 29–40. 10.1007/s40097-015-0175-9. [DOI] [Google Scholar]
- He Y.; Liao H.; Lyu S.; Xu X.-Q.; Li Z.; McCulloch I.; Yue W.; Wang Y. Coupling Molecular Rigidity and Flexibility on Fused Backbones for NIR-II Photothermal Conversion. Chem. Sci. 2021, 12 (14), 5177–5184. 10.1039/D1SC00060H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokalj A. On the Alleged Importance of the Molecular Electron-Donating Ability and the HOMO–LUMO Gap in Corrosion Inhibition Studies. Corros. Sci. 2021, 180, 109016. 10.1016/j.corsci.2020.109016. [DOI] [Google Scholar]
- Chortos A.; Pochorovski I.; Lin P.; Pitner G.; Yan X.; Gao T. Z.; To J. W. F.; Lei T.; Will J. W. III; Wong H. S. P.; et al. Universal Selective Dispersion of Semiconducting Carbon Nanotubes from Commercial Sources Using a Supramolecular Polymer. ACS Nano 2017, 11 (6), 5660–5669. 10.1021/acsnano.7b01076. [DOI] [PubMed] [Google Scholar]
- Ding J.; Li Z.; Lefebvre J.; Cheng F.; Dubey G.; Zou S.; Finnie P.; Hrdina A.; Scoles L.; Lopinski G. P.; et al. Enrichment of Large-Diameter Semiconducting SWCNTs by Polyfluorene Extraction for High Network Density Thin Film Transistors. Nanoscale 2014, 6 (4), 2328–2339. 10.1039/c3nr05511f. [DOI] [PubMed] [Google Scholar]
- Ouyang J.; Ding J.; Lefebvre J.; Li Z.; Guo C.; Kell A. J.; Malenfant P. R. L. Sorting of Semiconducting Single-Walled Carbon Nanotubes in Polar Solvents with an Amphiphilic Conjugated Polymer Provides General Guidelines for Enrichment. ACS Nano 2018, 12 (2), 1910–1919. 10.1021/acsnano.7b08818. [DOI] [PubMed] [Google Scholar]
- Jorio A.; Saito R. Raman spectroscopy for carbon nanotube applications. J. Appl. Phys. 2021, 129 (2), 021102 10.1063/5.0030809. [DOI] [Google Scholar]
- Su E.-J.; Chang T.-W.; Lin F.-Y.; Lu S.-T.; Tsai Y.-T.; Khan S.; Weng Y.-C.; Shih C.-C. Efficient Sorting of Semiconducting Single-Walled Carbon Nanotubes in Bio-Renewable Solvents Through Main-Chain Engineering of Conjugated Polymers. Small 2024, 20, 2403651. 10.1002/smll.202403651. [DOI] [PubMed] [Google Scholar]
- Dresselhaus M. S.; Dresselhaus G.; Saito R.; Jorio A. Raman Spectroscopy of Carbon Nanotubes. Phys. Rep. 2005, 409 (2), 47–99. 10.1016/j.physrep.2004.10.006. [DOI] [Google Scholar]
- Li Z.; Chen W.; Liu J.; Jiang D. Can Linear Conjugated Polymers Form Stable Helical Structures on the Carbon Nanotubes?. ACS Appl. Mater. Interfaces 2022, 14 (43), 49189–49198. 10.1021/acsami.2c14771. [DOI] [PubMed] [Google Scholar]
- Qiu S.; Wu K.; Gao B.; Li L.; Jin H.; Li Q. Solution-Processing of High-Purity Semiconducting Single-Walled Carbon Nanotubes for Electronics Devices. Adv. Mater. 2019, 31 (9), 1800750. 10.1002/adma.201800750. [DOI] [PubMed] [Google Scholar]
- Persson N. E.; McBride M. A.; Grover M. A.; Reichmanis E. Automated Analysis of Orientational Order in Images of Fibrillar Materials. Chem. Mater. 2017, 29 (1), 3–14. 10.1021/acs.chemmater.6b01825. [DOI] [Google Scholar]
- Noriega R.; Rivnay J.; Vandewal K.; Koch F. P. V.; Stingelin N.; Smith P.; Toney M. F.; Salleo A. A General Relationship Between Disorder, Aggregation and Charge Transport in Conjugated Polymers. Nat. Mater. 2013, 12 (11), 1038–1044. 10.1038/nmat3722. [DOI] [PubMed] [Google Scholar]
- Cai L.; Wang C. Carbon Nanotube Flexible and Stretchable Electronics. Nanoscale Res. Lett. 2015, 10 (1), 320. 10.1186/s11671-015-1013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Han J.; Xu L.; Zhou J.; Zhao C.; Ding S.; Shi H.; Xiao M.; Ding L.; Ma Z.; et al. Aligned, High-Density Semiconducting Carbon Nanotube Arrays for High-Performance Electronics. Science 2020, 368 (6493), 850–856. 10.1126/science.aba5980. [DOI] [PubMed] [Google Scholar]
- Dallaire N. J.; Mirka B.; Manion J. G.; Bodnaryk W. J.; Fong D.; Adronov A.; Hinzer K.; Lessard B. H. Conjugated Wrapping Polymer Influences on Photoexcitation of Single-Walled Carbon Nanotube-Based Thin Film Transistors. J. Mater. Chem. C 2023, 11 (27), 9161–9171. 10.1039/D3TC01484C. [DOI] [Google Scholar]
- Kim W.; Javey A.; Vermesh O.; Wang Q.; Li Y.; Dai H. Hysteresis Caused by Water Molecules in Carbon Nanotube Field-Effect Transistors. Nano Lett. 2003, 3 (2), 193–198. 10.1021/nl0259232. [DOI] [Google Scholar]
- Rice N. A.; Bodnaryk W. J.; Mirka B.; Melville O. A.; Adronov A.; Lessard B. H. Polycarbazole-Sorted Semiconducting Single-Walled Carbon Nanotubes for Incorporation into Organic Thin Film Transistors. Adv. Electron. Mater. 2019, 5 (1), 1800539. 10.1002/aelm.201800539. [DOI] [Google Scholar]
- Mirka B.; Fong D.; Rice N. A.; Melville O. A.; Adronov A.; Lessard B. H. Polyfluorene-Sorted Semiconducting Single-Walled Carbon Nanotubes for Applications in Thin-Film Transistors. Chem. Mater. 2019, 31 (8), 2863–2872. 10.1021/acs.chemmater.8b05357. [DOI] [Google Scholar]
- Srimani T.; Ding J.; Yu A.; Kanhaiya P.; Lau C.; Ho R.; Humes J.; Kingston C. T.; Malenfant P. R.L.; Shulaker M. M. Comprehensive Study on High Purity SemiconductingCarbon Nanotube Extraction. Adv. Electron. Mater. 2022, 8 (9), 2101377. 10.1002/aelm.202101377. [DOI] [Google Scholar]
- Park M.; Kim S.; Kwon H.; Hong S.; Im S.; Ju S.-Y. Selective Dispersion of Highly Pure Large-Diameter Semiconducting Carbon Nanotubes by a Flavin for Thin-Film Transistors. ACS Appl. Mater. Interfaces 2016, 8 (35), 23270–23280. 10.1021/acsami.6b06932. [DOI] [PubMed] [Google Scholar]
- Tour J. M. Seeds of Selective Nanotube Growth. Nature 2014, 512 (7512), 30–31. 10.1038/512030a. [DOI] [PubMed] [Google Scholar]
- Na J. Y.; Kang B.; Lee S. G.; Cho K.; Park Y. D. Surface-Mediated Solidification of a Semiconducting Polymer during Time-Controlled Spin-Coating. ACS Appl. Mater. Interfaces 2017, 9 (11), 9871–9879. 10.1021/acsami.6b11737. [DOI] [PubMed] [Google Scholar]
- Min H.; Kang B.; Shin Y. S.; Kim B.; Lee S. W.; Cho J. H. Transparent and Colorless Polyimides Containing Multiple Trifluoromethyl Groups as Gate Insulators for Flexible Organic Transistors with Superior Electrical Stability. ACS Appl. Mater. Interfaces 2020, 12 (16), 18739–18747. 10.1021/acsami.9b23318. [DOI] [PubMed] [Google Scholar]
- Guo X.; Watson M. D. Conjugated Polymers from Naphthalene Bisimide. Org. Lett. 2008, 10 (23), 5333–5336. 10.1021/ol801918y. [DOI] [PubMed] [Google Scholar]
- Mirka B.; Rice N. A.; Williams P.; Tousignant M. N.; Boileau N. T.; Bodnaryk W. J.; Fong D.; Adronov A.; Lessard B. H. Excess Polymer in Single-Walled Carbon Nanotube Thin-Film Transistors: Its Removal Prior to Fabrication Is Unnecessary. ACS Nano 2021, 15 (5), 8252–8266. 10.1021/acsnano.0c08584. [DOI] [PubMed] [Google Scholar]
- Wang S.; Li H.; Zhao K.; Zhang L.; Zhang Q.; Yu X.; Tian H.; Han Y. Increasing the Charge Transport of P(NDI2OD-T2) by Improving the Polarization of the NDI2OD Unit along the Backbone Direction and Preaggregation via H-Bonding. Macromolecules 2022, 55 (7), 2497–2508. 10.1021/acs.macromol.1c02329. [DOI] [Google Scholar]
- Rivnay J.; Noriega R.; Kline R. J.; Salleo A.; Toney M. F. Quantitative analysis of lattice disorder and crystallite size in organic semiconductor thin films. Phys. Rev. B 2011, 84 (4), 045203 10.1103/PhysRevB.84.045203. [DOI] [Google Scholar]
- Wang Y.; Zhang Z.; Zheng R.; Zhang Y. Calculation method for the dielectric constant of thioglycolic acid grafted modified SBS dielectric elastomer. Arabian J. Chem. 2021, 14 (10), 103361. 10.1016/j.arabjc.2021.103361. [DOI] [Google Scholar]
- Hanwell M. D.; Curtis D. E.; Lonie D. C.; Vandermeersch T.; Zurek E.; Hutchison G. R. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 2012, 4 (1), 17. 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102 (38), 7338–7364. 10.1021/jp980939v. [DOI] [Google Scholar]
- Sun H.; Ren P.; Fried J. R. The COMPASS Force Field: Parameterization and Validation for Phosphazenes. Comput. Theor. Polym. Sci. 1998, 8 (1), 229–246. 10.1016/S1089-3156(98)00042-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.