Abstract
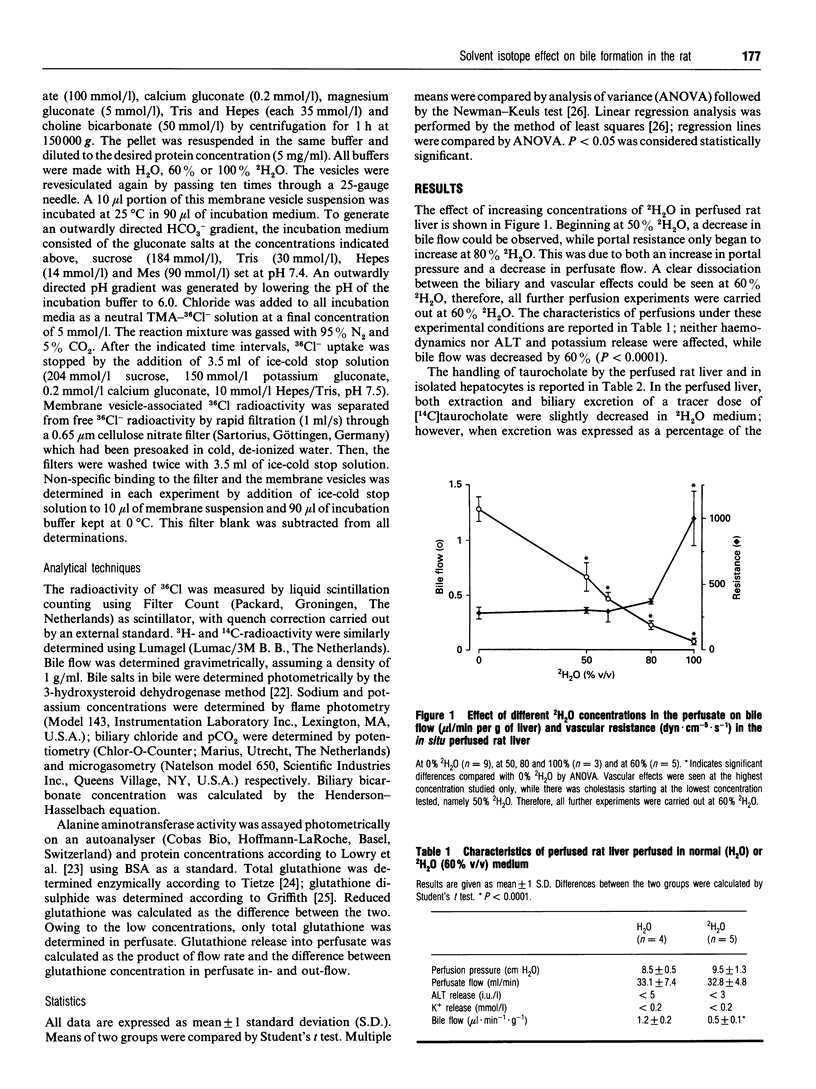
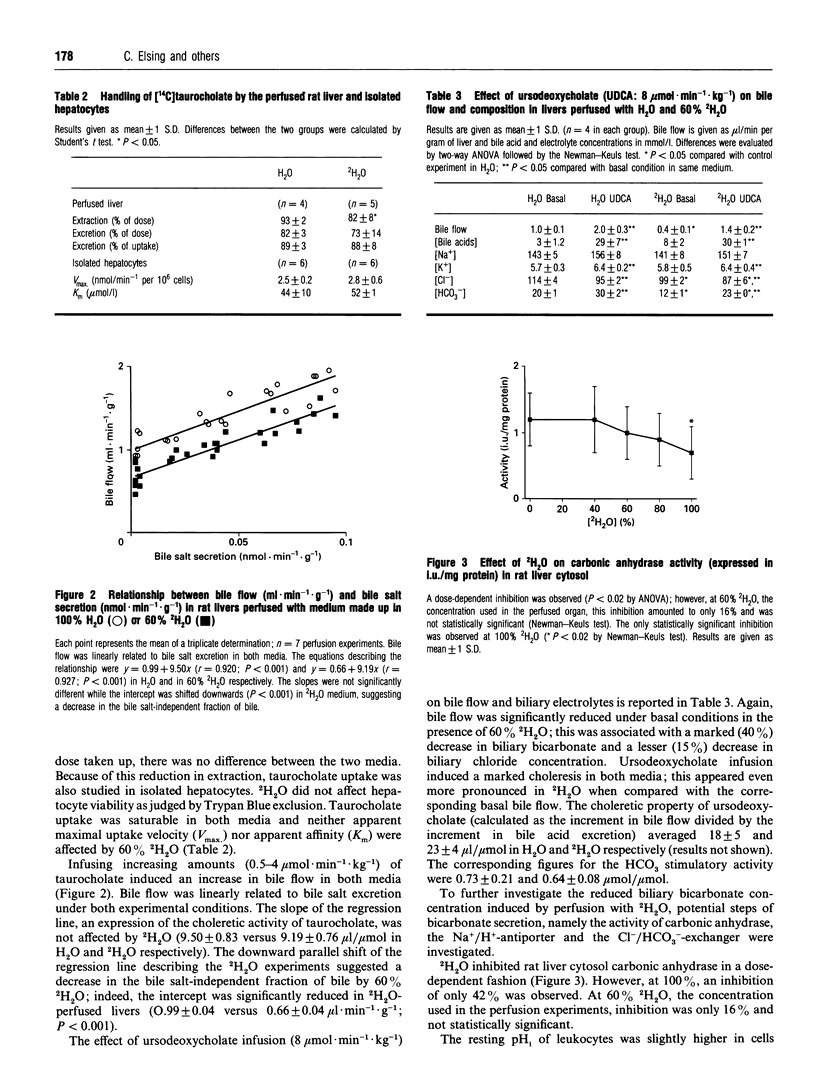
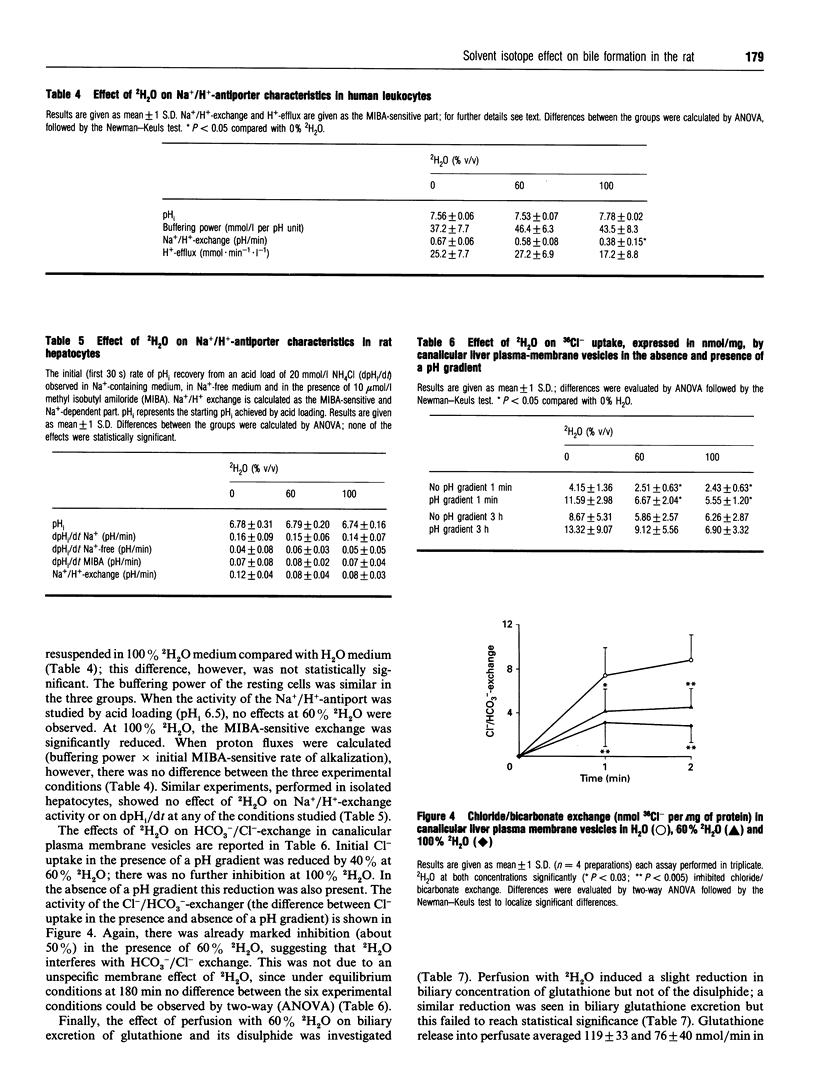
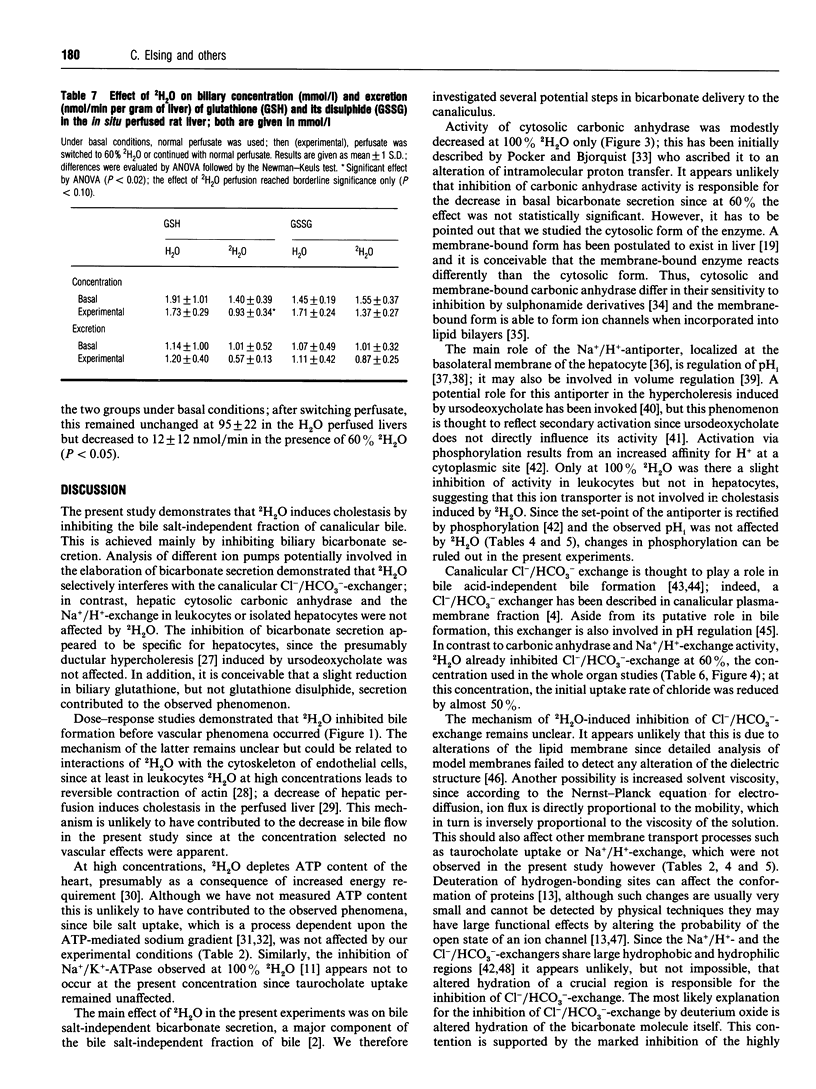
2H2O affects many membrane transport processes by solvent and kinetic isotope effects. Since bile formation is a process of osmotic filtration where such effects could be important, we investigated the effects of 2H2O on bile formation in the in situ perfused rat liver. Dose finding experiments showed that at high concentrations, 2H2O increased vascular resistance and induced cholestasis; at 60% 2H2O however, a clear dissociation between the vascular and biliary effects was observed. Therefore, further experiments were carried out at this concentration. The main finding was a reduction in bile salt-independent bile flow from 0.99 +/- 0.04 to 0.66 +/- 0.04 microliters.min-1.g-1 (P < 0.001). This was associated with a 40% reduction in biliary bicarbonate concentration (P < 0.001). Choleretic response to neither taurocholate nor ursodeoxycholate was altered by 2H2O; in particular, there was a similar stimulation of bicarbonate secretion by ursodeoxycholate in the presence of 60% 2H2O. To further elucidate this phenomenon, the effect of 2H2O on three proteins potentially involved in biliary bicarbonate secretion was studied in vitro. 2H2O slightly inhibited cytosolic carboanhydrase and leukocyte Na+/H(+)-exchange, these effects reached statistical significance at 100% 2H2O only, however. In contrast, Cl-/HCO(3-)-exchange in canalicular membrane vesicles was already inhibited by 50% (P < 0.001) at 60% 2H2O. Finally, there was a slight reduction in biliary glutathione secretion while that of the disulphide was not affected. Our results are compatible with an inhibition of canalicular Cl-/HCO(3-)-exchange by 2H2O. Whether this is due to altered hydration of the exchanger and/or of the transported bicarbonate remains to be determined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed K., Foster D. Studies of the effects of 2H2O on Na+, K+-ATPase. Ann N Y Acad Sci. 1974;242(0):280–292. doi: 10.1111/j.1749-6632.1974.tb19097.x. [DOI] [PubMed] [Google Scholar]
- Andjus P. R., Vucelić D. D2O-induced cell excitation. J Membr Biol. 1990 May;115(2):123–127. doi: 10.1007/BF01869451. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Hegner D. Role of inorganic electrolytes in bile acid-independent canalicular bile formation. Am J Physiol. 1983 Feb;244(2):G116–G124. doi: 10.1152/ajpgi.1983.244.2.G116. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Hondalus M. K., Atkinson J. M. Ursodeoxycholate-induced changes in hepatic Na+-H+ exchange and biliary HCO3- excretion. Am J Physiol. 1989 Sep;257(3 Pt 1):G371–G379. doi: 10.1152/ajpgi.1989.257.3.G371. [DOI] [PubMed] [Google Scholar]
- Arias I. M., Forgac M. The sinusoidal domain of the plasma membrane of rat hepatocytes contains an amiloride-sensitive Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5406–5408. [PubMed] [Google Scholar]
- Arányi P. Kinetic deuterium isotope effects in glucocorticoid receptor activation. J Recept Res. 1984;4(1-6):385–396. doi: 10.3109/10799898409042563. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Truong A. T. Relation between biliary glutathione excretion and bile acid-independent bile flow. Am J Physiol. 1989 Jan;256(1 Pt 1):G22–G30. doi: 10.1152/ajpgi.1989.256.1.G22. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Strazzabosco M., Corasanti J. G., Haddad P., Graf J., Boyer J. L. Cl(-)-HCO3- exchanger in isolated rat hepatocytes: role in regulation of intracellular pH. Am J Physiol. 1991 Sep;261(3 Pt 1):G512–G522. doi: 10.1152/ajpgi.1991.261.3.G512. [DOI] [PubMed] [Google Scholar]
- Brink P. R. Effect of deuterium oxide on junctional membrane channel permeability. J Membr Biol. 1983;71(1-2):79–87. doi: 10.1007/BF01870676. [DOI] [PubMed] [Google Scholar]
- Corasanti J. G., Gleeson D., Boyer J. L. Effects of osmotic stresses on isolated rat hepatocytes. I. Ionic mechanisms of cell volume regulation. Am J Physiol. 1990 Feb;258(2 Pt 1):G290–G298. doi: 10.1152/ajpgi.1990.258.2.G290. [DOI] [PubMed] [Google Scholar]
- Coster H. G., Laver D. R., Schoenborn B. P. Effect of 2H2O/H2O replacement on the dielectric structure of lipid bilayer membranes. Biochim Biophys Acta. 1982 Mar 23;686(1):141–143. doi: 10.1016/0005-2736(82)90161-4. [DOI] [PubMed] [Google Scholar]
- Dumont M., Erlinger S., Uchman S. Hypercholeresis induced by ursodeoxycholic acid and 7-ketolithocholic acid in the rat: possible role of bicarbonate transport. Gastroenterology. 1980 Jul;79(1):82–89. [PubMed] [Google Scholar]
- Dällenbach A., Marti U., Renner E. L. Hepatocellular Na+/H+ exchange is activated early, transiently and at a posttranscriptional level during rat liver regeneration. Hepatology. 1994 May;19(5):1290–1301. doi: 10.1002/hep.1840190530. [DOI] [PubMed] [Google Scholar]
- Döring O., Böttger M. Effect of D2O on maize plasmalemma ATPase and electron transport coupled proton pumping. Biochem Biophys Res Commun. 1992 Jan 31;182(2):870–876. doi: 10.1016/0006-291x(92)91813-6. [DOI] [PubMed] [Google Scholar]
- Fernley R. T. Non-cytoplasmic carbonic anhydrases. Trends Biochem Sci. 1988 Sep;13(9):356–359. doi: 10.1016/0968-0004(88)90107-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Marin J. J., Perez-Barriocanal F., Garcia A., Serrano M. A., Regueiro P., Esteller A. Evidence for the presence of carbonic anhydrase in the plasma membrane of rat hepatocytes. Biochim Biophys Acta. 1988 Nov 3;945(1):17–22. doi: 10.1016/0005-2736(88)90357-4. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Hahin R., Strichartz G. Effects of deuterium oxide on the rate and dissociation constants for saxitoxin and tetrodotoxin action. Voltage-clamp studies on frog myelinated nerve. J Gen Physiol. 1981 Aug;78(2):113–139. doi: 10.1085/jgp.78.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison W. G., Wood C. A. Importance of bicarbonate in bile salt independent fraction of bile flow. Am J Physiol. 1978 Aug;235(2):E158–E164. doi: 10.1152/ajpendo.1978.235.2.E158. [DOI] [PubMed] [Google Scholar]
- Henderson R. M., Graf J., Boyer J. L. Na-H exchange regulates intracellular pH in isolated rat hepatocyte couplets. Am J Physiol. 1987 Jan;252(1 Pt 1):G109–G113. doi: 10.1152/ajpgi.1987.252.1.G109. [DOI] [PubMed] [Google Scholar]
- Houston L. L., Odell J., Lee Y. C., Himes R. H. Solvent isotope effects on microtubule polymerization and depolymerization. J Mol Biol. 1974 Jul 25;87(1):141–146. doi: 10.1016/0022-2836(74)90566-x. [DOI] [PubMed] [Google Scholar]
- Klos C., Paumgartner G., Reichen J. Cation-anion gap and choleretic properties of rat bile. Am J Physiol. 1979 Apr;236(4):E434–E440. doi: 10.1152/ajpendo.1979.236.4.E434. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985 Jul 18;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lake J. R., Renner E. L., Scharschmidt B. F., Cragoe E. J., Jr, Hagey L. R., Lambert K. J., Gurantz D., Hofmann A. F. Inhibition of Na+/H+ exchange in the rat is associated with decreased ursodeoxycholate hypercholeresis, decreased secretion of unconjugated urodeoxycholate, and increased ursodeoxycholate glucuronidation. Gastroenterology. 1988 Aug;95(2):454–463. doi: 10.1016/0016-5085(88)90504-5. [DOI] [PubMed] [Google Scholar]
- Landowne D. D2O and the sodium pump in squid nerve membrane. J Membr Biol. 1987;96(3):277–281. doi: 10.1007/BF01869309. [DOI] [PubMed] [Google Scholar]
- Lawaczeck R. Water permeability through biological membranes by isotopic effects of fluorescence and light scattering. Biophys J. 1984 Mar;45(3):491–494. doi: 10.1016/S0006-3495(84)84184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Deuterium oxide and temperature effects on the properties of endplate channels at the frog neuromuscular junction. J Gen Physiol. 1985 Feb;85(2):137–156. doi: 10.1085/jgp.85.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P. J., Knickelbein R., Moseley R. H., Dobbins J. W., Boyer J. L. Evidence for carrier-mediated chloride/bicarbonate exchange in canalicular rat liver plasma membrane vesicles. J Clin Invest. 1985 Apr;75(4):1256–1263. doi: 10.1172/JCI111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson M. H., Boyer J. L. Mechanisms and regulation of bile secretion. Hepatology. 1991 Sep;14(3):551–566. [PubMed] [Google Scholar]
- Ng L. L., Dudley C. Intracellular pH clamping of human leucocytes: a technique for determination of cellular buffering power and Na+/H+ antiport characteristics. Clin Sci (Lond) 1989 Oct;77(4):417–423. doi: 10.1042/cs0770417. [DOI] [PubMed] [Google Scholar]
- Paumgartner G., Horak W., Probst P., Grabner G. Effect of phenobarbital on bile flow and bile salt excretion in the rat. Naunyn Schmiedebergs Arch Pharmakol. 1971;270(1):98–101. doi: 10.1007/BF00997305. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Bjorkquist D. W. Comparative studies of bovine carbonic anhydrase in H2O and D2O. Stopped-flow studies of the kinetics of interconversion of CO2 and HCO3. Biochemistry. 1977 Dec 27;16(26):5698–5707. doi: 10.1021/bi00645a008. [DOI] [PubMed] [Google Scholar]
- Reichen J., Berman M. D., Berk P. D. The role of microfilaments and microtubules in taurocholate uptake by isolated rat liver cells. Biochim Biophys Acta. 1981 Apr 22;643(1):126–133. doi: 10.1016/0005-2736(81)90224-8. [DOI] [PubMed] [Google Scholar]
- Reichen J., Le M. Verapamil favorably influences hepatic microvascular exchange and function in rats with cirrhosis of the liver. J Clin Invest. 1986 Aug;78(2):448–455. doi: 10.1172/JCI112596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichen J., Paumgartner G. Kinetics of taurocholate uptake by the perfused rat liver. Gastroenterology. 1975 Jan;68(1):132–136. [PubMed] [Google Scholar]
- Renner E. L., Lake J. R., Persico M., Scharschmidt B. F. Na+-H+ exchange activity in rat hepatocytes: role in regulation of intracellular pH. Am J Physiol. 1989 Jan;256(1 Pt 1):G44–G52. doi: 10.1152/ajpgi.1989.256.1.G44. [DOI] [PubMed] [Google Scholar]
- Reuter H. D., Fischer J. H., Thiele S. Investigations on the effects of heavy water (D2O) on the functional activity of human platelets. Haemostasis. 1985;15(3):157–163. doi: 10.1159/000215138. [DOI] [PubMed] [Google Scholar]
- Sardet C., Counillon L., Franchi A., Pouysségur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science. 1990 Feb 9;247(4943):723–726. doi: 10.1126/science.2154036. [DOI] [PubMed] [Google Scholar]
- Strasberg S. M., Ilson R. G., Paloheimo J. E. Bile salt-associated electrolyte secretion and the effect of sodium taurocholate on bile flow. J Lab Clin Med. 1983 Feb;101(2):317–326. [PubMed] [Google Scholar]
- Tavoloni N., Reed J. S., Boyer J. L. Hemodynamic effects on determinants of bile secretion in isolated rat liver. Am J Physiol. 1978 Jun;234(6):E584–E592. doi: 10.1152/ajpendo.1978.234.6.E584. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W., Stephens J. E., Scharschmidt B. F. Effects of ion substitution on bile acid-dependent and -independent bile formation by rat liver. J Clin Invest. 1982 Sep;70(3):505–517. doi: 10.1172/JCI110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilescu V., Katona E. Deuteration as a tool in investigating the role of water in the structure and function of excitable membranes. Methods Enzymol. 1986;127:662–678. doi: 10.1016/0076-6879(86)27052-4. [DOI] [PubMed] [Google Scholar]
- Verselis V., Brink P. R. The gap junction channel. Its aqueous nature as indicated by deuterium oxide effects. Biophys J. 1986 Nov;50(5):1003–1007. doi: 10.1016/S0006-3495(86)83542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistrand P. J. Properties of membrane-bound carbonic anhydrase. Ann N Y Acad Sci. 1984;429:195–206. doi: 10.1111/j.1749-6632.1984.tb12333.x. [DOI] [PubMed] [Google Scholar]
- Yoon Y. B., Hagey L. R., Hofmann A. F., Gurantz D., Michelotti E. L., Steinbach J. H. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology. 1986 Apr;90(4):837–852. doi: 10.1016/0016-5085(86)90859-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann A., Keller H. U., Cottier H. Heavy water (D2O)-induced shape changes, movements and F-actin redistribution in human neutrophil granulocytes. Eur J Cell Biol. 1988 Dec;47(2):320–326. [PubMed] [Google Scholar]


