Abstract
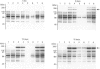
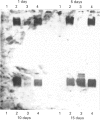
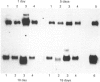
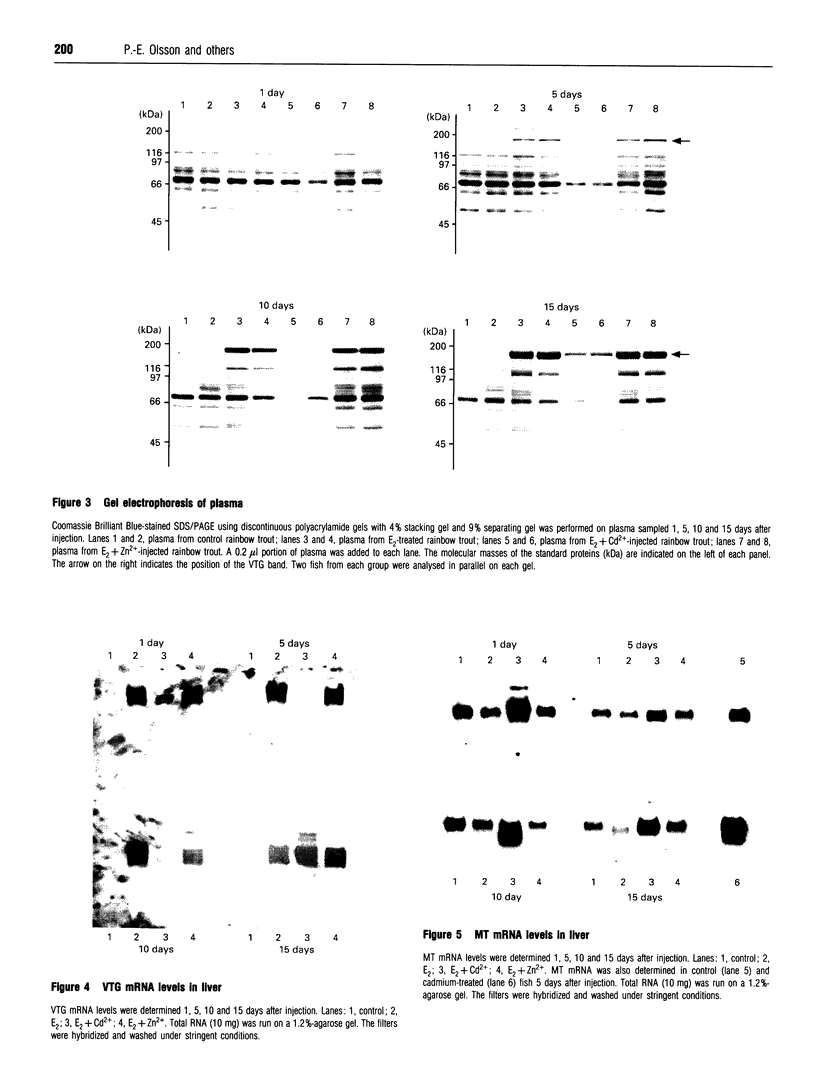
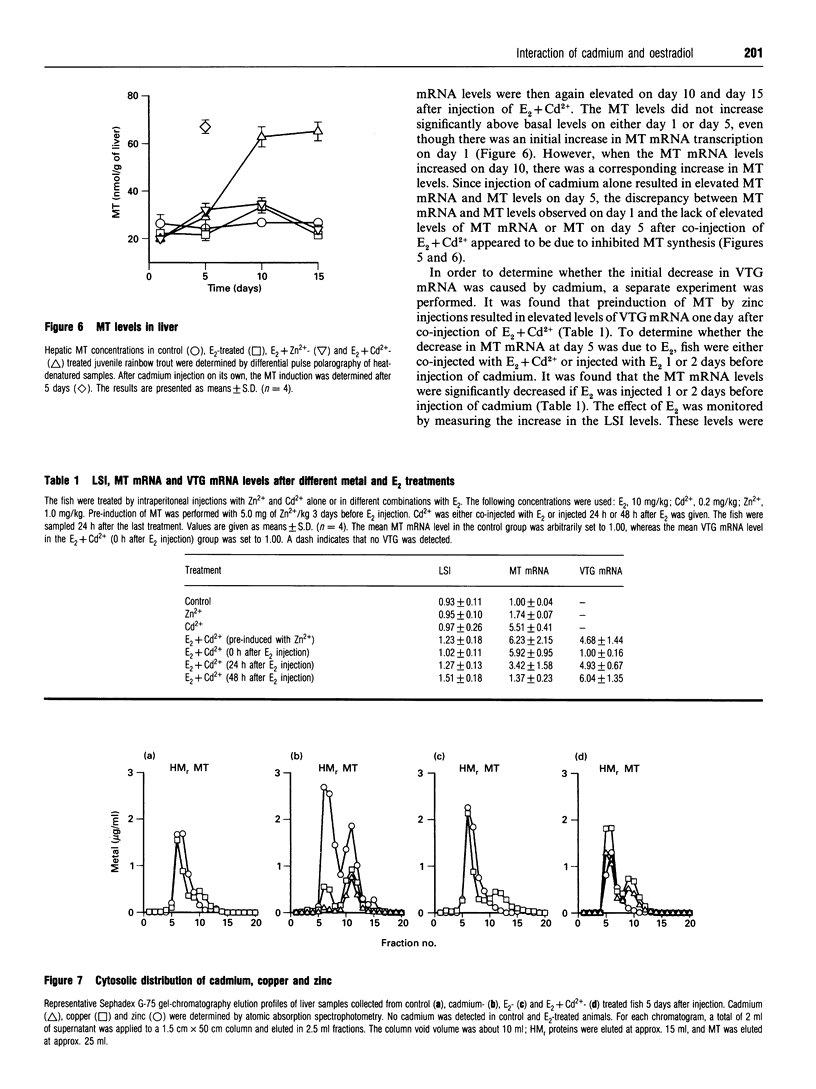
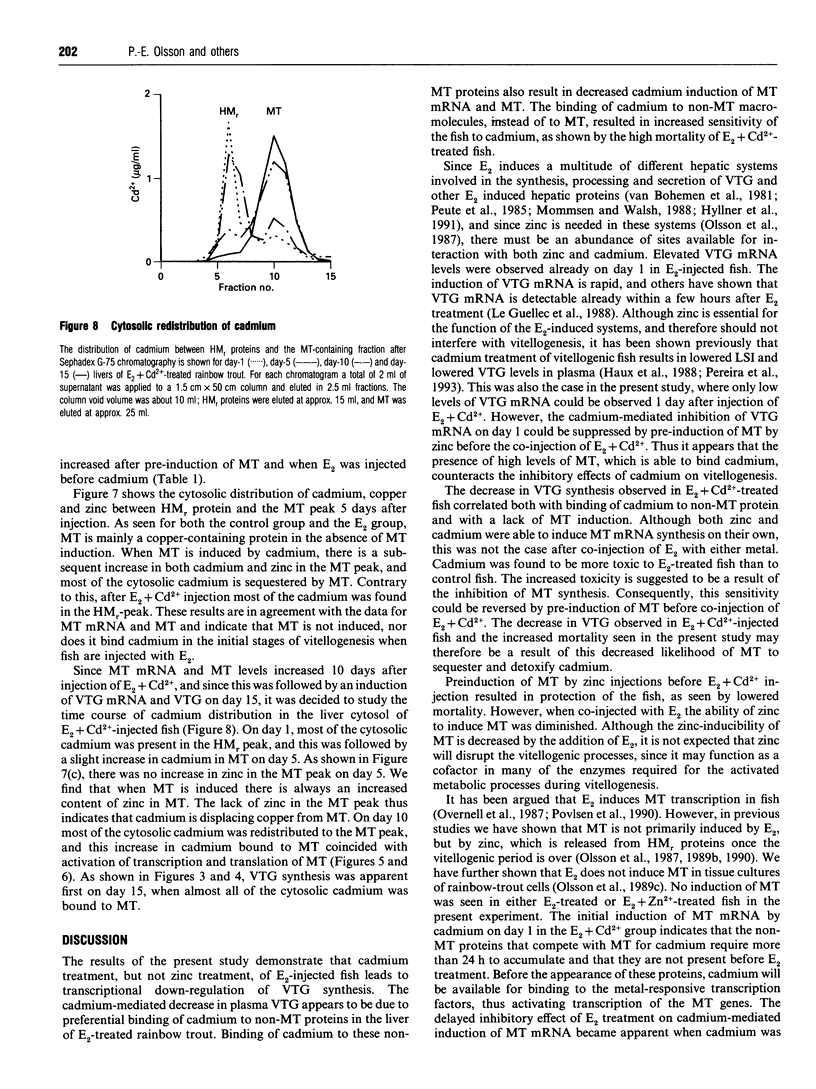
The induction of metallothionein and vitellogenin synthesis in rainbow trout liver was studied after injection of oestradiol-17 beta alone or in combination with cadmium or zinc. Intraperitoneal injection of oestradiol-17 beta increased the liver somatic index, with subsequent induction of vitellogenin synthesis. Oestradiol-17 beta did not induce metallothionein synthesis. Injection of cadmium induced the synthesis of metallothionein mRNA and metallothionein. Injection of oestradiol-17 beta in combination with cadmium resulted in inhibition of transcription and translation of both vitellogenin and metallothionein. Chromatography of liver cytosols revealed that cadmium, when co-injected with oestradiol-17 beta, did not bind to metallothionein but would initially bind to high-molecular-mass (HMr) cytosolic proteins. In fish injected with cadmium in combination with oestradiol-17 beta, cadmium was gradually redistributed from HMr proteins to metallothionein. This resulted in induction of metallothionein synthesis and in binding of most of the cadmium to metallothionein. Induction of vitellogenin mRNA was observed 15 days after injection, as cadmium was being redistributed to newly synthesized metallothionein. These findings indicate that cadmium inhibits the transcription of vitellogenin. The binding of cadmium to these non-metallothionein proteins represses the induction of metallothionein and results in increased toxicity of the metal. Preinduction of metallothionein by zinc injections resulted in decreased cadmium sensitivity of the fish and a decrease in the repression of vitellogenin mRNA. Furthermore, a role for metallothionein in the detoxification of cadmium is indicated by the induction of vitellogenin synthesis that occurs once metallothionein has begun sequestering cadmium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Hyllner S. J., Oppen-Berntsen D. O., Helvik J. V., Walther B. T., Haux C. Oestradiol-17 beta induces the major vitelline envelope proteins in both sexes in teleosts. J Endocrinol. 1991 Nov;131(2):229–236. doi: 10.1677/joe.0.1310229. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Guellec K., Lawless K., Valotaire Y., Kress M., Tenniswood M. Vitellogenin gene expression in male rainbow trout (Salmo gairdneri). Gen Comp Endocrinol. 1988 Sep;71(3):359–371. doi: 10.1016/0016-6480(88)90264-x. [DOI] [PubMed] [Google Scholar]
- McCarter J. A., Matheson A. T., Roch M., Olafson R. W., Buckley J. T. Chronic exposure of coho salmon to sublethal concentrations of copper--II. Distribution of copper between high- and low-molecular-weight proteins in liver cytosol and the possible role of metallothionein in detoxification. Comp Biochem Physiol C. 1982;72(1):21–26. doi: 10.1016/0306-4492(82)90199-x. [DOI] [PubMed] [Google Scholar]
- Olafson R. W., Olsson P. E. Electrochemical detection of metallothionein. Methods Enzymol. 1991;205:205–213. doi: 10.1016/0076-6879(91)05100-a. [DOI] [PubMed] [Google Scholar]
- Olsson P. E., Hyllner S. J., Zafarullah M., Andersson T., Gedamu L. Differences in metallothionein gene expression in primary cultures of rainbow trout hepatocytes and the RTH-149 cell line. Biochim Biophys Acta. 1990 May 24;1049(1):78–82. doi: 10.1016/0167-4781(90)90086-h. [DOI] [PubMed] [Google Scholar]
- Olsson P. E., Zafarullah M., Foster R., Hamor T., Gedamu L. Developmental regulation of metallothionein mRNA, zinc and copper levels in rainbow trout, Salmo gairdneri. Eur J Biochem. 1990 Oct 5;193(1):229–235. doi: 10.1111/j.1432-1033.1990.tb19327.x. [DOI] [PubMed] [Google Scholar]
- Olsson P. E., Zafarullah M., Gedamu L. A role of metallothionein in zinc regulation after oestradiol induction of vitellogenin synthesis in rainbow trout, Salmo gairdneri. Biochem J. 1989 Jan 15;257(2):555–559. doi: 10.1042/bj2570555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstead H. H. Some trace elements which are essential for human nutrition: zinc, copper, manganese, and chromium. Prog Food Nutr Sci. 1975;1(6):371–391. [PubMed] [Google Scholar]
- Verbost P. M., Flik G., Lock R. A., Wendelaar Bonga S. E. Cadmium inhibition of Ca2+ uptake in rainbow trout gills. Am J Physiol. 1987 Aug;253(2 Pt 2):R216–R221. doi: 10.1152/ajpregu.1987.253.2.R216. [DOI] [PubMed] [Google Scholar]
- Zafarullah M., Olsson P. E., Gedamu L. Endogenous and heavy-metal-ion-induced metallothionein gene expression in salmonid tissues and cell lines. Gene. 1989 Nov 15;83(1):85–93. doi: 10.1016/0378-1119(89)90406-x. [DOI] [PubMed] [Google Scholar]