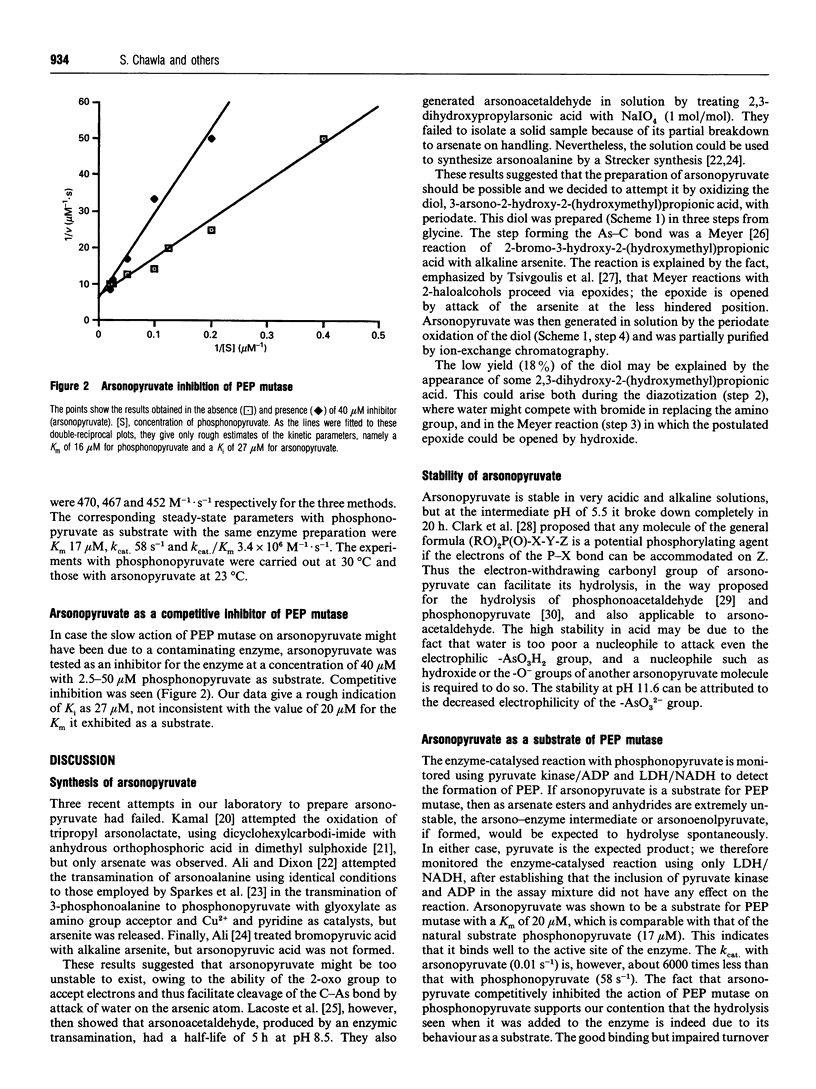
Abstract
3-Arsonopyruvate was prepared in four steps from glycine. The arsenic-carbon bond was formed by a Meyer reaction between alkaline arsenite and 2-bromo-3-hydroxy-2-(hydroxymethyl)propionic acid; the 3-arsono-2-hydroxy-2-(hydroxymethyl) propionic acid formed was oxidized with periodate to give 3-arsonopyruvate. This proves to be an alternative substrate for phosphoenolpyruvate mutase, giving pyruvate, which was assayed using lactate dehydrogenase. The K(m) is 20 microM, similar to that observed for the natural substrate phosphonopyruvate (17 microM), whereas the kcat. of 0.01 s-1 was much lower than that for phosphonopyruvate (58 s-1). Arsonopyruvate competitively inhibited the action of the mutase on phosphonopyruvate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. R., Sparkes M. J., Dixon H. B. The arsonomethyl analogue of adenosine 5'-phosphate. An uncoupler of adenylate kinase. Biochem J. 1984 Aug 1;221(3):829–836. doi: 10.1042/bj2210829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B. R., Dixon H. B. Synthesis of 3-arsonoalanine and its action on aspartate aminotransferase and aspartate ammonia-lyase. Comparison with arsenical analogues of malate and fumarate. Eur J Biochem. 1993 Jul 1;215(1):161–166. doi: 10.1111/j.1432-1033.1993.tb18018.x. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph O., Sparkes M. J., Dixon H. B. A rapid spectrophotometric estimation of individual phosphates and phosphonates: its application to chromatography of sugar phosphates and their phosphonate analogues. Anal Biochem. 1993 Apr;210(1):195–198. doi: 10.1006/abio.1993.1172. [DOI] [PubMed] [Google Scholar]
- Hidaka T., Hidaka M., Uozumi T., Seto H. Nucleotide sequence of a carboxyphosphonoenolpyruvate phosphonomutase gene isolated from a bialaphos-producing organism, Streptomyces hygroscopicus, and its expression in Streptomyces lividans. Mol Gen Genet. 1992 Jun;233(3):476–478. doi: 10.1007/BF00265446. [DOI] [PubMed] [Google Scholar]
- Hidaka T., Mori M., Imai S., Hara O., Nagaoka K., Seto H. Studies on the biosynthesis of bialaphos (SF-1293). 9. Biochemical mechanism of C-P bond formation in bialaphos: discovery of phosphoenolpyruvate phosphomutase which catalyzes the formation of phosphonopyruvate from phosphoenolpyruvate. J Antibiot (Tokyo) 1989 Mar;42(3):491–494. doi: 10.7164/antibiotics.42.491. [DOI] [PubMed] [Google Scholar]
- La Nauze J. M., Rosenberg H. The identification of 2-phosphonoacetaldehyde as an intermediate in the degradation of 2-aminoethylphosphonate by Bacillus cereus. Biochim Biophys Acta. 1968 Oct 15;165(3):438–447. doi: 10.1016/0304-4165(68)90223-7. [DOI] [PubMed] [Google Scholar]
- Lacoste A. M., Dumora C., Ali B. R., Neuzil E., Dixon H. B. Utilization of 2-aminoethylarsonic acid in Pseudomonas aeruginosa. J Gen Microbiol. 1992 Jun;138(6):1283–1287. doi: 10.1099/00221287-138-6-1283. [DOI] [PubMed] [Google Scholar]
- Lagunas R., Pestaña D., Diez-Masa J. C. Arsenic mononucleotides. Separation by high-performance liquid chromatography and identification with myokinase and adenylate deaminase. Biochemistry. 1984 Feb 28;23(5):955–960. doi: 10.1021/bi00300a024. [DOI] [PubMed] [Google Scholar]
- OTANI T. T., WINITZ M. Studies on hydroxyamino acids. I. Synthesis of some alpha-alkylated serines. Arch Biochem Biophys. 1960 Oct;90:254–259. doi: 10.1016/0003-9861(60)90577-4. [DOI] [PubMed] [Google Scholar]
- Pollack S. J., Freeman S., Pompliano D. L., Knowles J. R. Cloning, overexpression and mechanistic studies of carboxyphosphonoenolpyruvate mutase from Streptomyces hygroscopicus. Eur J Biochem. 1992 Oct 15;209(2):735–743. doi: 10.1111/j.1432-1033.1992.tb17342.x. [DOI] [PubMed] [Google Scholar]
- Rozovskaya T. A., Rechinsky V. O., Bibilashvili R. S., Karpeisky MYa, Tarusova N. B., Khomutov R. M., Dixon H. B. The mechanism of pyrophosphorolysis of RNA by RNA polymerase. Endowment of RNA polymerase with artificial exonuclease activity. Biochem J. 1984 Dec 1;224(2):645–650. doi: 10.1042/bj2240645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel H. M., Freeman S., Seto H., Knowles J. R. Phosphonate biosynthesis: isolation of the enzyme responsible for the formation of a carbon-phosphorus bond. Nature. 1988 Sep 29;335(6189):457–458. doi: 10.1038/335457a0. [DOI] [PubMed] [Google Scholar]
- Seidel H. M., Knowles J. R. Interaction of inhibitors with phosphoenolpyruvate mutase: implications for the reaction mechanism and the nature of the active site. Biochemistry. 1994 May 10;33(18):5641–5646. doi: 10.1021/bi00184a037. [DOI] [PubMed] [Google Scholar]
- Seidel H. M., Pompliano D. L., Knowles J. R. Phosphonate biosynthesis: molecular cloning of the gene for phosphoenolpyruvate mutase from Tetrahymena pyriformis and overexpression of the gene product in Escherichia coli. Biochemistry. 1992 Mar 10;31(9):2598–2608. doi: 10.1021/bi00124a021. [DOI] [PubMed] [Google Scholar]
- Sparkes M. J., Rogers K. L., Dixon H. B. The synthesis of 3-phosphonoalanine, phosphonopyruvic acid and phosphonolactic acid. Scission of the C-P bond during diazotization of phosphonoalanine. Eur J Biochem. 1990 Dec 12;194(2):373–376. doi: 10.1111/j.1432-1033.1990.tb15628.x. [DOI] [PubMed] [Google Scholar]
- Sparkes M. J., Rogers K. L., Dixon H. B. The synthesis of 3-phosphonoalanine, phosphonopyruvic acid and phosphonolactic acid. Scission of the C-P bond during diazotization of phosphonoalanine. Eur J Biochem. 1990 Dec 12;194(2):373–376. doi: 10.1111/j.1432-1033.1990.tb15628.x. [DOI] [PubMed] [Google Scholar]


