Abstract
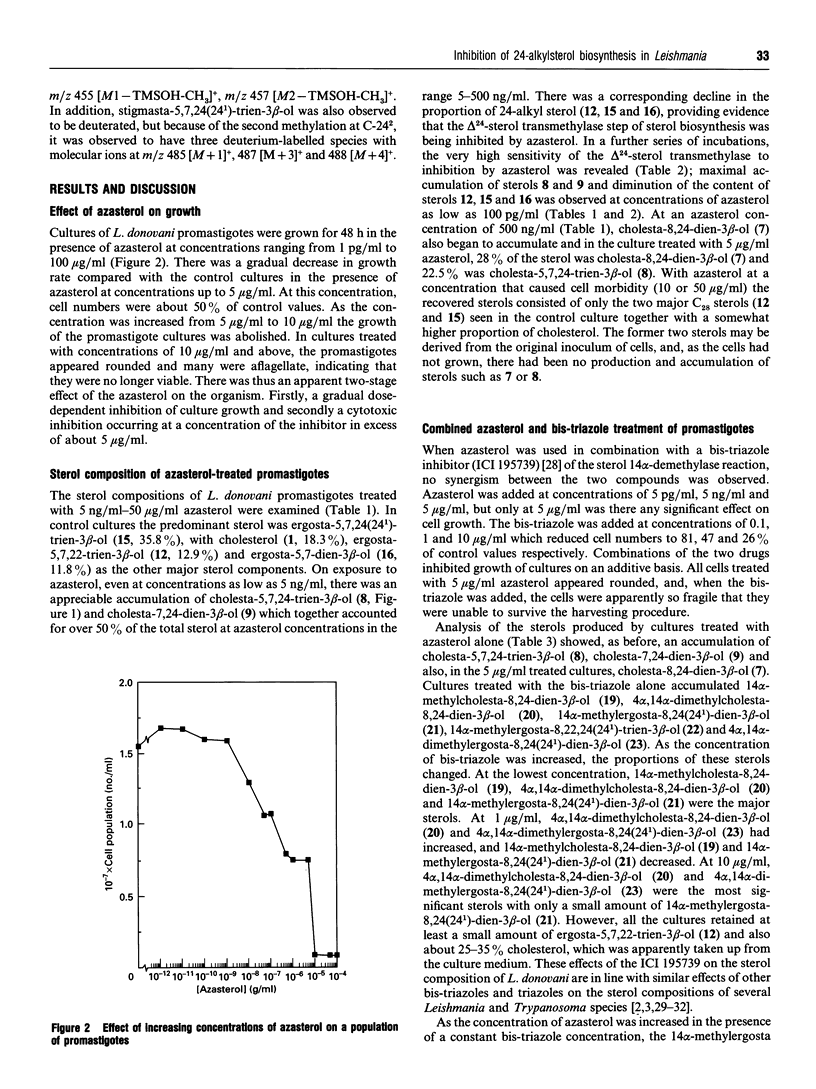
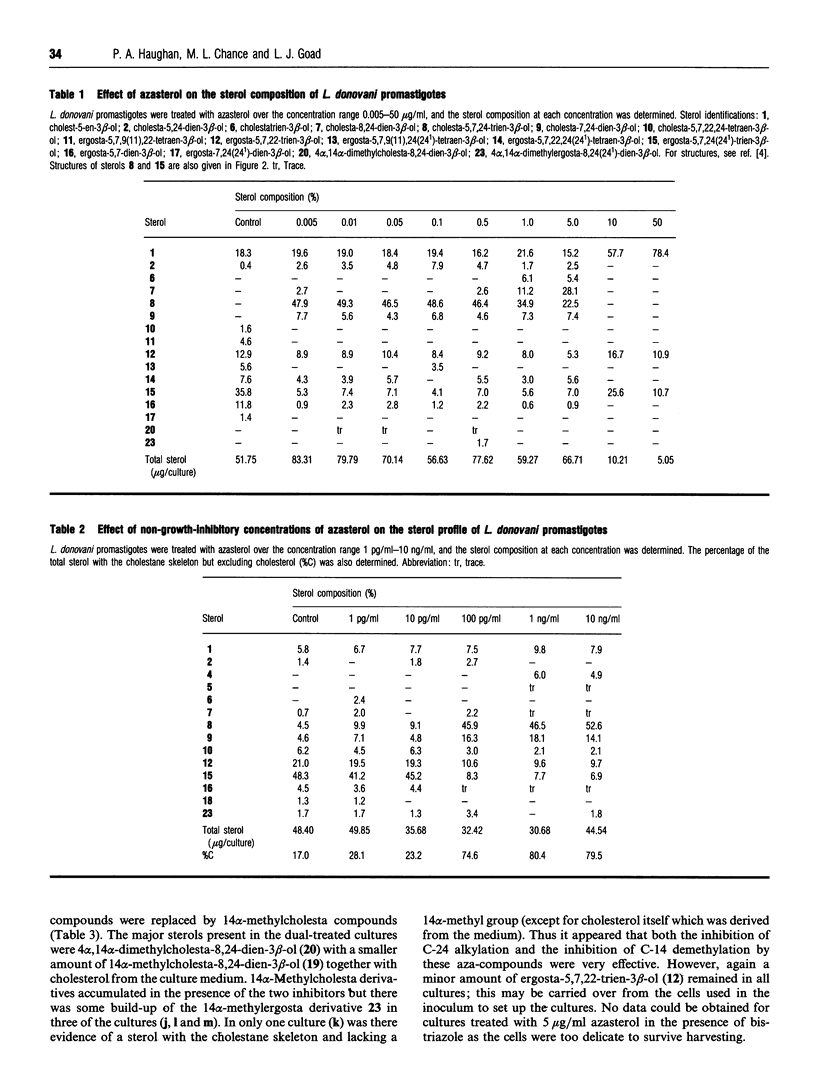
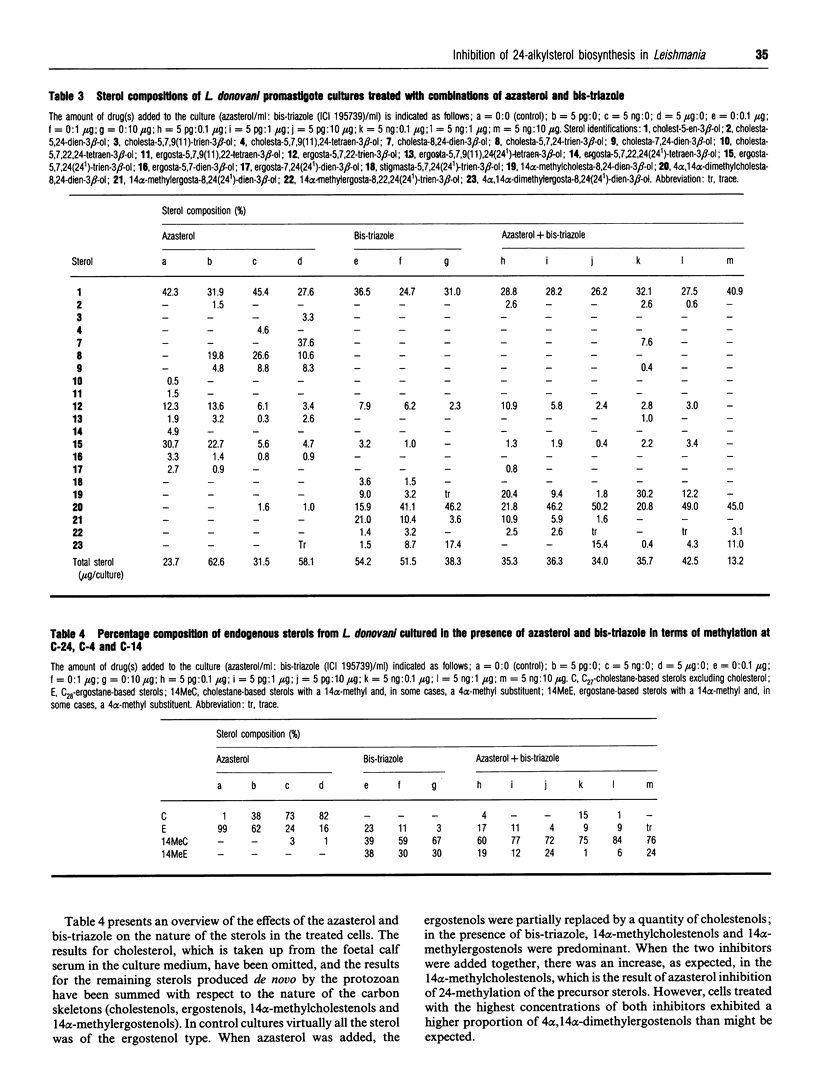
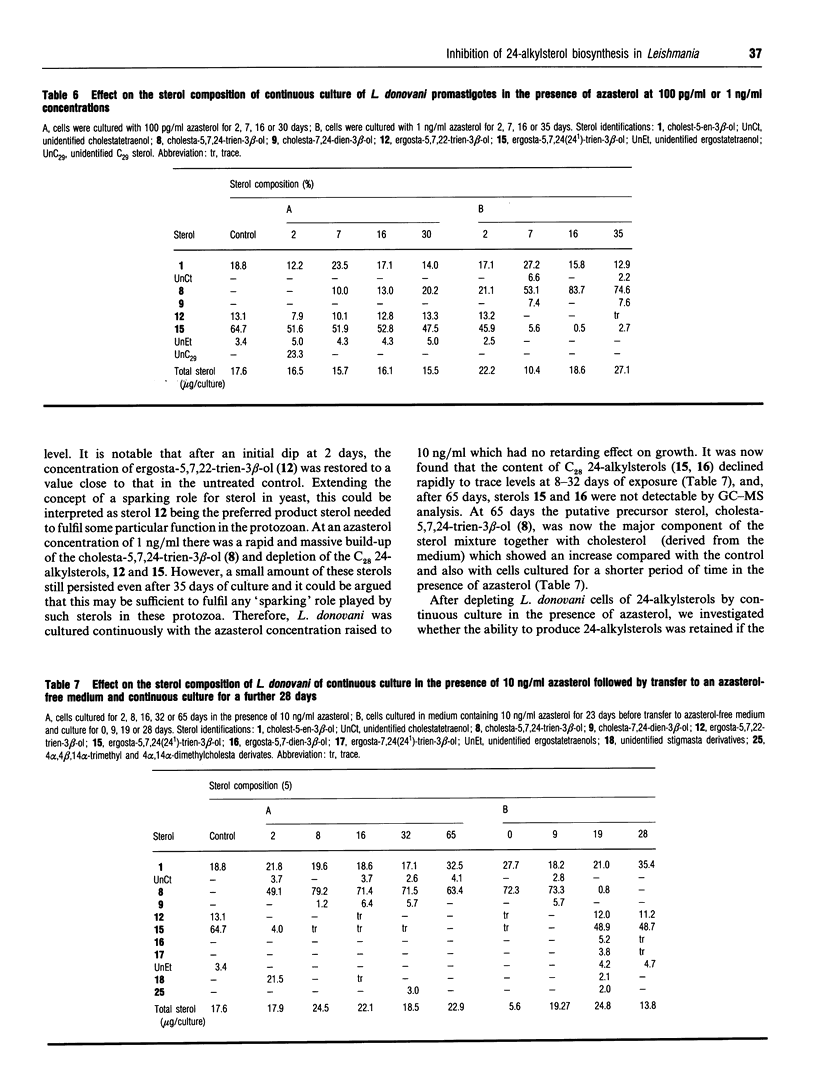
Leishmania donovani promastigotes were cultured in the presence of an azasterol (20-piperidin-2-yl-5 alpha-pregnane-3 beta,20-diol) to determine the effects on sterol biosynthesis and cell proliferation. Inhibition of growth increased gradually with azasterol concentrations up to 5 micrograms/ml; concentrations of azasterol exceeding 5 micrograms/ml were lethal. Sterol biosynthesis was affected by the azasterol when administered at concentrations as low as 100 pg/ml. The primary site of action was the alkylation at C-24 of a delta 24-sterol precursor. The 24-alkylated sterols [ergosta-5,7,24(24(1))-trien-3 beta-ol and ergosta-5,7,22-trien-3 beta-ol] of the protozoan were replaced by delta 24-cholesta-type sterols which then accumulated in the cells. Administration of the azasterol together with a bis-triazole inhibitor of the 14 alpha-methylsterol 14-demethylase reaction, which operates in sterol biosynthesis, resulted in depletion of 24-alkylsterols and their replacement with predominantly 14 alpha-methylsterols lacking a 24-alkyl group. Continuous subculture of promastigotes in the presence of the azasterol resulted in gradual depletion of 24-alkylsterols and their complete replacement by delta 24-cholesta-type sterols. Transfer of the azasterol-treated cells to medium lacking azasterol resulted in a gradual restoration, after several subcultures, of the normal 24-alkylsterol pattern. The results indicate that, although 24-alkylsterols are normally produced by the protozoan, it can nevertheless survive with sterols possessing only the cholestane skeleton. Thus there is no absolute requirement for 24-alkylsterols to fulfil some essential 'sparking' role associated with cell growth in promastigotes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthington B. A., Bennett L. G., Skatrud P. L., Guynn C. J., Barbuch R. J., Ulbright C. E., Bard M. Cloning, disruption and sequence of the gene encoding yeast C-5 sterol desaturase. Gene. 1991 Jun 15;102(1):39–44. doi: 10.1016/0378-1119(91)90535-j. [DOI] [PubMed] [Google Scholar]
- Ashman W. H., Barbuch R. J., Ulbright C. E., Jarrett H. W., Bard M. Cloning and disruption of the yeast C-8 sterol isomerase gene. Lipids. 1991 Aug;26(8):628–632. doi: 10.1007/BF02536427. [DOI] [PubMed] [Google Scholar]
- Bard M., Lees N. D., Turi T., Craft D., Cofrin L., Barbuch R., Koegel C., Loper J. C. Sterol synthesis and viability of erg11 (cytochrome P450 lanosterol demethylase) mutations in Saccharomyces cerevisiae and Candida albicans. Lipids. 1993 Nov;28(11):963–967. doi: 10.1007/BF02537115. [DOI] [PubMed] [Google Scholar]
- Beach D. H., Goad L. J., Holz G. G., Jr Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Mol Biochem Parasitol. 1988 Nov;31(2):149–162. doi: 10.1016/0166-6851(88)90166-1. [DOI] [PubMed] [Google Scholar]
- Berens R. L., Brun R., Krassner S. M. A simple monophasic medium for axenic culture of hemoflagellates. J Parasitol. 1976 Jun;62(3):360–365. [PubMed] [Google Scholar]
- Berman J. D., Goad L. J., Beach D. H., Holz G. G., Jr Effects of ketoconazole on sterol biosynthesis by Leishmania mexicana mexicana amastigotes in murine macrophage tumor cells. Mol Biochem Parasitol. 1986 Jul;20(1):85–92. doi: 10.1016/0166-6851(86)90145-3. [DOI] [PubMed] [Google Scholar]
- Boyle F. T., Gilman D. J., Gravestock M. B., Wardleworth J. M. Synthesis and structure-activity relationships of a novel antifungal agent, ICI 195,739. Ann N Y Acad Sci. 1988;544:86–100. doi: 10.1111/j.1749-6632.1988.tb40391.x. [DOI] [PubMed] [Google Scholar]
- Dahl C., Biemann H. P., Dahl J. A protein kinase antigenically related to pp60v-src possibly involved in yeast cell cycle control: positive in vivo regulation by sterol. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4012–4016. doi: 10.1073/pnas.84.12.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin D., Endo K. A convenient and economical preparation of L-methionine-methyl-d3. Anal Biochem. 1970 Aug;36(2):338–342. doi: 10.1016/0003-2697(70)90369-6. [DOI] [PubMed] [Google Scholar]
- Gaber R. F., Copple D. M., Kennedy B. K., Vidal M., Bard M. The yeast gene ERG6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol Cell Biol. 1989 Aug;9(8):3447–3456. doi: 10.1128/mcb.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad L. J., Holz G. G., Jr, Beach D. H. Sterols of Leishmania species. Implications for biosynthesis. Mol Biochem Parasitol. 1984 Feb;10(2):161–170. doi: 10.1016/0166-6851(84)90004-5. [DOI] [PubMed] [Google Scholar]
- Goad L. J., Holz G. G., Jr, Beach D. H. Sterols of ketoconazole-inhibited Leishmania mexicana mexicana promastigotes. Mol Biochem Parasitol. 1985 Jun;15(3):257–279. doi: 10.1016/0166-6851(85)90089-1. [DOI] [PubMed] [Google Scholar]
- Hart D. T., Lauwers W. J., Willemsens G., Vanden Bossche H., Opperdoes F. R. Perturbation of sterol biosynthesis by itraconazole and ketoconazole in Leishmania mexicana mexicana infected macrophages. Mol Biochem Parasitol. 1989 Mar 1;33(2):123–134. doi: 10.1016/0166-6851(89)90026-1. [DOI] [PubMed] [Google Scholar]
- Haughan P. A., Chance M. L., Goad L. J. Effects of sinefungin on growth and sterol composition of Leishmania promastigotes. Exp Parasitol. 1993 Sep;77(2):147–154. doi: 10.1006/expr.1993.1071. [DOI] [PubMed] [Google Scholar]
- Haughan P. A., Chance M. L., Goad L. J. Synergism in vitro of lovastatin and miconazole as anti-leishmanial agents. Biochem Pharmacol. 1992 Dec 1;44(11):2199–2206. doi: 10.1016/0006-2952(92)90347-l. [DOI] [PubMed] [Google Scholar]
- Lorenz R. T., Casey W. M., Parks L. W. Structural discrimination in the sparking function of sterols in the yeast Saccharomyces cerevisiae. J Bacteriol. 1989 Nov;171(11):6169–6173. doi: 10.1128/jb.171.11.6169-6173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A. M., Unrau A. M., Oehlschlager A. C., Woods R. A. Azasterol inhibitors in yeast. Inhibition of the delta 24-sterol methyltransferase and the 24-methylene sterol delta 24(28)-reductase in sterol mutants of Saccharomyces cerevisiae. Can J Biochem. 1979 Mar;57(3):201–208. doi: 10.1139/o79-025. [DOI] [PubMed] [Google Scholar]
- Pierce H. D., Jr, Pierce A. M., Srinivasan R., Unrau A. M., Oehlschlager A. C. Azasterol inhibitors in yeast. Inhibition of the 24-methylene sterol delta24(28)-reductase and delta24-sterol methyltransferase of Saccharomyces cerevisiae by 23-azacholesterol. Biochim Biophys Acta. 1978 Jun 23;529(3):429–437. doi: 10.1016/0005-2760(78)90087-5. [DOI] [PubMed] [Google Scholar]
- Pinto W. J., Lozano R., Sekula B. C., Nes W. R. Stereochemically distinct roles for sterol in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1983 Apr 15;112(1):47–54. doi: 10.1016/0006-291x(83)91795-3. [DOI] [PubMed] [Google Scholar]
- Pinto W. J., Nes W. R. Stereochemical specificity for sterols in Saccharomyces cerevisiae. J Biol Chem. 1983 Apr 10;258(7):4472–4476. [PubMed] [Google Scholar]
- Rahman M. D., Pascal R. A., Jr Inhibitors of ergosterol biosynthesis and growth of the trypanosomatid protozoan Crithidia fasciculata. J Biol Chem. 1990 Mar 25;265(9):4989–4996. [PubMed] [Google Scholar]
- Ramgopal M., Bloch K. Sterol synergism in yeast. Proc Natl Acad Sci U S A. 1983 Feb;80(3):712–715. doi: 10.1073/pnas.80.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. J., Low C., Bottema C. D., Parks L. W. Multiple functions for sterols in Saccharomyces cerevisiae. Biochim Biophys Acta. 1985 Dec 4;837(3):336–343. doi: 10.1016/0005-2760(85)90057-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Parks L. W. Structural and physiological features of sterols necessary to satisfy bulk membrane and sparking requirements in yeast sterol auxotrophs. Arch Biochem Biophys. 1983 Sep;225(2):861–871. doi: 10.1016/0003-9861(83)90099-1. [DOI] [PubMed] [Google Scholar]
- Whitaker B. D., Nelson D. L. Sterol synergism in Paramecium tetraurelia. J Gen Microbiol. 1988 Jun;134(6):1441–1447. doi: 10.1099/00221287-134-6-1441. [DOI] [PubMed] [Google Scholar]


