Abstract
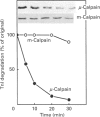
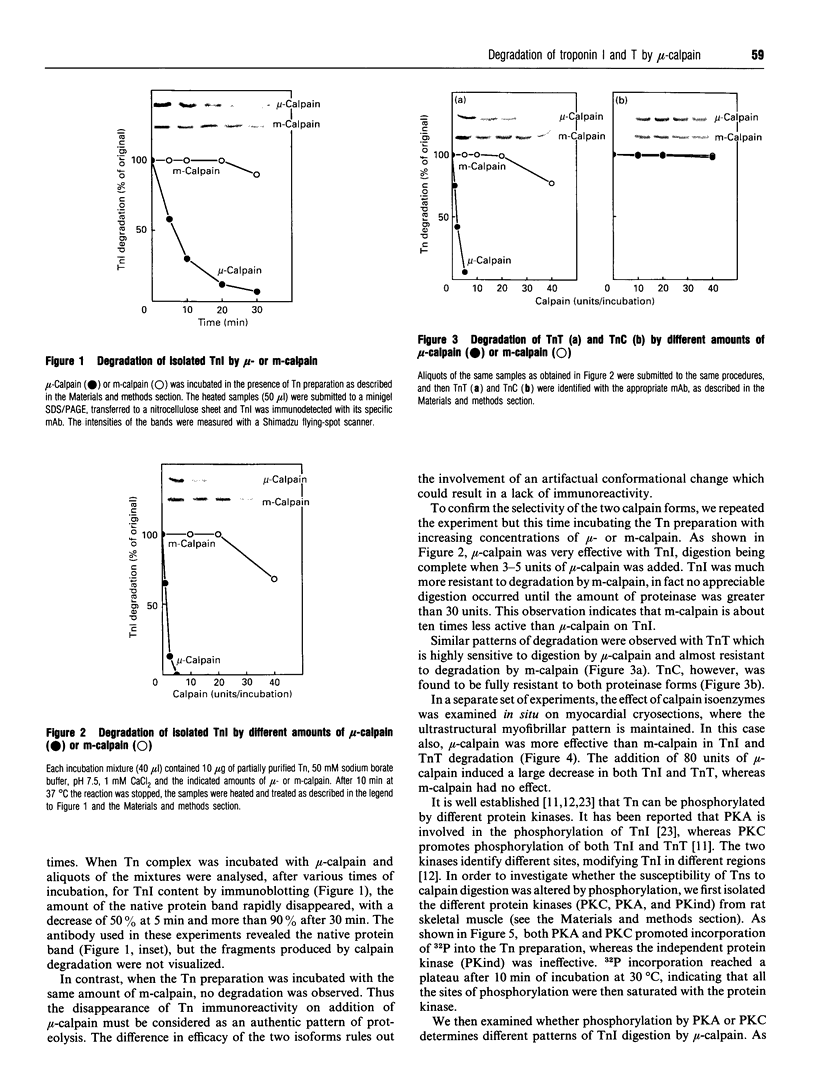
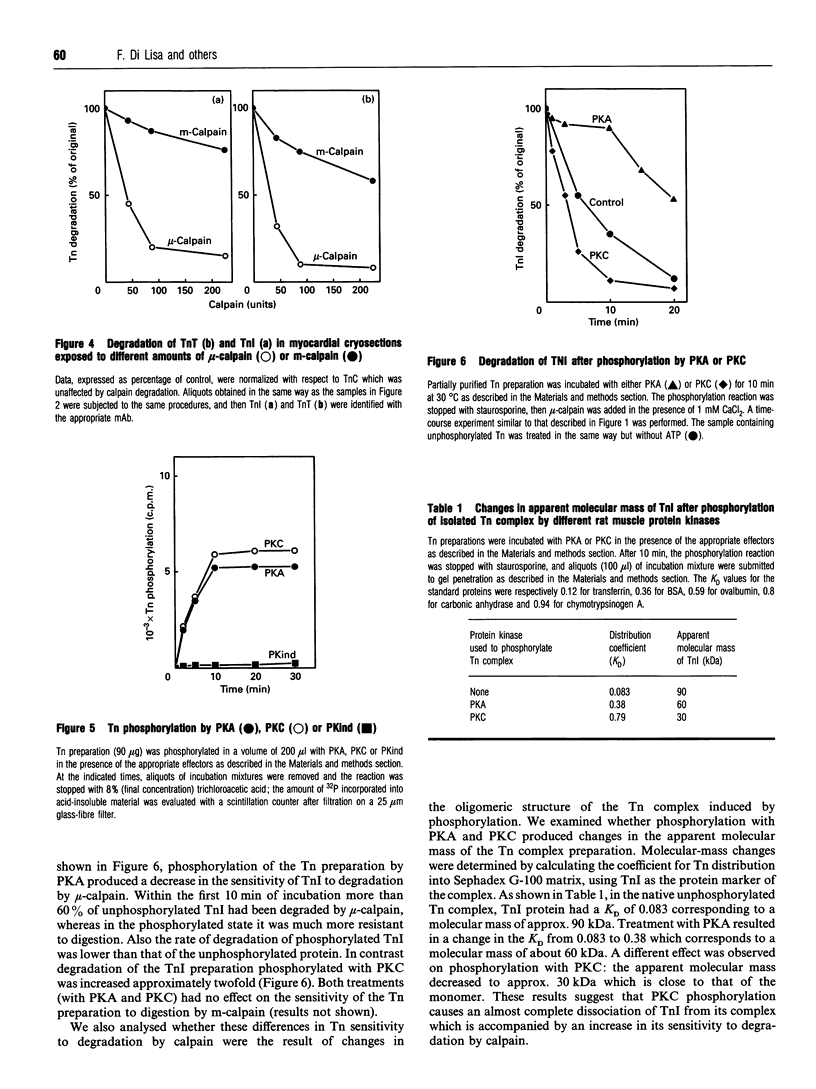
The degradation of troponin (Tn) subunits by calpain was studied by incubating either isolated cardiac Tns or myocardial cryosections with two different calpain isoenzymes isolated from rat skeletal muscle. Western-blot analysis with monoclonal antibodies against TnI and TnT showed that mu-calpain was at least ten times more active than m-calpain in degrading TnI and TnT both in vitro and in situ. TnC was completely resistant to both proteinase forms. Phosphorylation by cyclic AMP-dependent protein kinase (PKA) isolated from rat skeletal muscle reduced the sensitivity of TnI to degradation. This effect in combination with an increased efficiency of the endogenous inhibitor [Salamino, De Tullio, Michetti, Mengotti, Melloni and Pontremoli (1994) Biochem. Biophys. Res. Commun. 199, 1326-1332] probably reduces the proteolytic activity of calpain in cells on PKA stimulation. Conversely, phosphorylation by protein kinase C (PKC) resulted in a twofold increase in the degradation of TnI. Degradation by m-calpain was not modified by Tn phosphorylation. The different sensitivity to mu-calpain might be related to changes in TnI oligomeric structure. Indeed, on PKC phosphorylation, the apparent molecular mass of TnI calculated from the distribution coefficient of Tn complex in Sephadex G-100 matrix was reduced from 90 to 30 kDa suggesting dissociation of the Tn complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Perry S. V. The phosphorylation of troponin I from cardiac muscle. Biochem J. 1975 Sep;149(3):525–533. doi: 10.1042/bj1490525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Kodama A., Ebashi F. Troponin. I. Preparation and physiological function. J Biochem. 1968 Oct;64(4):465–477. doi: 10.1093/oxfordjournals.jbchem.a128918. [DOI] [PubMed] [Google Scholar]
- Geesink G. H., Ouali A., Smulders F. J., Talmant A., Tassy C., Guignot F., van Laack H. L. The role of ultimate pH in proteolysis and calpain/calpastatin activity in bovine muscle. Biochimie. 1992 Mar;74(3):283–289. doi: 10.1016/0300-9084(92)90127-z. [DOI] [PubMed] [Google Scholar]
- Goll D. E., Thompson V. F., Taylor R. G., Christiansen J. A. Role of the calpain system in muscle growth. Biochimie. 1992 Mar;74(3):225–237. doi: 10.1016/0300-9084(92)90121-t. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Katoh N., Wise B. C., Kuo J. F. Phosphorylation of cardiac troponin inhibitory subunit (troponin I) and tropomyosin-binding subunit (troponin T) by cardiac phospholipid-sensitive Ca2+-dependent protein kinase. Biochem J. 1983 Jan 1;209(1):189–195. doi: 10.1042/bj2090189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazón M. J., Gancedo J. M., Gancedo C. Inactivation of yeast fructose-1,6-bisphosphatase. In vivo phosphorylation of the enzyme. J Biol Chem. 1982 Feb 10;257(3):1128–1130. [PubMed] [Google Scholar]
- Mellgren R. L. Calcium-dependent proteases: an enzyme system active at cellular membranes? FASEB J. 1987 Aug;1(2):110–115. doi: 10.1096/fasebj.1.2.2886390. [DOI] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S. The calpains. Trends Neurosci. 1989 Nov;12(11):438–444. doi: 10.1016/0166-2236(89)90093-3. [DOI] [PubMed] [Google Scholar]
- Müller D., Holzer H. Regulation of fructose-1,6-bisphosphatase in yeast by phosphorylation/dephosphorylation. Biochem Biophys Res Commun. 1981 Dec 15;103(3):926–933. doi: 10.1016/0006-291x(81)90899-8. [DOI] [PubMed] [Google Scholar]
- Noland T. A., Jr, Kuo J. F. Protein kinase C phosphorylation of cardiac troponin I or troponin T inhibits Ca2(+)-stimulated actomyosin MgATPase activity. J Biol Chem. 1991 Mar 15;266(8):4974–4978. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., Sparatore B., Michetti M., Singh V. N., Horecker B. L. Evidence for an interaction between fructose 1,6-bisphosphatase and fructose 1,6-bisphosphate aldolase. Arch Biochem Biophys. 1979 Oct 1;197(1):356–363. doi: 10.1016/0003-9861(79)90256-x. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Viotti P. L., Michetti M., Di Lisa F., Siliprandi N. Isovalerylcarnitine is a specific activator of the high calcium requiring calpain forms. Biochem Biophys Res Commun. 1990 Feb 28;167(1):373–380. doi: 10.1016/0006-291x(90)91775-n. [DOI] [PubMed] [Google Scholar]
- Saggin L., Ausoni S., Gorza L., Sartore S., Schiaffino S. Troponin T switching in the developing rat heart. J Biol Chem. 1988 Dec 5;263(34):18488–18492. [PubMed] [Google Scholar]
- Saggin L., Gorza L., Ausoni S., Schiaffino S. Troponin I switching in the developing heart. J Biol Chem. 1989 Sep 25;264(27):16299–16302. [PubMed] [Google Scholar]
- Salamino F., De Tullio R., Michetti M., Mengotti P., Melloni E., Pontremoli S. Modulation of calpastatin specificity in rat tissues by reversible phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1326–1332. doi: 10.1006/bbrc.1994.1376. [DOI] [PubMed] [Google Scholar]
- Tobacman L. S., Adelstein R. S. Mechanism of regulation of cardiac actin-myosin subfragment 1 by troponin-tropomyosin. Biochemistry. 1986 Feb 25;25(4):798–802. doi: 10.1021/bi00352a010. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo-Oka T. Phosphorylation with cyclic adenosine 3':5' monophosphate-dependent protein kinase renders bovine cardiac troponin sensitive to the degradation by calcium-activated neutral protease. Biochem Biophys Res Commun. 1982 Jul 16;107(1):44–50. doi: 10.1016/0006-291x(82)91667-9. [DOI] [PubMed] [Google Scholar]
- Venema R. C., Kuo J. F. Protein kinase C-mediated phosphorylation of troponin I and C-protein in isolated myocardial cells is associated with inhibition of myofibrillar actomyosin MgATPase. J Biol Chem. 1993 Feb 5;268(4):2705–2711. [PubMed] [Google Scholar]
- Wang K. K., Villalobo A., Roufogalis B. D. Calmodulin-binding proteins as calpain substrates. Biochem J. 1989 Sep 15;262(3):693–706. doi: 10.1042/bj2620693. [DOI] [PMC free article] [PubMed] [Google Scholar]