Abstract
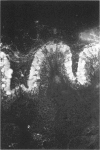
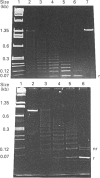
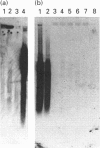
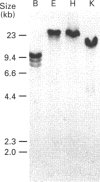
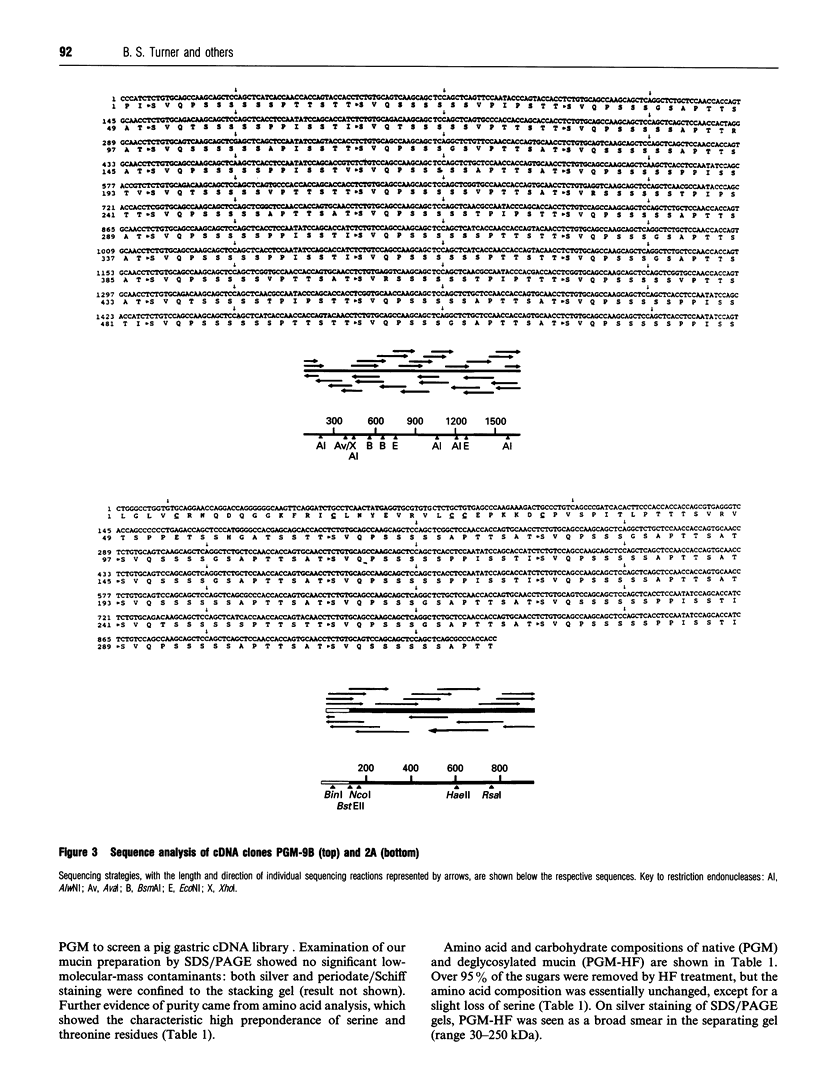
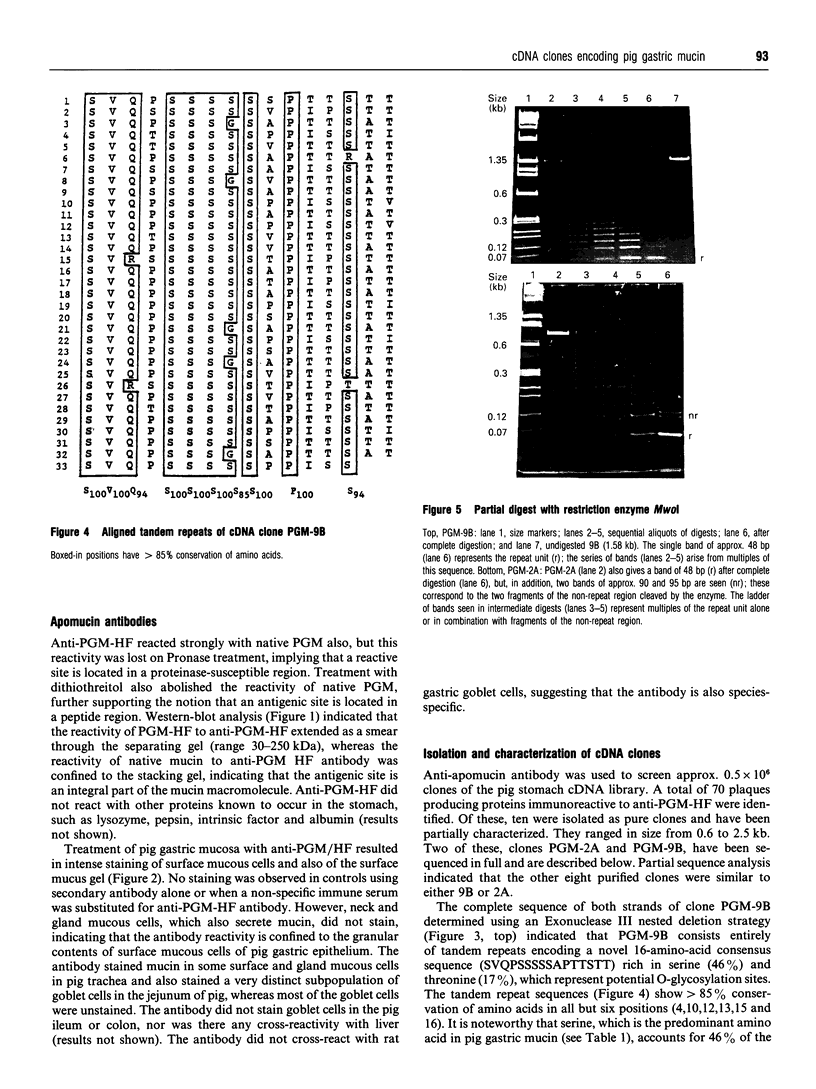
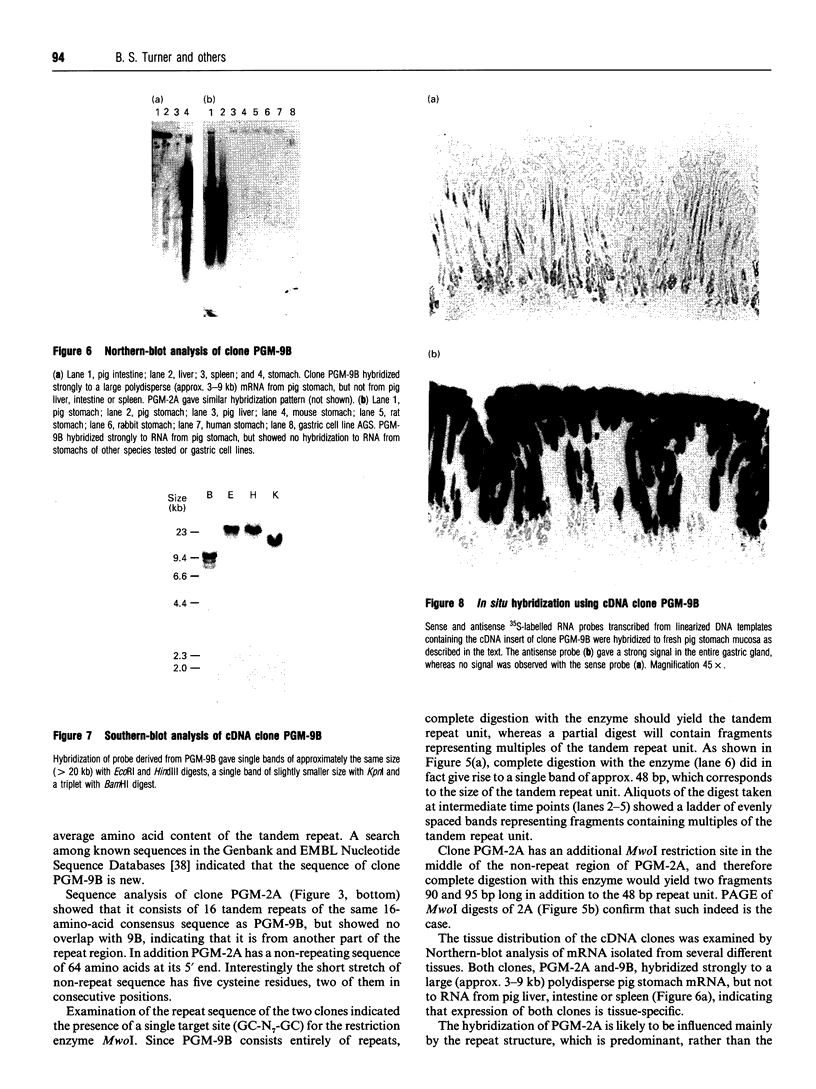
Polyclonal antibodies raised to deglycosylated pig gastric mucin were used to screen a cDNA library constructed with pig stomach mucosal mRNA. Immunocytochemistry indicated that the antibody recognizes intracellular and secreted mucin in surface mucous cells of pig gastric epithelium. A total of 70 clones producing proteins immunoreactive to this antibody were identified, two of which (PGM-2A,9B) were fully sequenced from both ends. Clone PGM-9B hybridized to a polydisperse mRNA (3-9 kb) from pig stomach, but not liver, intestine or spleen, nor to mRNA from human, mouse, rabbit or rat stomach. Sequence analysis indicated that PGM-9B encodes 33 tandem repeats of a 16-amino-acid consensus sequence rich in serine (46%) and threonine (17%). Using the restriction enzyme MwoI, which has a single target site in the repeat, it was demonstrated that PGM-9B consists entirely of this tandem repeat. Southern-blot analysis indicated that the repeat region is contained in a 20 kb HindIII-EcoRI fragment, and BamHI digestion suggested that most of the repeats are contained in a 10 kb fragment. In situ hybridization with an antisense probe to PGM-9B showed an intense signal in the entire gastric gland. Clone PGM-2A also contains the same repeat sequence as 9B, but, in addition, has a 64-amino-acid-long non-repeat region at its 5' end. Interestingly the non-repeat region of PGM-2A has five cysteine residues, the arrangement of which is identical with that reported for human intestinal mucin gene MUC2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. Structure of gastrointestinal mucus glycoproteins and the viscous and gel-forming properties of mucus. Br Med Bull. 1978 Jan;34(1):28–33. [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar K. R., Garik P., Turner B. S., Bradley J. D., Bansil R., Stanley H. E., LaMont J. T. Viscous fingering of HCl through gastric mucin. Nature. 1992 Dec 3;360(6403):458–461. doi: 10.1038/360458a0. [DOI] [PubMed] [Google Scholar]
- Bhaskar K. R., Gong D. H., Bansil R., Pajevic S., Hamilton J. A., Turner B. S., LaMont J. T. Profound increase in viscosity and aggregation of pig gastric mucin at low pH. Am J Physiol. 1991 Nov;261(5 Pt 1):G827–G832. doi: 10.1152/ajpgi.1991.261.5.G827. [DOI] [PubMed] [Google Scholar]
- Bhaskar K. R., Reid L. Application of density gradient methods for the study of mucus glycoprotein and other macromolecular components of the sol and gel phases of asthmatic sputa. J Biol Chem. 1981 Jul 25;256(14):7583–7589. [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K., Corfield A. P., Gallagher J. T. Mucous glycoproteins: a gel of a problem. Essays Biochem. 1985;20:40–76. [PubMed] [Google Scholar]
- Chomczynski P. One-hour downward alkaline capillary transfer for blotting of DNA and RNA. Anal Biochem. 1992 Feb 14;201(1):134–139. doi: 10.1016/0003-2697(92)90185-a. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gong D. H., Turner B., Bhaskar K. R., Lamont J. T. Lipid binding to gastric mucin: protective effect against oxygen radicals. Am J Physiol. 1990 Oct;259(4 Pt 1):G681–G686. doi: 10.1152/ajpgi.1990.259.4.G681. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Lagace R. E., Byrd J. C., Toribara N. W., Siddiki B., Fearney F. J., Lamport D. T., Kim Y. S. Molecular cloning of rat intestinal mucin. Lack of conservation between mammalian species. J Biol Chem. 1991 Nov 25;266(33):22733–22738. [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Toribara N. W., Siddiki B., Kim Y. S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994 Jan 28;269(4):2440–2446. [PubMed] [Google Scholar]
- Gum J. R., Jr Mucin genes and the proteins they encode: structure, diversity, and regulation. Am J Respir Cell Mol Biol. 1992 Dec;7(6):557–564. doi: 10.1165/ajrcmb/7.6.557. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Inoue H., Nojima H., Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990 Nov 30;96(1):23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- Lichtenberger L. M., Romero J. J., Kao Y. C., Dial E. J. Gastric protective activity of mixtures of saturated polar and neutral lipids in rats. Gastroenterology. 1990 Aug;99(2):311–326. doi: 10.1016/0016-5085(90)91011-t. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Lamport D. T. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem. 1977 Oct;82(2):289–309. doi: 10.1016/0003-2697(77)90165-8. [DOI] [PubMed] [Google Scholar]
- Oliver M. G., Specian R. D. Intracellular variation of rat intestinal mucin granules localized by monoclonal antibodies. Anat Rec. 1991 Aug;230(4):513–518. doi: 10.1002/ar.1092300410. [DOI] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Pearson J., Allen A., Venables C. Gastric mucus: isolation and polymeric structure of the undegraded glycoprotein: its breakdown by pepsin. Gastroenterology. 1980 Apr;78(4):709–715. [PubMed] [Google Scholar]
- Porchet N., Nguyen V. C., Dufosse J., Audie J. P., Guyonnet-Duperat V., Gross M. S., Denis C., Degand P., Bernheim A., Aubert J. P. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun. 1991 Mar 15;175(2):414–422. doi: 10.1016/0006-291x(91)91580-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steggles A. W. A rapid procedure for creating nested sets of deletions using mini-prep plasmid DNA samples. Biotechniques. 1989 Mar;7(3):241–242. [PubMed] [Google Scholar]
- Strous G. J., Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27(1-2):57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Timpte C. S., Eckhardt A. E., Abernethy J. L., Hill R. L. Porcine submaxillary gland apomucin contains tandemly repeated, identical sequences of 81 residues. J Biol Chem. 1988 Jan 15;263(2):1081–1088. [PubMed] [Google Scholar]
- Toribara N. W., Gum J. R., Jr, Culhane P. J., Lagace R. E., Hicks J. W., Petersen G. M., Kim Y. S. MUC-2 human small intestinal mucin gene structure. Repeated arrays and polymorphism. J Clin Invest. 1991 Sep;88(3):1005–1013. doi: 10.1172/JCI115360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribara N. W., Roberton A. M., Ho S. B., Kuo W. L., Gum E., Hicks J. W., Gum J. R., Jr, Byrd J. C., Siddiki B., Kim Y. S. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993 Mar 15;268(8):5879–5885. [PubMed] [Google Scholar]
- Xu G., Huan L. J., Khatri I. A., Wang D., Bennick A., Fahim R. E., Forstner G. G., Forstner J. F. cDNA for the carboxyl-terminal region of a rat intestinal mucin-like peptide. J Biol Chem. 1992 Mar 15;267(8):5401–5407. [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]