Abstract
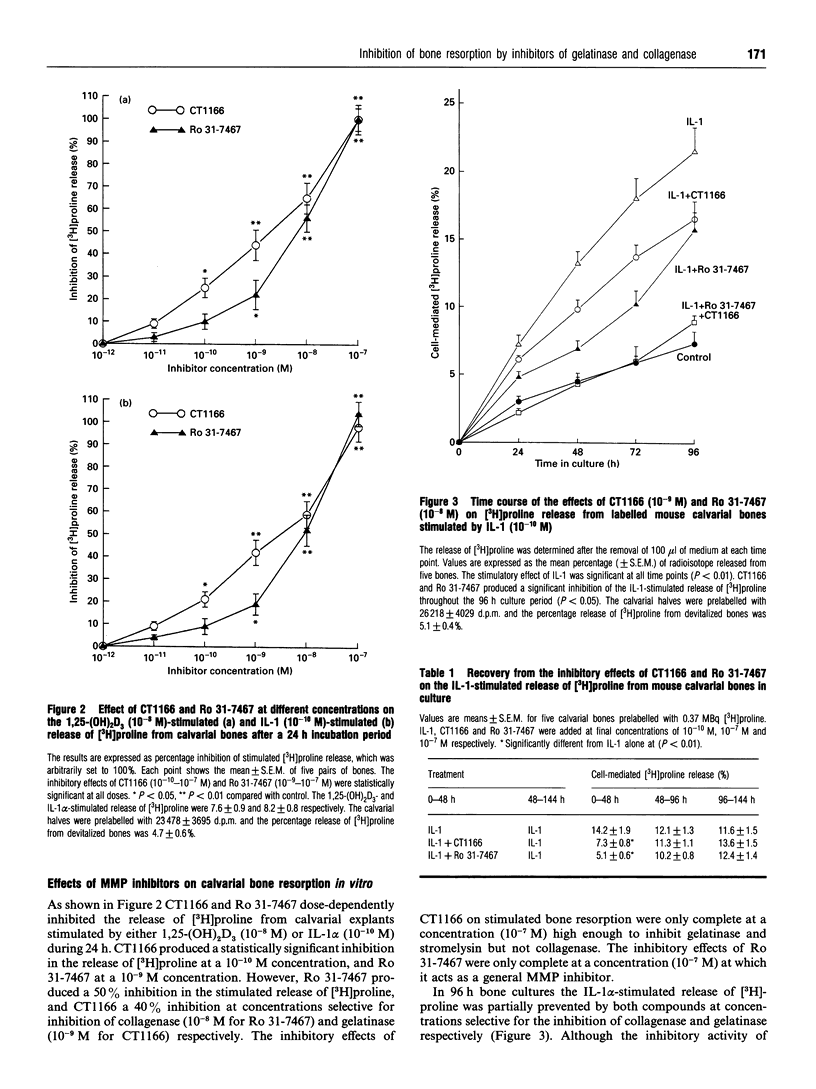
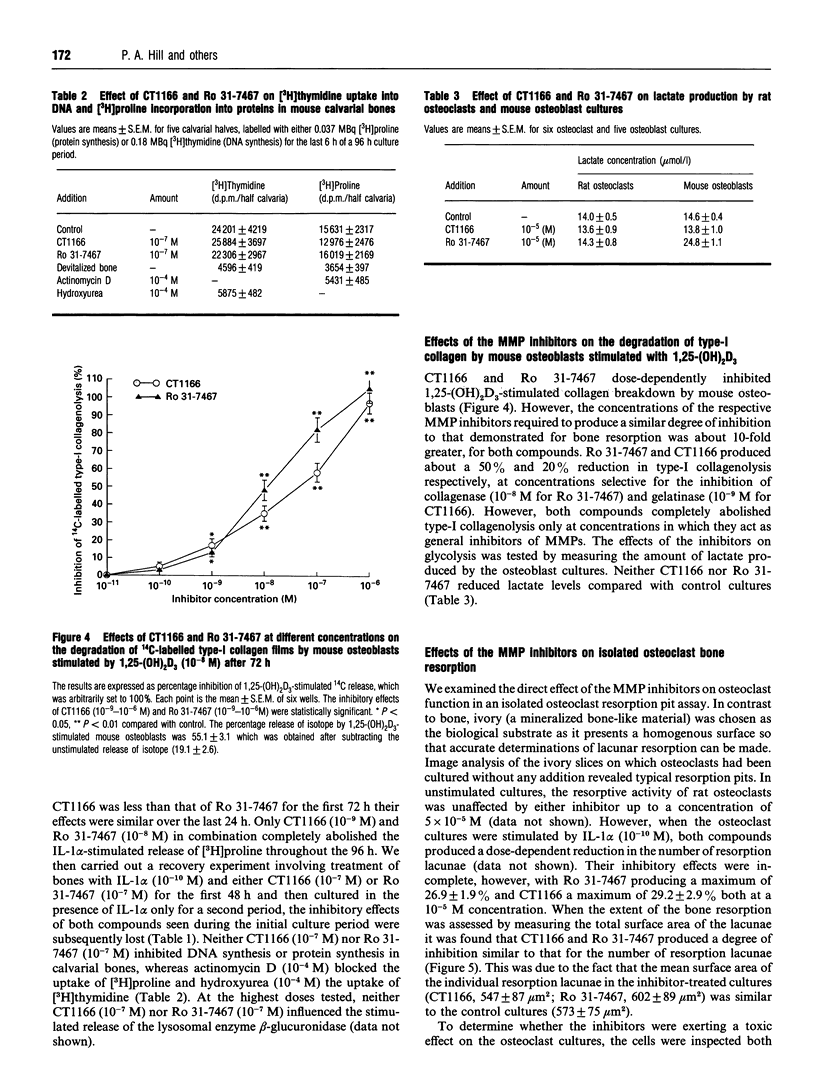
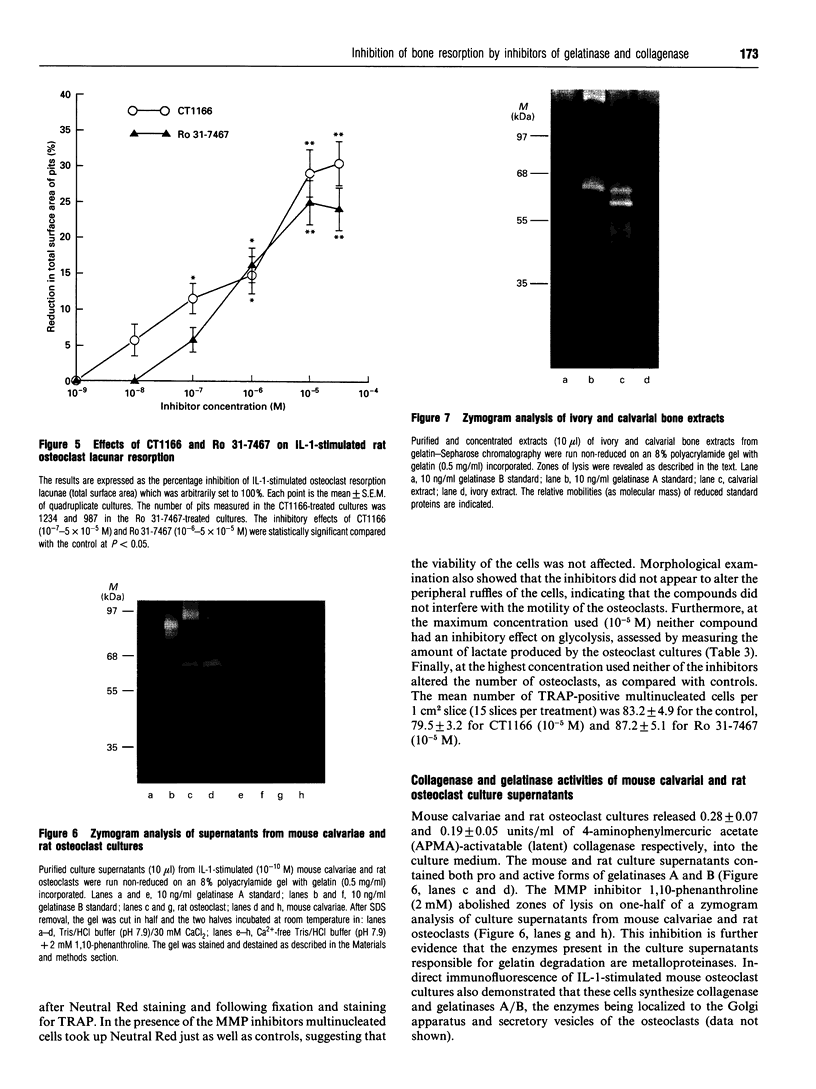
Two low-molecular-mass inhibitors of matrix metalloproteinases (MMPs), CT1166, a concentration-dependent selective inhibitor of gelatinases A and B, and Ro 31-7467, a concentration-dependent selective inhibitor of collagenase, were examined for their effects on bone resorption and type-I collagenolysis. The test systems consisted of measuring (1) the release of [3H]proline from prelabelled mouse calvarial explants; (2) the release of 14C from prelabelled type-I collagen films by mouse calvarial osteoblasts; and (3) lacunar resorption by isolated rat osteoclasts cultured on ivory slices. In 24 h cultures, CT1166 and Ro 31-7467 inhibited both interleukin-1 alpha- (IL-1 alpha; 10(-10) M) and 1,25-dihydroxyvitamin D3 (10(-8) M)-stimulated bone resorption in cultured neonatal mouse calvariae at concentration selective for the inhibition of gelatinase (10(-9) M for CT1166) and collagenase (10(-8) M for Ro 31-7467) respectively. For each compound the inhibition was dose-dependent, reversible, and complete at a 10(-7) M concentration. However, CT1166 (10(-9) M) and Ro 31-7467 (10(-8) M) in combination were required to completely abolish IL-1 alpha-stimulated bone resorption in mouse calvariae throughout a 96 h culture period. Neither of the inhibitors affected protein synthesis, DNA synthesis nor the IL-1 alpha-stimulated secretion of the lysosomal enzyme, beta-glucuronidase. Both CT1166 and Ro 31-7467 partially inhibited IL-1 alpha-stimulated lacunar resorption by isolated osteoclasts, but were without effect on unstimulated lacunar resorption. Rodent osteoclasts produced collagenase and gelatinases-A and -B activity. In contrast the substrate used to assess osteoclast lacunar resorption contained no detectable collagenase or gelatinase activity. Both compounds dose-dependently inhibited 1,25-dihydroxyvitamin D3 (10(-8) M)-stimulated degradation of type-I collagen by mouse calvarial osteoblasts; however, complete inhibition of collagenolysis was only achieved at concentrations at which CT1166 and Ro 31-7467 act as general MMP inhibitors. This study demonstrates that collagenase and gelatinases A and/or B participate in bone resorption. While these MMPs may be primarily involved in osteoid removal, we conclude that they may also be released by osteoclasts, where they participate in bone collagen degradation within the resorption lacunae.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Bell C. E., Sly W. S. Human beta-glucuronidase: in vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell. 1978 Sep;15(1):269–278. doi: 10.1016/0092-8674(78)90102-2. [DOI] [PubMed] [Google Scholar]
- Barham D., Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972 Feb;97(151):142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- Baron R. Molecular mechanisms of bone resorption by the osteoclast. Anat Rec. 1989 Jun;224(2):317–324. doi: 10.1002/ar.1092240220. [DOI] [PubMed] [Google Scholar]
- Blair H. C., Kahn A. J., Crouch E. C., Jeffrey J. J., Teitelbaum S. L. Isolated osteoclasts resorb the organic and inorganic components of bone. J Cell Biol. 1986 Apr;102(4):1164–1172. doi: 10.1083/jcb.102.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. C., Teitelbaum S. L., Ghiselli R., Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989 Aug 25;245(4920):855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- Boyde A., Ali N. N., Jones S. J. Resorption of dentine by isolated osteoclasts in vitro. Br Dent J. 1984 Mar 24;156(6):216–220. doi: 10.1038/sj.bdj.4805313. [DOI] [PubMed] [Google Scholar]
- Case J. P., Sano H., Lafyatis R., Remmers E. F., Kumkumian G. K., Wilder R. L. Transin/stromelysin expression in the synovium of rats with experimental erosive arthritis. In situ localization and kinetics of expression of the transformation-associated metalloproteinase in euthymic and athymic Lewis rats. J Clin Invest. 1989 Dec;84(6):1731–1740. doi: 10.1172/JCI114356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., McSheehy P. M., Thomson B. M., Fuller K. The effect of calcium-regulating hormones and prostaglandins on bone resorption by osteoclasts disaggregated from neonatal rabbit bones. Endocrinology. 1985 Jan;116(1):234–239. doi: 10.1210/endo-116-1-234. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Thomson B. M., Fuller K. Effect of substrate composition on bone resorption by rabbit osteoclasts. J Cell Sci. 1984 Aug;70:61–71. doi: 10.1242/jcs.70.1.61. [DOI] [PubMed] [Google Scholar]
- Cury J. D., Campbell E. J., Lazarus C. J., Albin R. J., Welgus H. G. Selective up-regulation of human alveolar macrophage collagenase production by lipopolysaccharide and comparison to collagenase production by fibroblasts. J Immunol. 1988 Dec 15;141(12):4306–4312. [PubMed] [Google Scholar]
- Delaisse J. M., Boyde A., Maconnachie E., Ali N. N., Sear C. H., Eeckhout Y., Vaes G., Jones S. J. The effects of inhibitors of cysteine-proteinases and collagenase on the resorptive activity of isolated osteoclasts. Bone. 1987;8(5):305–313. doi: 10.1016/8756-3282(87)90007-x. [DOI] [PubMed] [Google Scholar]
- Delaisse J. M., Eeckhout Y., Vaes G. Bone-resorbing agents affect the production and distribution of procollagenase as well as the activity of collagenase in bone tissue. Endocrinology. 1988 Jul;123(1):264–276. doi: 10.1210/endo-123-1-264. [DOI] [PubMed] [Google Scholar]
- Delaissé J. M., Eeckhout Y., Sear C., Galloway A., McCullagh K., Vaes G. A new synthetic inhibitor of mammalian tissue collagenase inhibits bone resorption in culture. Biochem Biophys Res Commun. 1985 Dec 17;133(2):483–490. doi: 10.1016/0006-291x(85)90932-5. [DOI] [PubMed] [Google Scholar]
- Delaissé J. M., Ledent P., Vaes G. Collagenolytic cysteine proteinases of bone tissue. Cathepsin B, (pro)cathepsin L and a cathepsin L-like 70 kDa proteinase. Biochem J. 1991 Oct 1;279(Pt 1):167–174. doi: 10.1042/bj2790167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Eeckhout Y., Delaissé J. M., Vaes G. Direct extraction and assay of bone tissue collagenase and its relation to parathyroid-hormone-induced bone resorption. Biochem J. 1986 Nov 1;239(3):793–796. doi: 10.1042/bj2390793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y. Possible role and mechanism of action of dissolved calcium in the degradation of bone collagen by lysosomal cathepsins and collagenase. Biochem J. 1990 Dec 1;272(2):529–532. doi: 10.1042/bj2720529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington D. J., Birkedahl-Hansen H. The influence of dissolved calcium salts on the degradation of hard-tissue collagens by lysosomal cathepsins. Coll Relat Res. 1987 Aug;7(3):185–199. doi: 10.1016/s0174-173x(87)80009-2. [DOI] [PubMed] [Google Scholar]
- Everts V., Beertsen W., Schröder R. Effects of the proteinase inhibitors leupeptin and E-64 on osteoclastic bone resorption. Calcif Tissue Int. 1988 Sep;43(3):172–178. doi: 10.1007/BF02571316. [DOI] [PubMed] [Google Scholar]
- Everts V., Delaissé J. M., Korper W., Niehof A., Vaes G., Beertsen W. Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J Cell Physiol. 1992 Feb;150(2):221–231. doi: 10.1002/jcp.1041500202. [DOI] [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovíc J., Reynolds J. J., Murphy G. Inhibition of type I collagen film degradation by tumour cells using a specific antibody to collagenase and the specific tissue inhibitor of metalloproteinases (TIMP). Cell Biol Int Rep. 1985 Dec;9(12):1097–1107. doi: 10.1016/s0309-1651(85)80007-2. [DOI] [PubMed] [Google Scholar]
- Heath J. K., Atkinson S. J., Meikle M. C., Reynolds J. J. Mouse osteoblasts synthesize collagenase in response to bone resorbing agents. Biochim Biophys Acta. 1984 Nov 6;802(1):151–154. doi: 10.1016/0304-4165(84)90046-1. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Cawston T. E., Dingle J. T., Reynolds J. J. Characterization of a specific antiserum for mammalian collagenase from several species: immunolocalization of collagenase in rabbit chondrocytes and uterus. J Cell Sci. 1986 Mar;81:105–123. doi: 10.1242/jcs.81.1.105. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Reynolds J. J. Immunolocalization of tissue inhibitor of metalloproteinases (TIMP) in human cells. Characterization and use of a specific antiserum. J Cell Sci. 1985 Feb;73:105–119. doi: 10.1242/jcs.73.1.105. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hill P. A., Buttle D. J., Jones S. J., Boyde A., Murata M., Reynolds J. J., Meikle M. C. Inhibition of bone resorption by selective inactivators of cysteine proteinases. J Cell Biochem. 1994 Sep;56(1):118–130. doi: 10.1002/jcb.240560116. [DOI] [PubMed] [Google Scholar]
- Hill P. A., Reynolds J. J., Meikle M. C. Inhibition of stimulated bone resorption in vitro by TIMP-1 and TIMP-2. Biochim Biophys Acta. 1993 May 8;1177(1):71–74. doi: 10.1016/0167-4889(93)90159-m. [DOI] [PubMed] [Google Scholar]
- Johnson-Wint B. A quantitative collagen film collagenase assay for large numbers of samples. Anal Biochem. 1980 May 1;104(1):175–181. doi: 10.1016/0003-2697(80)90295-x. [DOI] [PubMed] [Google Scholar]
- Johnson W. H., Roberts N. A., Borkakoti N. Collagenase inhibitors: their design and potential therapeutic use. J Enzyme Inhib. 1987;2(1):1–22. doi: 10.3109/14756368709030352. [DOI] [PubMed] [Google Scholar]
- Knight C. G., Willenbrock F., Murphy G. A novel coumarin-labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett. 1992 Jan 27;296(3):263–266. doi: 10.1016/0014-5793(92)80300-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lerner U. H. Modifications of the mouse calvarial technique improve the responsiveness to stimulators of bone resorption. J Bone Miner Res. 1987 Oct;2(5):375–383. doi: 10.1002/jbmr.5650020504. [DOI] [PubMed] [Google Scholar]
- Ljunggren O., Ransjö M., Lerner U. H. In vitro studies on bone resorption in neonatal mouse calvariae using a modified dissection technique giving four samples of bone from each calvaria. J Bone Miner Res. 1991 Jun;6(6):543–550. doi: 10.1002/jbmr.5650060604. [DOI] [PubMed] [Google Scholar]
- Lorenzo J. A., Pilbeam C. C., Kalinowski J. F., Hibbs M. S. Production of both 92- and 72-kDa gelatinases by bone cells. Matrix. 1992 Aug;12(4):282–290. doi: 10.1016/s0934-8832(11)80080-6. [DOI] [PubMed] [Google Scholar]
- Meikle M. C., Bord S., Hembry R. M., Compston J., Croucher P. I., Reynolds J. J. Human osteoblasts in culture synthesize collagenase and other matrix metalloproteinases in response to osteotropic hormones and cytokines. J Cell Sci. 1992 Dec;103(Pt 4):1093–1099. doi: 10.1242/jcs.103.4.1093. [DOI] [PubMed] [Google Scholar]
- Moore W. M., Spilburg C. A. Purification of human collagenases with a hydroxamic acid affinity column. Biochemistry. 1986 Sep 9;25(18):5189–5195. doi: 10.1021/bi00366a031. [DOI] [PubMed] [Google Scholar]
- Morrison J. F., Walsh C. T. The behavior and significance of slow-binding enzyme inhibitors. Adv Enzymol Relat Areas Mol Biol. 1988;61:201–301. doi: 10.1002/9780470123072.ch5. [DOI] [PubMed] [Google Scholar]
- Murphy G., Allan J. A., Willenbrock F., Cockett M. I., O'Connell J. P., Docherty A. J. The role of the C-terminal domain in collagenase and stromelysin specificity. J Biol Chem. 1992 May 15;267(14):9612–9618. [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., McGarrity A. M., Reynolds J. J., Henderson B. Gelatinase (type IV collagenase) immunolocalization in cells and tissues: use of an antiserum to rabbit bone gelatinase that identifies high and low Mr forms. J Cell Sci. 1989 Mar;92(Pt 3):487–495. doi: 10.1242/jcs.92.3.487. [DOI] [PubMed] [Google Scholar]
- Murphy G., McAlpine C. G., Poll C. T., Reynolds J. J. Purification and characterization of a bone metalloproteinase that degrades gelatin and types IV and V collagen. Biochim Biophys Acta. 1985 Sep 20;831(1):49–58. doi: 10.1016/0167-4838(85)90148-7. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ward R., Gavrilovic J., Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992;1:224–230. [PubMed] [Google Scholar]
- Murphy G., Willenbrock F., Ward R. V., Cockett M. I., Eaton D., Docherty A. J. The C-terminal domain of 72 kDa gelatinase A is not required for catalysis, but is essential for membrane activation and modulates interactions with tissue inhibitors of metalloproteinases. Biochem J. 1992 May 1;283(Pt 3):637–641. doi: 10.1042/bj2830637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell J. P., Willenbrock F., Docherty A. J., Eaton D., Murphy G. Analysis of the role of the COOH-terminal domain in the activation, proteolytic activity, and tissue inhibitor of metalloproteinase interactions of gelatinase B. J Biol Chem. 1994 May 27;269(21):14967–14973. [PubMed] [Google Scholar]
- Otsuka K., Sodek J., Limeback H. Synthesis of collagenase and collagenase inhibitors by osteoblast-like cells in culture. Eur J Biochem. 1984 Nov 15;145(1):123–129. doi: 10.1111/j.1432-1033.1984.tb08530.x. [DOI] [PubMed] [Google Scholar]
- Reynolds J. J., Dingle J. T. A sensitive in vitro method for studying the induction and inhibition of bone resorption. Calcif Tissue Res. 1970;4(4):339–349. doi: 10.1007/BF02279136. [DOI] [PubMed] [Google Scholar]
- Rifas L., Halstead L. R., Peck W. A., Avioli L. V., Welgus H. G. Human osteoblasts in vitro secrete tissue inhibitor of metalloproteinases and gelatinase but not interstitial collagenase as major cellular products. J Clin Invest. 1989 Aug;84(2):686–694. doi: 10.1172/JCI114216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin B. R., Vernillo A. T., Kleckner A. P., Auszmann J. M., Rosenberg L. R., Zimmerman M. Cathepsin B and L activities in isolated osteoclasts. Biochem Biophys Res Commun. 1991 Aug 30;179(1):63–69. doi: 10.1016/0006-291x(91)91334-9. [DOI] [PubMed] [Google Scholar]
- Sellers A., Reynolds J. J. Identification and partial characterization of an inhibitor of collagenase from rabbit bone. Biochem J. 1977 Nov 1;167(2):353–360. doi: 10.1042/bj1670353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers A., Reynolds J. J., Meikle M. C. Neutral metallo-proteinases of rabbit bone. Separation in latent forms of distinct enzymes that when activated degrade collagen, gelatin and proteoglycans. Biochem J. 1978 May 1;171(2):493–496. doi: 10.1042/bj1710493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer J. L., Adams S. A., Grant G. A., Eisen A. Z. Purification and properties of a gelatin-specific neutral protease from human skin. J Biol Chem. 1981 May 10;256(9):4662–4668. [PubMed] [Google Scholar]
- Shapiro S. D., Campbell E. J., Kobayashi D. K., Welgus H. G. Immune modulation of metalloproteinase production in human macrophages. Selective pretranslational suppression of interstitial collagenase and stromelysin biosynthesis by interferon-gamma. J Clin Invest. 1990 Oct;86(4):1204–1210. doi: 10.1172/JCI114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack M. S., Gray R. D. Comparison of vertebrate collagenase and gelatinase using a new fluorogenic substrate peptide. J Biol Chem. 1989 Mar 15;264(8):4277–4281. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Brown P. D., Onisto M., Levy A. T., Liotta L. A. Tissue inhibitor of metalloproteinases-2 (TIMP-2) mRNA expression in tumor cell lines and human tumor tissues. J Biol Chem. 1990 Aug 15;265(23):13933–13938. [PubMed] [Google Scholar]
- Thomson B. M., Atkinson S. J., Reynolds J. J., Meikle M. C. Degradation of type I collagen films by mouse osteoblasts is stimulated by 1,25 dihydroxyvitamin D3 and inhibited by human recombinant TIMP (tissue inhibitor of metalloproteinases). Biochem Biophys Res Commun. 1987 Oct 29;148(2):596–602. doi: 10.1016/0006-291x(87)90918-1. [DOI] [PubMed] [Google Scholar]
- Vaes G. The release of collagenase as an inactive proenzyme by bone explants in culture. Biochem J. 1972 Jan;126(2):275–289. doi: 10.1042/bj1260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]