Summary
Microgravity has been shown to lead to both muscle atrophy and impaired muscle regeneration. The purpose was to study the efficacy of microgravity to model impaired muscle regeneration in an engineered muscle platform and then to demonstrate the feasibility of performing drug screening in this model. Engineered human muscle was launched to the International Space Station National Laboratory, where the effect of microgravity exposure for 7 days was examined by transcriptomics and proteomics approaches. Gene set enrichment analysis of engineered muscle cultured in microgravity, compared to normal gravity conditions, highlighted a metabolic shift toward lipid and fatty acid metabolism, along with increased apoptotic gene expression. The addition of pro-regenerative drugs, insulin-like growth factor-1 (IGF-1) and a 15-hydroxyprostaglandin dehydrogenase inhibitor (15-PGDH-i), partially inhibited the effects of microgravity. In summary, microgravity mimics aspects of impaired myogenesis, and the addition of these drugs could partially inhibit the effects induced by microgravity.
Keywords: skeletal muscle, tissue engineering, microgravity, regeneration, atrophy, aging, transcriptomics
Graphical abstract
Highlights
-
•
A muscle-on-a-chip platform launched to the International Space Station National Laboratory
-
•
Muscle bioconstructs in microgravity underwent impaired regeneration
-
•
Drug testing in this microgravity platform was feasible
Kim and colleagues examined the effect of microgravity in engineered muscle bioconstructs aboard the International Station National Laboratory. The transcriptional results show a metabolic shift toward lipid and fatty acid metabolism and increased apoptotic pathways in microgravity. This signature was partially inhibited by pro-regenerative drugs like insulin-like growth factor-1 and a 15-hydroxyprostaglandin dehydrogenase inhibitor.
Introduction
Representing approximately 40% of body weight, skeletal muscle is one of the most abundant tissues in the human body (Reid and Fielding, 2012). Skeletal muscle regenerates itself through satellite cells, a reservoir of quiescent muscle stem cells that can be activated with injury or disease and give rise to newly formed multi-nucleated myotubes and myofibers (Mauro, 1961; Morgan and Partridge, 2003). As civilian space travel to the International Space Station and beyond will become increasingly more common in the next century, it is important to better understand how microgravity affects the regenerative capacity of muscle. Mice exposed to microgravity have shown reduced muscle regeneration, compared to those in normal gravity, as demonstrated by relatively fewer postmitotic nuclei within myofibers as an indicator of impaired de novo muscle fiber formation (Radugina et al., 2018). Similarly, in vitro studies composed of murine myoblasts seeded within three-dimensional hydrogels in simulated microgravity also show reduced myogenesis, based on the formation of shorter and thinner myotubes with reduced fusion capacity (Ren et al., 2024). These data support a larger body of literature showing that microgravity exerts profound effects on skeletal muscle (Fitts et al., 2010; Juhl et al., 2021; Trappe, 2009).
The identification of countermeasures against the effects of microgravity on muscle regeneration is an increasing priority for continued space travel. Developing drugs that counteract the effects of microgravity is a promising strategy, but no Food and Drug Administration (FDA)-approved drug currently exists, partially due to the limited access of participants to microgravity conditions. In contrast, a muscle-on-a-chip in microgravity platform can be performed in a facile manner in vitro and can allow for screening many drugs in parallel. Through the US National Science Foundation and the Center for the Advancement of Science in Space (CASIS), we were offered a unique opportunity to perform tissue engineering and mechanobiology research aboard the International Space Station National Laboratory (ISSNL). With the assistance of astronauts, we applied our expertise in the engineering of skeletal muscle (Huang et al., 2006; Nakayama et al., 2019) toward the study of muscle myogenesis using a muscle-on-a-chip platform. By performing multi-omics analyses, we show that the muscle-on-a-chip platform in microgravity mimics salient aspects of impaired muscle regeneration after merely 7 days in microgravity. Furthermore, we demonstrate the feasibility of drug screening in this microgravity platform by using two drugs that partially prevent the negative effects of microgravity in the muscle-on-a-chip bioconstructs.
Results
Engineering of muscle-on-a-chip system in microgravity
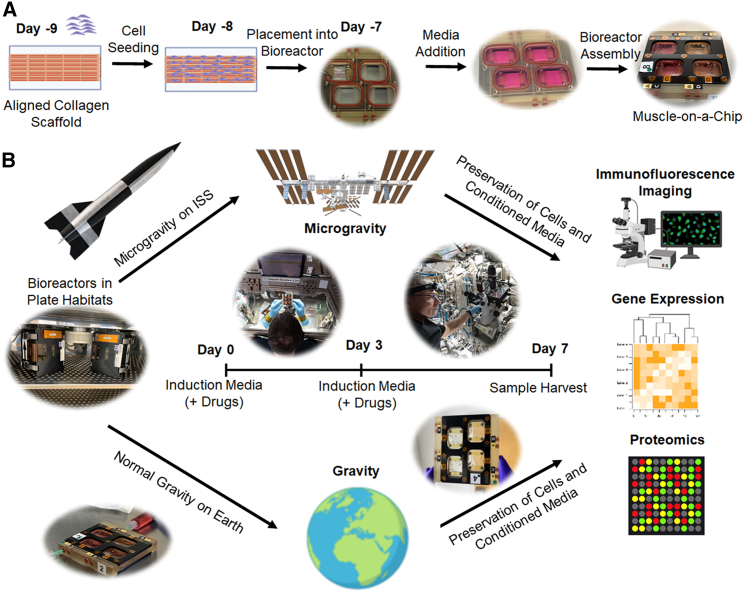
To adapt engineered muscle for microgravity conditions aboard the ISSNL, we developed a muscle-on-a-chip platform to immobilize the engineered muscle onto rigid hydrophobic glass for culture within a flight-compatible custom-made bioreactor. Skeletal muscle constructs composed of primary human myotubes on parallel-aligned collagen nanofibrils were generated by a facile shear-based collagen extrusion assay. Taking advantage of collagen fibrillogenesis being controlled by both pH and shear, we sheared acidic monomeric collagen type I from a blunt needle onto a rigid glass substrate bathed in pH neutral buffer (Figure 1A), forming strips of collagen with highly organized nanofibrils. The collagen strips were composed of nanofibrils that were <100 nm in diameter, and the strips were then bundled together (Figures 1B and 1C). The anisotropic organization of the collagen fibrils provides topographical patterning cues to induce primary human skeletal muscle cells to organize along the direction of the nanofibrils (Figure 1D) and subsequently to form aligned myotubes (Figure 1E). The cell-seeded scaffolds were denoted as a “muscle-on-a-chip.”
Figure 1.
Generation and characterization of biomimetic skeletal muscle-on-a-chip platform
(A) Schematic depicting the fabrication of aligned collagen nanofibrils using shear-mediated extrusion of monomeric collagen into pH 7 buffer.
(B) Gross image and schematic of anisotropic collagen strips deposited onto hydrophobic glass chips.
(C) Scanning electron microscopy analysis shows highly organized anisotropic collagen fibrils.
(D) Seeding of primary human skeletal muscle cells shows cell alignment along the direction of the collagen nanofibrils (denoted by arrow).
(E) In induction media, the cells fuse to form parallel-aligned myotubes, as shown by myosin heavy-chain staining (green). Scale bars: (B) 5 mm, (C) 1 μm, (D–E) 100 μm.
To adapt the muscle-on-a-chip platform to microgravity, microgravity-compatible bioreactors were custom-made by BioServe Space Technologies. Prior to launch, the flight hardware was assembled by securing the muscle-on-a-chip bioconstructs within the chamber, filling the chamber with medium, and sealing with an air-permeable membrane stabilized with a solid frame (Figures 2A, S1A, and S1B). During transit to the ISSNL on a Cygnus vehicle, the bioreactors were transported within gas-sealed space habitats that maintained temperature and CO2 levels (Figures 2 and S1C). Upon arrival at the ISSNL, the space habitats housing the bioreactors were placed into the Space Automated Bioproduct Lab incubator and maintained at 37°C and 5% CO2. The experiments were initiated (day 0) in microgravity with the syringe-mediated replacement of the media with induction media that supports myotube formation (Figure 2B). Comparable bioreactors were assembled on Earth in normal gravity conditions, and the same experiments were performed in parallel to samples in microgravity.
Figure 2.
Preparation of muscle-on-a-chip devices for microgravity studies and experimental overview
(A) The overview of muscle-on-a-chip preparation consists of seeding human muscle cells onto immobilized aligned nanofibrillar collagen scaffolds followed by cell seeding. After one day, the cell-seeded scaffolds are placed into bioreactors and filled with growth media. The bioreactor is then secured with screws for transportation to the ISSNL.
(B) The experimental overview consists of incubating the bioreactor during transit to the ISSNL in plate habitats that provide temperature maintenance during transport to the ISSNL or in normal gravity conditions on Earth. Upon docking at the ISSNL, microgravity experiments began (day 0) with media change into an induction media that supports myotube formation. In some experiments, drugs were added to the media on day 0 and again on day 3. On day 7, the conditioned media were extracted, and the samples were stabilized in RNAlater or fixed in paraformaldehyde for analysis on by RNA sequencing, proteomics analysis, or myotube formation.
Microgravity alters myogenesis and genetic program in skeletal muscle-on-A-chip
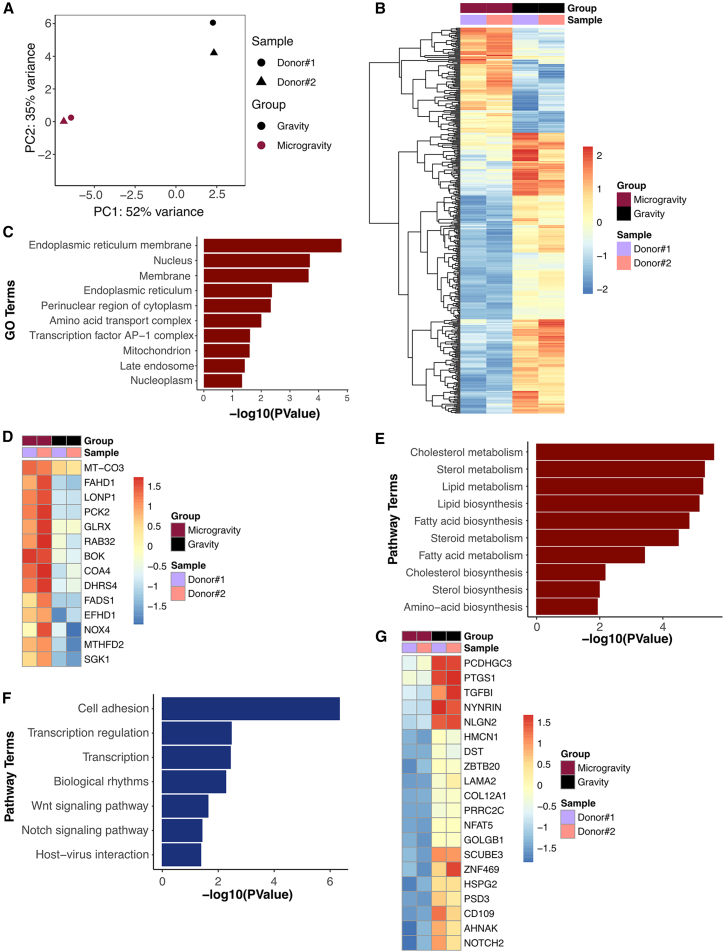
We first quantified myotube formation from healthy donor-derived engineered muscle after 7 days of exposure to microgravity. Our results showed reduced myotube length, width, and fusion index in microgravity, compared to gravity (Figure S2; Table S1). This finding of reduced myogenesis in microgravity was then corroborated by transcriptional analysis. Following 7 days of differentiation and myotube formation, the muscle-on-a-chip bioconstructs in both microgravity and gravity from two healthy donors were assessed by RNA sequencing (RNA-seq). We detected 58,735 genes, of which 25,914 passed the threshold for expression. Transcripts of low count, expressed in less than two samples, were excluded. Samples were clustered by the gravity condition (Figure 3A), where 107 genes were upregulated and 286 genes were downregulated in microgravity samples with a false-discovery rate (FDR) < 10% (Figure 3B and Table S2). Database for Annotation, Visualization and Integrated Discovery (DAVID) analysis classified 104 upregulated genes in microgravity into the Cellular Component Gene Ontology (GO-CC) term. The analysis shows significant enrichment in genes encoding proteins located in various subcellular structures (Figure 3C). Among these genes, 14 of them were annotated under the “mitochondrion” GO-CC (Figure 3D). For example, mitochondrially encoded cytochrome c oxidase III (MT-CO3) has previously been reported to increase in muscle stem cells during aging (Perez et al., 2022), which is associated with impaired regeneration. Since muscle has high metabolic function, proper mitochondrial biogenesis is necessary for regeneration.
Figure 3.
Transcriptional differences between microgravity and gravity control biomimetic skeletal muscle
(A) Principal-component analysis of the transcriptome of biomimetic skeletal muscle samples after 7 days in microgravity or control conditions (gravity).
(B) Heatmap shows all differentially expressed genes after 7 days in microgravity, compared to gravity (FDR 10%).
(C) Gene Ontology (GO) enrichment terms of differentially upregulated genes in microgravity (p < 0.05).
(D) Heatmap shows the expression of genes found in the mitochondrion GO enrichment term.
(E) Pathway enrichment terms of differentially upregulated genes in microgravity (p < 0.05).
(F) Pathway enrichment terms of differentially downregulated genes in microgravity (p < 0.05).
(G) Heatmap shows expression of top 20 differentially downregulated genes in microgravity (n = 2 donors per group).
Pathway enrichment analysis also supported changes in metabolic pathways, including genes implicated in cholesterol, lipid, and fatty acid metabolism (Figure 3E). Among them, fatty acid desaturase 1 (FADS1) and mitochondrial matrix Lon peptidase 1 (LONP1) were also implicated in cellular responses to muscle regeneration (Huang et al., 2020). Additionally, the pro-apoptotic gene, Bcl-2 related ovarian killer (BOK), along with cell survival and differentiation-associated genes, serum/glucocorticoid regulated kinase 1 (SGK1) and NADPH oxidase 4 (NOX4) (Dehner et al., 2008; Pedruzzi et al., 2004; Yakovlev et al., 2004), was upregulated in microgravity. SGK1 and NOX4 are associated with muscle mass maintenance and myoblast differentiation, respectively (Andres-Mateos et al., 2013; Youm et al., 2019). In particular, SGK1 regulates muscle homeostasis by the downregulation of proteolysis and autophagy (Andres-Mateos et al., 2013). NOX4-derived reactive oxygen species (ROS) positively regulate myotube formation (Youm et al., 2019), which may be important for muscle regeneration.
Pathway analysis of decreased genes in microgravity highlights significant changes in biological processes, including genes involved in cell adhesion and cell fate decision pathways, including Wnt and Notch signaling pathway (Figure 3F). The top 20 downregulated genes included those involved in cell adhesion (protocadherin gamma C3 [PCDHGC3], transforming growth factor β induced [TGFBI], neuroligin 2 [NLGN2], dystonin [DST], laminin α2 [LAMA2], collagen type XII alpha 1 chain [COL12A1]), Notch signaling pathway (NOTCH2), and inflammation (prostaglandin-endoperoxide synthase 1 [PTGS1/COX1]) (Figure 3G). In particular, NOTCH2, which is an important marker of muscle stem cell self-renewal and stem cell fate decision (Yartseva et al., 2020), was significantly downregulated in microgravity. Downregulation of NOTCH2 gene expression in satellite cells leads to a decrease in their ability to differentiate (Fujimaki et al., 2018), suggesting an impairment in the balance between proliferation and differentiation of primary human skeletal muscle cells in microgravity. The downregulation of NOTCH2 concurs with our in vitro findings of a significantly lower fusion index in the engineered muscles in microgravity (Figure S2). Additionally, extracellular matrix (ECM) remodeling is associated with muscle regeneration (Calve et al., 2010), and we observed significant downregulation of ECMs such as COL12A1, LAMA2, and heparan sulfate proteoglycan 2 (HSPG2) genes. Together, this analysis suggested that microgravity conditions induced a cellular phenotype that is associated with hallmarks of impaired regeneration, including metabolic remodeling, imbalance between proliferation and differentiation, and decreased cell-cell and cell-ECM adhesion.
To further examine the effects of microgravity, we performed proteomic analysis of 200 proteins from the conditioned media of bioconstructs cultured in microgravity or gravity conditions for 7 days. In comparing the microgravity versus gravity samples with FDR <25%, we identified 5 proteins that were secreted in greater abundance and 4 proteins that were of reduced abundance. The proteins of greater abundance in microgravity include Eotaxin-3 (CCL26), C-X-C motif chemokine ligand 16 (CXCL16), growth differentiation factor 15 (GDF-15), tumor necrosis factor superfamily member 14 (LIGHT/TNSF14), and pupoid fetus (PF). Of these proteins, CCL26 and CXCL16 are chemoattractants known to induce immune cell infiltration and are associated with chronic inflammation (Scholz et al., 2007; Yamada et al., 2019). In addition, GDF-15 is a biomarker for mitochondrial dysfunction and cellular senescence (Fujita et al., 2016). The proteins of reduced abundance in microgravity include bone morphogenetic protein 4 (BMP-4), Resistin, C-X-C motif chemokine ligand 12 (CXCL12/SDF-1b), interleukin-16 (IL-16), and CD40. CXCL12, in particular, is an important player in the maintenance of muscle and myogenesis (Gilbert et al., 2019; Puchert et al., 2016).
To determine whether the secreted proteomes were related to their RNA expression levels, we compared their relative expression between microgravity and gravity samples. We observed that some differential protein changes generally correlated with their corresponding RNA expression level changes (Figure S3). For example, GDF-15 was consistently increased in microgravity conditions at both the protein and RNA levels (Figure S3), whereas BMP-4 and SDF-1b (CXCL12) levels were consistently downregulated in microgravity at both the protein and RNA levels (Figure S3). Since GDF-15 has previously been reported as a diagnostic biomarker for conditions associated with impaired muscle regeneration such as aging (Ito et al., 2018; Kim et al., 2020), we searched for potential interacting genes of GDF-15 in microgravity samples. The protein-protein interaction (PPI) network was constructed by the STRING database using 107 genes that were upregulated with microgravity, including GDF-15. The unconnected genes were then removed. The resulting PPI network contained 57 nodes and 135 edges (Figure S4), where a node represents a gene and an edge represents the relationship between the two inter-connected genes. From this analysis, we identified early growth response factor 1 (EGR1) and tribbles homolog 3 (TRIB3) as the first neighbors of GDF-15. These results suggest that GDF-15 and its immediate neighbors may play an important role in microgravity-induced impairment in myogenesis.
Microgravity partially mimics genomic features of impaired muscle regeneration in sarcopenia
Since impaired muscle regeneration is associated with clinical conditions such as sarcopenia, we assessed whether the muscle-on-a-chip in microgravity platform mimics regeneration-related signaling pathways in clinical sarcopenia muscle samples. We compared the transcriptome of human muscle tissue obtained from either healthy donors (denoted as “Normal”; [n = 4]), or those with sarcopenia, as defined by accepted cutoffs for the relative skeletal muscle (RSM) index (n = 4, Table S3; Figure 4A). RNA-seq detected 58,735 genes, of which 29,313 passed the threshold for expression. In particular, 48 genes were upregulated and 113 genes were downregulated in sarcopenia samples with an FDR <25% (Figure 4B; Table S4). Among the top upregulated genes were those involved in apoptotic signaling pathways in response to DNA damage (Apoptosis enhancing nuclease [AEN] and PHLDA3), ECM organization (TIMP1, COL1A1, and COL14A1), and the VEGFA/VEGFR2 signaling pathway (GIPC1, CTGF, GPC1, and MAP2K3). Interestingly, more genes were downregulated in sarcopenia and they included genes that are implicated in Wnt signaling (mutated in colorectal cancers [MCC]) and associated with rhabdomyosarcoma (FHL2) (Figure 4C). Importantly, an increase in apoptosis or impairment in skeletal muscle cell function could contribute to the development or progression of sarcopenia (Chung and Ng, 2006; Leeuwenburgh, 2003; Siu et al., 2005), in which the upregulation of markers such as AEN and PHLDA3 is implicated in p53-dependent apoptosis (Cruz-Jentoft and Sayer, 2019; Kawase et al., 2008, 2009).
Figure 4.
Transcriptional similarities between clinical sarcopenia muscle and engineered skeletal muscle in microgravity
(A) Principal-component analysis of the transcriptome in healthy vs. sarcopenia clinical samples from both sexes.
(B) Volcano plot shows differentially expressed genes in sarcopenia, compared to normal conditions (p < 0.05, log2 FC > 1.0). Genes involved in DNA damage response, extracellular matrix organization, VEGFA-VEGFR2 signaling pathway, and Wnt signaling are labeled. Genes showing statistically significant expression (FDR 25%) are indicated in red.
(C) Heatmap shows the genes involved in Wnt signaling, rhabdomyosarcoma, DNA damage response, extracellular matrix organization, and VEGFA-VEGFR2 signaling.
(D–F) Enrichment plots for custom gene set enrichment analysis (GSEA). The plots report positive enrichment of Old_MuSC_UP gene set (D) and negative enrichment of Sarcopenia_UP and Sarcopenia-DOWN gene sets (E–F) in microgravity, compared to gravity samples. The enrichment plot shows the gene set names (top) and the running enrichment score. The red curve indicates positive enrichment, whereas the blue curve indicates negative enrichment scores. Black bars indicate the positions of the gene set on the rank-ordered list in GSEA, and the signal-to-noise metrics are shown at the bottom.
We next established a custom gene set library to assess whether engineered muscle in microgravity mimics features of sarcopenia from clinical samples. In brief, we used the up- and downregulated gene list from sarcopenia versus normal samples and converted the list into a gene set enrichment analysis (GSEA) analysis-compatible format. We also analyzed publicly available datasets derived from old adult muscle myoblasts (Gene Expression Omnibus repository: GSE52699) and generated an enriched gene set list compared to young myoblasts (see Supplemental Information). As shown in Figure 4D, custom GSEA results indicate that genes upregulated in old human myoblasts (“Old_MuSC_UP” gene set) were over-represented in the muscle-on-a-chip bioconstruct in microgravity, as indicated by the positive running enrichment score (RES) (Figure 4D). Although some of the sarcopenia-related genes were significantly decreased in gravity, compared to microgravity conditions, the enrichment plot for Sarcopenia_vs_Normal_UP shown in Figure 4E suggests that the genes upregulated by sarcopenia were not enriched by microgravity. However, the genes downregulated by sarcopenia were also downregulated with microgravity exposure (Figure 4F). These custom GSEA results indicate that the muscle-on-a-chip bioconstructs in microgravity had a negative enrichment of genes that were downregulated in sarcopenia (“Sarcopenia_VS_Normal_DOWN” gene set [Figures 4E and 4F]). These data reveal a similarity in the transcriptional signature between the clinical sarcopenia samples and engineered muscle in microgravity samples, in which the genes that were downregulated in sarcopenia muscle tissue were also downregulated in the engineered muscle in microgravity conditions, compared to those of the gravity condition (Figure 4F). Conversely, the genes that were upregulated in sarcopenia were not enriched in the microgravity condition compared to those of gravity conditions (Figure 4E). Overall, the custom GSEA results suggest that space flight mimics in part the genomic signature of old adult myoblasts and sarcopenia.
Drug treatment partially prevents adverse effects of microgravity
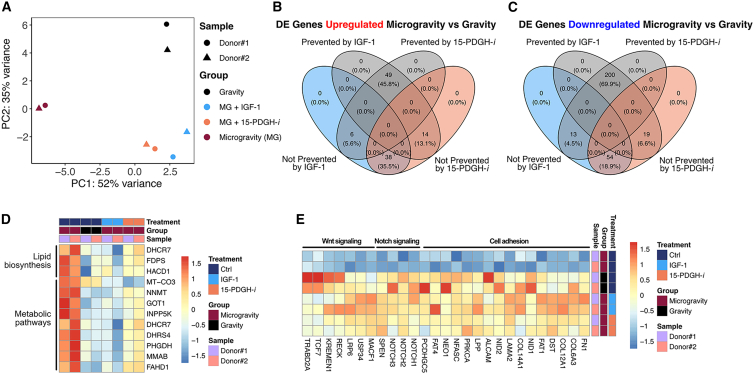
We next used the muscle-on-a-chip platform to perform proof-of-concept drug screening studies. Recently published studies showed that a 15-PDGH inhibitor (15-PDGH-i) could stimulate myogenesis as well as abrogate sarcopenia in preclinical models through the regulation of prostaglandin E2 signaling (Ho et al., 2017; Palla et al., 2021). Besides 15-PDGH-i, we also examined insulin-like growth factor-1 (IGF-1) in the muscle-on-a-chip platform. The effects of IGF-1 in promoting muscle myogenesis and inhibition of muscle atrophy are well established (Alcazar et al., 2020; Ascenzi et al., 2019; Ma et al., 2009; Machida and Booth, 2004; Yu et al., 2015). To examine the potential use of these two drugs to regulate myogenesis, the engineered skeletal muscle-on-a-chip bioconstructs were treated with either IGF-1 or 15-PDGH-i for 7 days in microgravity conditions, followed by RNA-seq analysis. Through principal-component analysis, we found that, in microgravity, the drug-treated groups were generally more transcriptionally similar to the samples in gravity, rather than their microgravity counterparts (Figure 5A). Out of 107 genes that were upregulated in microgravity compared to gravity counterparts, 49 genes showed similar expression levels to those of gravity conditions with IGF-1 and 15-PDGH-i treatment, suggesting that the drugs could prevent the transcriptional upregulation effects of microgravity. In addition, IGF-1 and 15-PDGH-i treatment prevented the transcriptional upregulation effects of microgravity in 6 and 14 genes, respectively (Figure 5B).
Figure 5.
IGF-1 and 15-PDGFH inhibitor treatment can partially prevent microgravity effect
(A) Principal-component analysis of transcriptome in 7 days in microgravity (MG) or gravity control conditions in the presence of drug treatment (IGF-1 or 15-PDGH-i) in biomimetic skeletal muscle samples.
(B) The 4-way Venn diagram shows the number of differentially expressed genes that were upregulated in microgravity, compared to gravity conditions, or whose differential expression could be either prevented by IGF-1 or 15-PDGH-i treatment (gray) or not prevented by drug treatment (IGF-1 in blue and 15-PDGH-i in orange).
(C) 4-way Venn diagram showing the number of differentially expressed genes downregulated in microgravity, compared to gravity conditions, or whose differential expression could be either prevented by IGF-1 or 15-PDGH-i treatment (gray) or not prevented by drug treatment (IGF-1 in blue and 15-PDGH-i in orange).
(D) Heatmap showing genes implicated in lipid biosynthesis, metabolic pathways, and adipogenesis.
(E) Heatmap showing genes implicated in cell adhesion, Notch signaling, and Wnt signaling (n = 2 donors per group).
Of the 286 downregulated genes with microgravity, 200 genes showed similar expression levels to those of gravity conditions with IGF-1 and 15-PDGH-i treatment, suggesting that the drugs could prevent the transcriptional downregulation effects of microgravity. In particular, IGF-1 and 15-PDGH-i treatment prevented the transcriptional downregulation effects of microgravity of 13 genes and 19 genes, respectively (Figure 5C). As noted earlier, pathway enrichment analysis highlighted an enrichment of genes associated with metabolic pathways including lipid and fatty acid metabolism in microgravity samples. We found that the drug treatment prevented a shift in metabolic profiles and maintained normal expression of genes involved in lipid biosynthesis (DHCR7, FDPS, HACD1) and metabolic pathways (MT-CO3, NNMT, GOT1, INPP5K, DHCR7, DHRS4, PHGDH, MMAB, FAHD1) (Figure 5D). Moreover, we also observed that many genes implicated in cell adhesion, the Notch signaling pathway (NOTCH1, NOTCH2, NOTCH3, SPEN), and the Wnt signaling pathway (MACF1, USP34, LRP6, RECK, KREMEN1, TCF7, TRABD2A) were downregulated with space flight but were similar in expression level to gravity samples upon drug treatment (Figures 5E, S5A, and S5B). Overall, the drug-treated samples displayed a signature that more closely resembled gravity samples, compared to their microgravity counterparts.
Since the addition of IGF-1 and 15-PDGH-i prevented some effects of microgravity at the transcriptional level, we further assessed their effect on proteins found in conditioned media at 7 days after drug treatment. Interestingly, we found that IGF-1 and 15-PDGH-i treatment prevented the ability of microgravity to increase GDF-15 protein levels (Figure S5C; Table S5). Additionally, 15-PDGH-i treatment protected CXCL16 and PF4 protein levels from rising to levels, similar to those of microgravity samples. Despite the ability of the drugs to prevent an increase in protein levels to reach those of microgravity samples, none of the proteins that were decreased in microgravity compared to gravity conditions could be normalized to the levels associated with gravity samples by the addition of drugs (Figure S5D). We further tested whether the first neighbors of GDF-15 (EGR1 and TRIB3) are affected with drug treatment. As described earlier, EGR1 was not affected by drug treatment, whereas IGF-1 addition prevented TRIB3 level from reaching that of microgravity samples (Figure S5E). Taken together, our multi-omics analysis demonstrates that microgravity induces in engineered muscle a signature that reflects salient aspects of impaired myogenesis, which could be partially prevented by IGF-1 or 15-PDGH-i.
Discussion
The salient findings of the work are the following: (1) an engineered muscle-on-a-chip platform facilitates the study of microgravity effects on myotube formation and muscle-related biological processes; (2) microgravity mimics salient aspects of impaired regeneration, based on RNA-seq and proteomic analysis; (3) microgravity effects on engineered muscle can be prevented in part by IGF-1 and PDGH-i addition; and (4) drug screening in microgravity using an engineered muscle-on-a-chip platform is feasible. These findings suggest that aspects of impaired muscle myogenesis can be modeled in just one week of exposure in microgravity and that drug screening in microgravity may be a useful platform for identifying potential drug targets that regulate myogenesis.
The study of the effects of microgravity on engineered muscle-on-a-chip is an emerging area of research. Our findings using engineered muscle in microgravity concur with Vandenburgh et al., whose engineered avian muscle aboard the Space Transportation Shuttle for 12 days showed evidence of reduced myofiber size and cross-sectional area (Vandenburgh et al., 1999). Additionally, a larger body of work derived from in vitro cultured myoblasts in microgravity or simulated microgravity further supports the finding that microgravity abrogates myogenic differentiation and myotube size, owing to cellular senescence and epigenetic modifications (Furukawa et al., 2018; Parafati et al., 2023; Takahashi et al., 2021). Our findings of impaired myotube formation in the presence of microgravity are in alignment with these studies.
Previous studies have reported multiple effects of spaceflight on human physiology. Most recently, large-scale multi-omics, systems biology analytical approaches profiled 59 astronauts from the National Aeronautics and Space Administration (NASA)’s GeneLab to determine transcriptomic, proteomic, metabolomic, and epigenetic responses to spaceflight, which highlighted a significant enrichment for mitochondrial processes and DNA damage (da Silveira et al., 2020). Alterations in mitochondrial activity and impaired lipid metabolism are associated with muscle atrophy (Nikawa et al., 2004; Vitry et al., 2022). In line with these reports, our engineered muscle-on-a-chip platform exposed to microgravity for 7 days also demonstrated changes in mitochondrial genes (Figure 3C), a shift in metabolism toward lipid and fatty acid biosynthesis (Figure 3E), and an increase in apoptotic processes (BOK, SGK1, and NOX4). The existence of mitochondrial dysfunction was further supported by proteomic studies reporting an increase in GDF-15. GDF-15 is a biomarker of dysfunctional mitochondria and cellular senescence (Fujita et al., 2016). Increased GDF-15 levels are associated with muscle atrophy in cancer models, and GDF-15 treatment was shown to induce muscle atrophy in C2C12 myotubes (Zhang et al., 2022). GDF-15 neutralization could restore muscle mass and function (Kim-Muller et al., 2023). More importantly, the addition of pro-regenerative drugs such as IGF-1 and 15-PGDH-i could partially prevent the effects of microgravity on our engineered muscle-on-a-chip platform.
Notch signaling is major regulator of myogenesis (Luo et al., 2005). The disruption in Notch signaling in aging muscle compromises new myofiber formation and impairs tissue regeneration as a potential contributing factor to sarcopenia (Arthur and Cooley, 2012; Carlson et al., 2008; Conboy et al., 2003). Notch inhibition also contributes to the dysregulation of cellular quiescence and can perturb the quiescent state of the muscle stem cells (Bjornson et al., 2012; van Velthoven and Rando, 2019). Notch signaling can also regulate the stability of a tumor suppressor, p53. The Notch-p53 axis declines with age and is detrimental to cell survival and expansion (Liu et al., 2018). Our data are consistent with the literature in demonstrating a downregulation in Notch signaling in microgravity (Figure 3F). The loss of such a signal is correlated with reduced differentiation potential, as demonstrated by the impairment in myotube formation.
More genes were downregulated than upregulated in both microgravity versus gravityand the comparison of sarcopenia versus normal muscle samples. Microgravity samples were more sarcopenia like in terms of genes that are important for apoptosis and Wnt signaling. The genes involved in apoptosis were upregulated, whereas the genes involved in Wnt signaling were downregulated in both microgravity and sarcopenia samples. Although the cellular response to gamma radiation and apoptosis processes increased with microgravity but were not prevented with IGF-1 or 15-PGDH-i treatment, the gene expression of Wnt signaling components including Lrp6 was prevented with drug treatment (Figure 5E). Lrp6 is one of the two co-receptors involved in canonical Wnt signaling in vertebrates and conditional Lrp6 knockout reduced fiber size in mice (Gessler et al., 2022; Ren et al., 2021), highlighting its importance in muscle mass maintenance. Interestingly, microgravity increased the expression level of TRIB3, which negatively regulates cell survival and inhibits myogenic differentiation (Kato and Du, 2007; Saleem and Biswas, 2017). TRIB3 was identified in the PPI network as one of the two immediate neighboring gene of GDF-15. IGF-1 treatment prevented the elevation of TRIB3 gene expression in microgravity. In a mouse model, Trib3 is implicated in both age-related and food deprivation-induced muscle fiber atrophy and fibrosis (Choi et al., 2019; Shang et al., 2020). Furthermore, our findings align with a recent report that also demonstrated differential expression of TRIB3 and PCK2 under microgravity conditions (Parafati et al., 2023), further supporting the impact of microgravity on myogenesis and metabolism.
We acknowledge the limitations associated with this study. Owing to constraints associated with space research, only one launch was possible. For this reason, the data were derived from two primary human muscle donors as a measure of variance in the dataset. Furthermore, we acknowledge that measurements of muscle contractility could be informative, but such a functional assay would be most appropriate for engineered muscles that are more mature, which would have further increased the duration of the studies. Finally, the findings from the data in microgravity do not preclude the possibility of other contributing factors such as ionizing radiation. Despite these limitations, we believe that the observed effects of microgravity on engineered muscle are compelling and provide an avenue for future drug screening explorations in space for identifying drug targets that can enhance muscle myogenesis.
In conclusion, we show that engineered muscle-on-a-chip bioconstructs exposed to microgravity induced prominent changes to their transcriptome that mimic aspects of impaired myogenesis. GSEA demonstrated a shift in metabolic pathways toward lipid metabolism, along with downregulated Notch signaling pathways and the increased expression of apoptotic genes. Importantly, microgravity exposure to engineered muscle-on-a-chip bioconstructs resembled some similar features to that of clinical sarcopenia muscle. The effects of microgravity could be partly prevented by the addition of IGF-1 or a 15-PGDH-i. Together, this transcriptomic and proteomics analyses of microgravity effects using a muscle-on-a-chip platform demonstrate that microgravity mimics aspects of impaired myogenesis. This work further highlights the utility of microgravity as a unique environment for drug discovery.
Experimental procedures
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Ngan F. Huang (ngantina@stanford.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The accession number for the RNA-seq raw data reported in this paper is GSE238215. The accession number will be accesible to readers upon publication. All data may be made available by request to the corresponding author.
Flight experiment in microgravity
The experiments were initiated in microgravity with the exchange of media into induction media consisting of DMEM supplemented with 2% horse serum and 1% penicillin/streptomycin (day 0), according to Figure 2B. The media were exchanged again on day 3. The viability of samples was qualitatively monitored using an on-orbit phase-contrast microscope (Figure S1F). On day 7, the conditioned media within the bioreactors were harvested and stored at −80°C. The muscle-on-a-chip devices within each chamber were either treated with RNAlater stabilization agent (Fisher Scientific) and then stored at −80°C or fixed in 4% paraformaldehyde (Fisher Scientific) for 15 min and then stored at 4°C. Samples were maintained on the ISSNL at the indicated temperatures and then returned to Earth on SpaceX Cargo Resupply Service 23 about two months later. Semi-synchronous ground control experiments were performed on a separate set of muscle-on-a-chip bioconstructs from the same donors in identical bioreactors and syringe media exchange procedures. The bioreactors in normal gravity conditions were cultured in conventional incubators but were handled otherwise in the same manner and with the same time stamps, as in microgravity.
Statistical analysis
Statistical analysis of transcriptional and proteomics data is described earlier or in the supplemental experimental procedures. Where applicable, data are shown as mean ± standard deviation. Statistical significance was accepted at p < 0.05.
Acknowledgments
This work was supported in part by grants to N.F.H. from the US National Institutes of Health (R01 HL142718, R41 HL170875, and R21 HL172096-01), the US Department of Veterans Affairs (1I01BX004259, 5I01RX001222, and 1I21RX004898), the National Science Foundation (1829534 and 2227614), the Center for the Advancement of Science in Space (CASIS, 80JSC018M0005), and the American Heart Association (20IPA35360085 and 20IPA35310731) and by grants to T.A.R. from the US National Institutes of Health (R01 AG068667 and P01 AG036695) and the US Department of Veterans Affairs (I01BX002324 and I01RX001222). N.F.H. is a recipient of a Research Career Scientist award (IK6 BX006309) from the Department of Veterans Affairs. We acknowledge the technical assistance of astronauts, namely, Thomas Pesquet, K. Megan McArthur, and Mark Vande Hei who performed the experiments on the ISSNL. We also acknowledge members from BioServe Space Technologies for technical and logistical support associated with the launch, namely Matthew Vellone, Shankini Doraisingam, Mark Rupert, Stefanie Countryman, and Sheila Murphy. We acknowledge project management assistance of Kristin Kopperud and Marc Giulianotti from CASIS. Finally, we acknowledge the clinical muscle RNA samples from the University of Kentucky Center for Muscle Biology. Figures 1 and 2, and the graphical abstract were created with Biorender.com.
Author contributions
Conceptualization: N.F.H. and T.A.R. Funding acquisition: N.F.H. and T.A.R. Methodology: B.A., M.S., T.A.R., and N.F.H. Investigation: S.K., B.A., and M.S. Resources: N.F.H. and T.A.R. Supervision: N.F.H. and T.A.R. Visualization: S.K., B.A., and M.S. Writing – original draft: S.K. and N.F.H. Writing – review and editing: S.K., B.A., M.S., T.A.R., and N.F.H.
Declaration of interests
The authors declare no competing interests.
Published: July 25, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2024.06.010.
Contributor Information
Thomas A. Rando, Email: trando@mednet.ucla.edu.
Ngan F. Huang, Email: ngantina@stanford.edu.
Supplemental information
References
- Alcazar C.A., Hu C., Rando T.A., Huang N.F., Nakayama K.H. Transplantation of insulin-like growth factor-1 laden scaffolds combined with exercise promotes neuroregeneration and angiogenesis in a preclinical muscle injury model. Biomater. Sci. 2020;8:5376–5389. doi: 10.1039/d0bm00990c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Mateos E., Brinkmeier H., Burks T.N., Mejias R., Files D.C., Steinberger M., Soleimani A., Marx R., Simmers J.L., Lin B., et al. Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Mol. Med. 2013;5:80–91. doi: 10.1002/emmm.201201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur S.T., Cooley I.D. The effect of physiological stimuli on sarcopenia; impact of Notch and Wnt signaling on impaired aged skeletal muscle repair. Int. J. Biol. Sci. 2012;8:731–760. doi: 10.7150/ijbs.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascenzi F., Barberi L., Dobrowolny G., Villa Nova Bacurau A., Nicoletti C., Rizzuto E., Rosenthal N., Scicchitano B.M., Musaro A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell. 2019;18:e12954. doi: 10.1111/acel.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson C.R., Cheung T.H., Liu L., Tripathi P.V., Steeper K.M., Rando T.A. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cell. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calve S., Odelberg S.J., Simon H.G. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev. Biol. 2010;344:259–271. doi: 10.1016/j.ydbio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M.E., Hsu M., Conboy I.M. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi R.H., McConahay A., Silvestre J.G., Moriscot A.S., Carson J.A., Koh H.J. TRB3 regulates skeletal muscle mass in food deprivation-induced atrophy. FASEB J. 2019;33:5654–5666. doi: 10.1096/fj.201802145RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L., Ng Y.C. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim. Biophys. Acta. 2006;1762:103–109. doi: 10.1016/j.bbadis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Smythe G.M., Rando T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- da Silveira W.A., Fazelinia H., Rosenthal S.B., Laiakis E.C., Kim M.S., Meydan C., Kidane Y., Rathi K.S., Smith S.M., Stear B., et al. Comprehensive Multi-omics Analysis Reveals Mitochondrial Stress as a Central Biological Hub for Spaceflight Impact. Cell. 2020;183:1185–1201.e20. doi: 10.1016/j.cell.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehner M., Hadjihannas M., Weiske J., Huber O., Behrens J. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J. Biol. Chem. 2008;283:19201–19210. doi: 10.1074/jbc.M710366200. [DOI] [PubMed] [Google Scholar]
- Fitts R.H., Trappe S.W., Costill D.L., Gallagher P.M., Creer A.C., Colloton P.A., Peters J.R., Romatowski J.G., Bain J.L., Riley D.A. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 2010;588:3567–3592. doi: 10.1113/jphysiol.2010.188508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki S., Seko D., Kitajima Y., Yoshioka K., Tsuchiya Y., Masuda S., Ono Y. Notch1 and Notch2 Coordinately Regulate Stem Cell Function in the Quiescent and Activated States of Muscle Satellite Cells. Stem Cell. 2018;36:278–285. doi: 10.1002/stem.2743. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Taniguchi Y., Shinkai S., Tanaka M., Ito M. Secreted growth differentiation factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatr. Gerontol. Int. 2016;16:17–29. doi: 10.1111/ggi.12724. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Tanimoto K., Fukazawa T., Imura T., Kawahara Y., Yuge L. Simulated microgravity attenuates myogenic differentiation via epigenetic regulations. NPJ Microgravity. 2018;4:11. doi: 10.1038/s41526-018-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler L., Kurtek C., Merholz M., Jian Y., Hashemolhosseini S. In Adult Skeletal Muscles, the Co-Receptors of Canonical Wnt Signaling, Lrp5 and Lrp6, Determine the Distribution and Size of Fiber Types, and Structure and Function of Neuromuscular Junctions. Cells. 2022;11:3698. doi: 10.3390/cells11243968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Bragg R., Elmansi A.M., McGee-Lawrence M.E., Isales C.M., Hamrick M.W., Hill W.D., Fulzele S. Stromal cell-derived factor-1 (CXCL12) and its role in bone and muscle biology. Cytokine. 2019;123:154783. doi: 10.1016/j.cyto.2019.154783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A.T.V., Palla A.R., Blake M.R., Yucel N.D., Wang Y.X., Magnusson K.E.G., Holbrook C.A., Kraft P.E., Delp S.L., Blau H.M. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc. Natl. Acad. Sci. USA. 2017;114:6675–6684. doi: 10.1073/pnas.1705420114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N.F., Patel S., Thakar R.G., Wu J., Hsiao B.S., Chu B., Lee R.J., Li S. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006;6:537–542. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- Huang S., Wang X., Yu J., Tian Y., Yang C., Chen Y., Chen H., Ge H. LonP1 regulates mitochondrial network remodeling through the PINK1/Parkin pathway during myoblast differentiation. Am J. Physiol. Cell Physiol. 2020;319:C1020–C1028. doi: 10.1152/ajpcell.00589.2019. [DOI] [PubMed] [Google Scholar]
- Ito T., Nakanishi Y., Yamaji N., Murakami S., Schaffer S.W. Induction of Growth Differentiation Factor 15 in Skeletal Muscle of Old Taurine Transporter Knockout Mouse. Biol. Pharm. Bull. 2018;41:435–439. doi: 10.1248/bpb.b17-00969. [DOI] [PubMed] [Google Scholar]
- Juhl O.J.t., Buettmann E.G., Friedman M.A., DeNapoli R.C., Hoppock G.A., Donahue H.J. Update on the effects of microgravity on the musculoskeletal system. NPJ Microgravity. 2021;7:28. doi: 10.1038/s41526-021-00158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Du K. TRB3 modulates C2C12 differentiation by interfering with Akt activation. Biochem. Biophys. Res. Commun. 2007;353:933–938. doi: 10.1016/j.bbrc.2006.12.161. [DOI] [PubMed] [Google Scholar]
- Kawase T., Ichikawa H., Ohta T., Nozaki N., Tashiro F., Ohki R., Taya Y. p53 target gene AEN is a nuclear exonuclease required for p53-dependent apoptosis. Oncogene. 2008;27:3797–3810. doi: 10.1038/onc.2008.32. [DOI] [PubMed] [Google Scholar]
- Kawase T., Ohki R., Shibata T., Tsutsumi S., Kamimura N., Inazawa J., Ohta T., Ichikawa H., Aburatani H., Tashiro F., Taya Y. PH domain-only protein PHLDA3 is a p53-regulated repressor of Akt. Cell. 2009;136:535–550. doi: 10.1016/j.cell.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Kim-Muller J.Y., Song L., LaCarubba Paulhus B., Pashos E., Li X., Rinaldi A., Joaquim S., Stansfield J.C., Zhang J., Robertson A., et al. GDF15 neutralization restores muscle function and physical performance in a mouse model of cancer cachexia. Cell Rep. 2023;42:111947. doi: 10.1016/j.celrep.2022.111947. [DOI] [PubMed] [Google Scholar]
- Kim H., Kim K.M., Kang M.J., Lim S. Growth differentiation factor-15 as a biomarker for sarcopenia in aging humans and mice. Exp. Gerontol. 2020;142:111115. doi: 10.1016/j.exger.2020.111115. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C. Role of apoptosis in sarcopenia. J. Gerontol. A. Biol. Sci. Med. Sci. 2003;58:999–1001. doi: 10.1093/gerona/58.11.m999. [DOI] [PubMed] [Google Scholar]
- Liu L., Charville G.W., Cheung T.H., Yoo B., Santos P.J., Schroeder M., Rando T.A. Impaired Notch Signaling Leads to a Decrease in p53 Activity and Mitotic Catastrophe in Aged Muscle Stem Cells. Cell Stem Cell. 2018;23:544–556.e4. doi: 10.1016/j.stem.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Renault V.M., Rando T.A. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin. Cell Dev. Biol. 2005;16:612–622. doi: 10.1016/j.semcdb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Ma Q.L., Yang T.L., Yin J.Y., Peng Z.Y., Yu M., Liu Z.Q., Chen F.P. Role of insulin-like growth factor-1 (IGF-1) in regulating cell cycle progression. Biochem. Biophys. Res. Commun. 2009;389:150–155. doi: 10.1016/j.bbrc.2009.08.114. [DOI] [PubMed] [Google Scholar]
- Machida S., Booth F.W. Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation. Proc. Nutr. Soc. 2004;63:337–340. doi: 10.1079/PNS2004354. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J.E., Partridge T.A. Muscle satellite cells. Int. J. Biochem. Cell Biol. 2003;35:1151–1156. doi: 10.1016/s1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- Nakayama K.H., Quarta M., Paine P., Alcazar C., Karakikes I., Garcia V., Abilez O.J., Calvo N.S., Simmons C.S., Rando T.A., Huang N.F. Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration. Commun. Biol. 2019;2:170. doi: 10.1038/s42003-019-0416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa T., Ishidoh K., Hirasaka K., Ishihara I., Ikemoto M., Kano M., Kominami E., Nonaka I., Ogawa T., Adams G.R., et al. Skeletal muscle gene expression in space-flown rats. FASEB J. 2004;18:522–524. doi: 10.1096/fj.03-0419fje. [DOI] [PubMed] [Google Scholar]
- Palla A.R., Ravichandran M., Wang Y.X., Alexandrova L., Yang A.V., Kraft P., Holbrook C.A., Schurch C.M., Ho A.T.V., Blau H.M. Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science. 2021;371:abc8059. doi: 10.1126/science.abc8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parafati M., Giza S., Shenoy T.S., Mojica-Santiago J.A., Hopf M., Malany L.K., Platt D., Moore I., Jacobs Z.A., Kuehl P., et al. Human skeletal muscle tissue chip autonomous payload reveals changes in fiber type and metabolic gene expression due to spaceflight. NPJ Microgravity. 2023;9:77. doi: 10.1038/s41526-023-00322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J.C., Pouzet C., Samadi M., Elbim C., et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez K., Ciotlos S., McGirr J., Limbad C., Doi R., Nederveen J.P., Nilsson M.I., Winer D.A., Evans W., Tarnopolsky M., et al. Single nuclei profiling identifies cell specific markers of skeletal muscle aging, frailty, and senescence. Aging (Albany NY) 2022;14:9393–9422. doi: 10.18632/aging.204435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchert M., Adams V., Linke A., Engele J. Evidence for the involvement of the CXCL12 system in the adaptation of skeletal muscles to physical exercise. Cell. Signal. 2016;28:1205–1215. doi: 10.1016/j.cellsig.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Radugina E.A., Almeida E.A.C., Blaber E., Poplinskaya V.A., Markitantova Y.V., Grigoryan E.N. Exposure to microgravity for 30 days onboard Bion M1 caused muscle atrophy and impaired regeneration in murine femoral Quadriceps. Life Sci. Space Res. 2018;16:18–25. doi: 10.1016/j.lssr.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Reid K.F., Fielding R.A. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc. Sport Sci. Rev. 2012;40:4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q., Chen J., Liu Y. LRP5 and LRP6 in Wnt Signaling: Similarity and Divergence. Front. Cell Dev. Biol. 2021;9:670960. doi: 10.3389/fcell.2021.670960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z., Ahn E.H., Do M., Mair D.B., Monemianesfahani A., Lee P.H.U., Kim D.H. Simulated microgravity attenuates myogenesis and contractile function of 3D engineered skeletal muscle tissues. NPJ Microgravity. 2024;10:18. doi: 10.1038/s41526-024-00353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem S., Biswas S.C. Tribbles Pseudokinase 3 Induces Both Apoptosis and Autophagy in Amyloid-beta-induced Neuronal Death. J. Biol. Chem. 2017;292:2571–2585. doi: 10.1074/jbc.M116.744730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz F., Schulte A., Adamski F., Hundhausen C., Mittag J., Schwarz A., Kruse M.L., Proksch E., Ludwig A. Constitutive expression and regulated release of the transmembrane chemokine CXCL16 in human and murine skin. J. Invest. Dermatol. 2007;127:1444–1455. doi: 10.1038/sj.jid.5700751. [DOI] [PubMed] [Google Scholar]
- Shang G.K., Han L., Wang Z.H., Liu Y.P., Yan S.B., Sai W.W., Wang D., Li Y.H., Zhang W., Zhong M. Sarcopenia is attenuated by TRB3 knockout in aging mice via the alleviation of atrophy and fibrosis of skeletal muscles. J. Cachexia Sarcopenia Muscle. 2020;11:1104–1120. doi: 10.1002/jcsm.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu P.M., Pistilli E.E., Alway S.E. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1015–R1026. doi: 10.1152/ajpregu.00198.2005. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Nakamura A., Shimizu T. Simulated microgravity accelerates aging of human skeletal muscle myoblasts at the single cell level. Biochem. Biophys. Res. Commun. 2021;578:115–121. doi: 10.1016/j.bbrc.2021.09.037. [DOI] [PubMed] [Google Scholar]
- Trappe T. Influence of aging and long-term unloading on the structure and function of human skeletal muscle. Appl. Physiol. Nutr. Metab. 2009;34:459–464. doi: 10.1139/H09-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velthoven C.T.J., Rando T.A. Stem Cell Quiescence: Dynamism, Restraint, and Cellular Idling. Cell Stem Cell. 2019;24:213–225. doi: 10.1016/j.stem.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenburgh H., Chromiak J., Shansky J., Del Tatto M., Lemaire J. Space travel directly induces skeletal muscle atrophy. FASEB J. 1999;13:1031–1038. doi: 10.1096/fasebj.13.9.1031. [DOI] [PubMed] [Google Scholar]
- Vitry G., Finch R., McStay G., Behesti A., Dejean S., Larose T., Wotring V., da Silveira W.A. Muscle atrophy phenotype gene expression during spaceflight is linked to a metabolic crosstalk in both the liver and the muscle in mice. iScience. 2022;25:105213. doi: 10.1016/j.isci.2022.105213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev A.G., Di Giovanni S., Wang G., Liu W., Stoica B., Faden A.I. BOK and NOXA are essential mediators of p53-dependent apoptosis. J. Biol. Chem. 2004;279:28367–28374. doi: 10.1074/jbc.M313526200. [DOI] [PubMed] [Google Scholar]
- Yamada T., Miyabe Y., Ueki S., Fujieda S., Tokunaga T., Sakashita M., Kato Y., Ninomiya T., Kawasaki Y., Suzuki S., Saito H. Eotaxin-3 as a Plasma Biomarker for Mucosal Eosinophil Infiltration in Chronic Rhinosinusitis. Front. Immunol. 2019;10:74. doi: 10.3389/fimmu.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yartseva V., Goldstein L.D., Rodman J., Kates L., Chen M.Z., Chen Y.J., Foreman O., Siebel C.W., Modrusan Z., Peterson A.S., Jovicic A. Heterogeneity of Satellite Cells Implicates DELTA1/NOTCH2 Signaling in Self-Renewal. Cell Rep. 2020;30:1491–1503.e6. doi: 10.1016/j.celrep.2019.12.100. [DOI] [PubMed] [Google Scholar]
- Youm T.H., Woo S.H., Kwon E.S., Park S.S. NADPH Oxidase 4 Contributes to Myoblast Fusion and Skeletal Muscle Regeneration. Oxid. Med. Cell. Longev. 2019;2019:3585390. doi: 10.1155/2019/3585390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Wang H., Xu Y., Yu D., Li D., Liu X., Du W. Insulin-like growth factor-1 (IGF-1) promotes myoblast proliferation and skeletal muscle growth of embryonic chickens via the PI3K/Akt signalling pathway. Cell Biol. Int. 2015;39:910–922. doi: 10.1002/cbin.10466. [DOI] [PubMed] [Google Scholar]
- Zhang W., Sun W., Gu X., Miao C., Feng L., Shen Q., Liu X., Zhang X. GDF-15 in tumor-derived exosomes promotes muscle atrophy via Bcl-2/caspase-3 pathway. Cell Death Discov. 2022;8:162. doi: 10.1038/s41420-022-00972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the RNA-seq raw data reported in this paper is GSE238215. The accession number will be accesible to readers upon publication. All data may be made available by request to the corresponding author.