Abstract
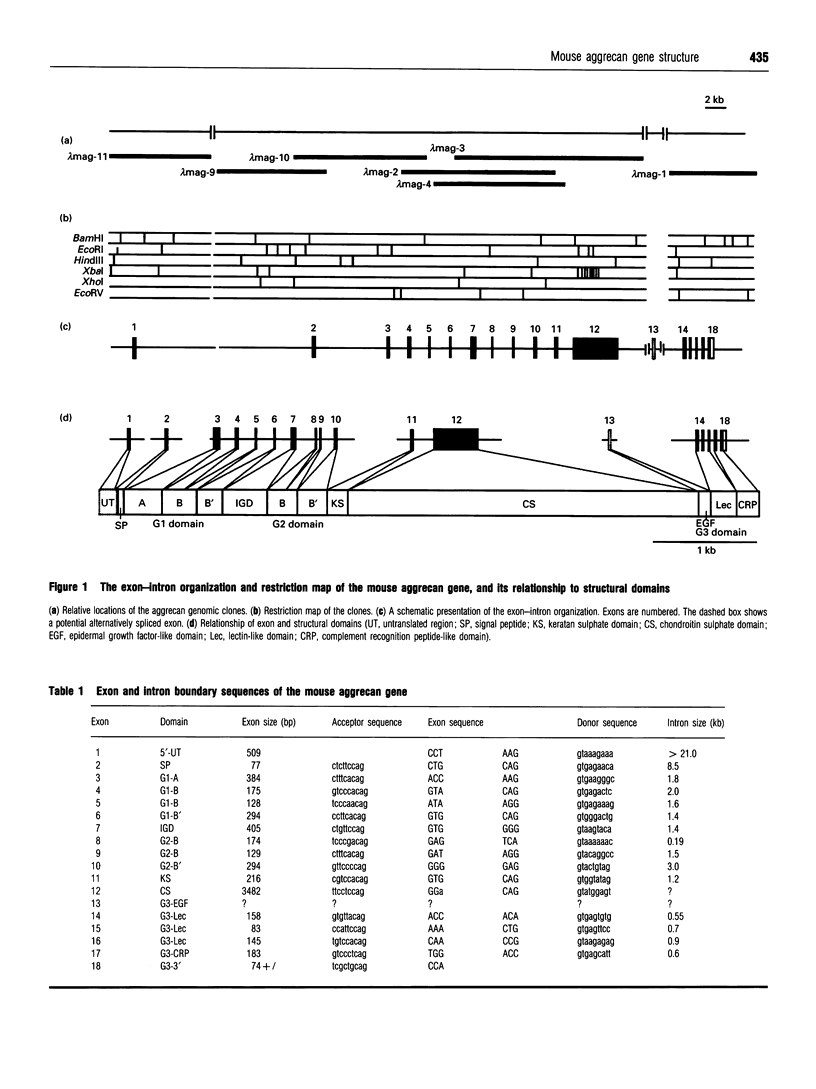
Seven genomic clones for mouse aggrecan core protein have been isolated including 3 kb of 5'- and 7 kb of 3'-flanking sequences. All exon sequences and their intron boundary sequences in these clones were identified and mapped by DNA sequencing. The gene spans at least 61 kb and contains 18 exons. Exon 1 encodes 5'-untranslated sequence and exon 2 contains a translation start codon, methionine. The coding sequence is 6545 bp for a 2132-amino-acid protein with calculated M(r) = 259,131 including an 18-amino-acid signal peptide. There is a strong correlation between structural domains and exons. Notably, the chondroitin sulphate domain consisting of 1161 amino acids is encoded by a single exon of 3.6 kb. Although link protein has similar structural domains and subdomains, the sequence identity and the organization of exons encoding the subdomains B and B' of G1 and G2 domains revealed a strong similarity of mouse aggrecan to both human versican and rat neurocan. Primer extension analysis identified four transcription start sites which are close together. The promoter sequence showed high G/C content (65%) and contained several consensus binding motifs for transcription factors including Sp-1 and the glucocorticoid receptor. There are stretches of sequences similar to the promoter region of both the type-II collagen and link protein genes. These sequences may be important for cartilage gene expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonsson P., Heinegård D., Oldberg A. The keratan sulfate-enriched region of bovine cartilage proteoglycan consists of a consecutively repeated hexapeptide motif. J Biol Chem. 1989 Sep 25;264(27):16170–16173. [PubMed] [Google Scholar]
- Barry F. P., Gaw J. U., Young C. N., Neame P. J. Hyaluronan-binding region of aggrecan from pig laryngeal cartilage. Amino acid sequence, analysis of N-linked oligosaccharides and location of the keratan sulphate. Biochem J. 1992 Sep 15;286(Pt 3):761–769. doi: 10.1042/bj2860761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezouska K., Crichlow G. V., Rose J. M., Taylor M. E., Drickamer K. Evolutionary conservation of intron position in a subfamily of genes encoding carbohydrate-recognition domains. J Biol Chem. 1991 Jun 25;266(18):11604–11609. [PubMed] [Google Scholar]
- Bourdon M. A., Krusius T., Campbell S., Schwartz N. B., Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran L., Tanzer M. L. Molecular cloning of chicken aggrecan. Structural analyses. Biochem J. 1992 Dec 15;288(Pt 3):903–910. doi: 10.1042/bj2880903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Doege K., Sasaki M., Horigan E., Hassell J. R., Yamada Y. Complete primary structure of the rat cartilage proteoglycan core protein deduced from cDNA clones. J Biol Chem. 1987 Dec 25;262(36):17757–17767. [PubMed] [Google Scholar]
- Fosang A. J., Last K., Knäuper V., Neame P. J., Murphy G., Hardingham T. E., Tschesche H., Hamilton J. A. Fibroblast and neutrophil collagenases cleave at two sites in the cartilage aggrecan interglobular domain. Biochem J. 1993 Oct 1;295(Pt 1):273–276. doi: 10.1042/bj2950273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Hardingham T. E., Murphy G., Hamilton J. A. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991 Aug 25;266(24):15579–15582. [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Last K., Hardingham T. E., Murphy G., Hamilton J. A. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992 Sep 25;267(27):19470–19474. [PubMed] [Google Scholar]
- Fülöp C., Walcz E., Valyon M., Glant T. T. Expression of alternatively spliced epidermal growth factor-like domains in aggrecans of different species. Evidence for a novel module. J Biol Chem. 1993 Aug 15;268(23):17377–17383. [PubMed] [Google Scholar]
- Goetinck P. F., Stirpe N. S., Tsonis P. A., Carlone D. The tandemly repeated sequences of cartilage link protein contain the sites for interaction with hyaluronic acid. J Cell Biol. 1987 Nov;105(5):2403–2408. doi: 10.1083/jcb.105.5.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Hascall V. C., Kimura J. H. Proteoglycans: isolation and characterization. Methods Enzymol. 1982;82(Pt A):769–800. doi: 10.1016/0076-6879(82)82102-2. [DOI] [PubMed] [Google Scholar]
- Hoyle G. W., Hill R. L. Structure of the gene for a carbohydrate-binding receptor unique to rat kupffer cells. J Biol Chem. 1991 Jan 25;266(3):1850–1857. [PubMed] [Google Scholar]
- Iozzo R. V., Naso M. F., Cannizzaro L. A., Wasmuth J. J., McPherson J. D. Mapping of the versican proteoglycan gene (CSPG2) to the long arm of human chromosome 5 (5q12-5q14). Genomics. 1992 Dec;14(4):845–851. doi: 10.1016/s0888-7543(05)80103-x. [DOI] [PubMed] [Google Scholar]
- Iwata M., Wight T. N., Carlson S. S. A brain extracellular matrix proteoglycan forms aggregates with hyaluronan. J Biol Chem. 1993 Jul 15;268(20):15061–15069. [PubMed] [Google Scholar]
- Kimata K., Barrach H. J., Brown K. S., Pennypacker J. P. Absence of proteoglycan core protein in cartilage from the cmd/cmd (cartilage matrix deficiency) mouse. J Biol Chem. 1981 Jul 10;256(13):6961–6968. [PubMed] [Google Scholar]
- Krueger R. C., Jr, Fields T. A., Hildreth J., 4th, Schwartz N. B. Chick cartilage chondroitin sulfate proteoglycan core protein. I. Generation and characterization of peptides and specificity for glycosaminoglycan attachment. J Biol Chem. 1990 Jul 15;265(20):12075–12087. [PubMed] [Google Scholar]
- Krueger R. C., Jr, Fields T. A., Mensch J. R., Jr, Schwartz N. B. Chick cartilage chondroitin sulfate proteoglycan core protein. II. Nucleotide sequence of cDNA clone and localization of the S103L epitope. J Biol Chem. 1990 Jul 15;265(20):12088–12097. [PubMed] [Google Scholar]
- Li H., Schwartz N. B., Vertel B. M. cDNA cloning of chick cartilage chondroitin sulfate (aggrecan) core protein and identification of a stop codon in the aggrecan gene associated with the chondrodystrophy, nanomelia. J Biol Chem. 1993 Nov 5;268(31):23504–23511. [PubMed] [Google Scholar]
- Metsäranta M., Toman D., de Crombrugghe B., Vuorio E. Mouse type II collagen gene. Complete nucleotide sequence, exon structure, and alternative splicing. J Biol Chem. 1991 Sep 5;266(25):16862–16869. [PubMed] [Google Scholar]
- Mok M. T., Ilic M. Z., Handley C. J., Robinson H. C. Cleavage of proteoglycan aggregate by leucocyte elastase. Arch Biochem Biophys. 1992 Feb 1;292(2):442–447. doi: 10.1016/0003-9861(92)90014-n. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch U., Karthikeyan L., Maurel P., Margolis R. U., Margolis R. K. Cloning and primary structure of neurocan, a developmentally regulated, aggregating chondroitin sulfate proteoglycan of brain. J Biol Chem. 1992 Sep 25;267(27):19536–19547. [PubMed] [Google Scholar]
- Rhodes C., Savagner P., Line S., Sasaki M., Chirigos M., Doege K., Yamada Y. Characterization of the promoter for the rat and human link protein gene. Nucleic Acids Res. 1991 Apr 25;19(8):1933–1939. doi: 10.1093/nar/19.8.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Neame P. J., Boynton R. E., Flannery C. R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991 May 15;266(14):8683–8685. [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Har-el R., Tanzer M. L. Partial structure of the gene for chicken cartilage proteoglycan core protein. J Biol Chem. 1988 Oct 25;263(30):15831–15835. [PubMed] [Google Scholar]
- Watanabe H., Kimata K., Line S., Strong D., Gao L. Y., Kozak C. A., Yamada Y. Mouse cartilage matrix deficiency (cmd) caused by a 7 bp deletion in the aggrecan gene. Nat Genet. 1994 Jun;7(2):154–157. doi: 10.1038/ng0694-154. [DOI] [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



