Abstract
Cow's milk allergy (CMA) is one of the most common presentations of food allergy in early childhood. Management of CMA involves individualized avoidance of cow's milk and other mammalian milk and foods containing these. Optimal elimination of cow's milk avoidance includes: label reading; information about safe and nutritious substitute foods; appropriate choice of infant formula or a plant-based food; establishing tolerance to baked milk and monitoring nutritional intake and growth. Substitute formulas are divided into soy formula (not hydrolyzed), milk-based extensively hydrolyzed formulas, rice based extensive, and partially hydrolyzed formulas and amino acid-based formulas. The use of other mammalian milks is not recommended for the management of cow's milk allergy due to a high level of cross-reactivity and nutritional concerns. For toddlers who are eating well, children, and adults, a suitable plant-based beverage may be a suitable alternative to a specialized formula, following careful nutritional considerations. Families need to be instructed on finding suitable nutritious foods and how to prepare suitable meals at home. Individuals with CMA also need to know how to identify and treat acute severe reactions.
Keywords: Cow's milk allergy, Nutrition, Baked milk, Hypoallergenic formulas, Plant based alternatives
Introduction
Cow's milk allergy (CMA)1 is one of the most common presentations of allergy in early childhood.2 It is estimated that CMA affects 2% of infants and 4.5% of children.3 Consumption of cow's milk and foods that contain cow's milk have increased rapidly in the developed and developing world in recent years with the highest level of consumption in Europe, followed by Oceana, the United States, Asia, and Africa.4 Cow's milk contains a range of proteins of which 80% are casein proteins and 20% whey proteins. Caseins (Bos d8) are the major cows' milk allergens, and include 4 isoforms: a-S1-casein (Bos d9), a-S2-casein (Bos d10), b-casein (Bos d11), and k-casein. Whey proteins consist of a-lactalbumin (Bos d4), b-lactoglobulin (Bos d5), immunoglobulins (Bos d7), bovine serum albumin (BSA, Bos d6), and traces of lactoferrin.5
CMA can present as IgE and non-IgE mediated forms of food allergy (Table 1). Non-IgE mediated food allergies are further classified into food protein induced enterocolitis syndrome, food protein induced proctocolitis (confirmed through elimination and reintroduction and in some cases endoscopy), food protein induced enteropathy, and other forms of mild to moderate non-IgE mediated food allergies. Symptoms of IgE mediated food allergies typically presents within 2 h of consumption and symptoms may include the skin, gastro-intestinal track, and respiratory tract, or they can be systemic. Non-IgE mediated food allergies have a longer temporal relationship and then involve mainly the gastro-intestinal track. It is, however, important to avoid overdiagnosis and only make a diagnosis of non-IgE mediated CMA after performing on a thorough clinical evaluation, including dietary history, symptomatology, and in some cases, endoscopic and histological findings.6, 7, 8, 9, 10
Table 1.
Different presentations of cow's milk allergy
| Mechanism | IgE-mediated CMA | Non-IgE, cell mediated or mixed pathophysiology CMA |
|---|---|---|
| Symptoms | Skin: urticaria, angioedema; GI: throat swelling, crampy abdominal pain, immediate emesis, diarrhea; Respiratory: rhinorrhea, sneezing, laryngospasm, cough, bronchspasm; CV: tachycardia, low blood pressure; Generalized: anaphylaxis |
Chronic: Skin: eczematous skin rash; GI: nausea, emesis, reflux, abdominal pain, diarrhea, malabsorption, rectal bleeding Generalized: poor weight gain, failure to thrive, stunting Acute-FPIES: GI: projectile emesis, abdominal pain, diarrhea; CV: tachycardia, hypotension, hypovolemic/distributive shock; Generalized: pallor, lethargy |
| Onset of symptoms following food ingestion | Within minutes to 2 h | Generally within hours to days; exception acute FPIES-onset of emesis within 2–4 h |
| Diagnosis | Detection of food-sIgE by skin prick test and serologic tests, oral food challenge | Recognition of symptoms, response to diagnostic elimination diet (2–4 weeks); FPIES-meeting the diagnostic criteria |
| Management -acute symptoms | Antihistamines, epinephrine, inhaled bronchodilator, oxygen, intravenous fluids | FPIES: rehydration (oral or intravenous), ondansetron (oral or parenteral), single dose of a steroid for severe symptoms |
| Management-dietary | Dietary food avoidance, majority tolerate cow's milk in the form of baked foods | Dietary food avoidance; a subset of patients might tolerate cow's milk in the form of baked foods |
| Natural history | Favorable, generally outgrown before school age; children reacting to baked milk or with peak cow's milk-sIgE >50 kUa/L tend to have a more prolonged course | Favorable, usually outgrown by age 1–3 years |
Management of cow's milk allergy
Management of CMA includes avoidance of cow's milk and foods containing cow's milk proteins by following an individualized avoidance strategy.11,12 Optimal management of CMA includes education, onlabel reading, information about safe and nutritious substitute foods, appropriate choice of infant formula or a plant-based food, establishing tolerance to baked milk, and monitoring nutritional intake and growth. Individuals with CMA also need to know how to identify and treat acute severe reactions.
Foods containing cow's milk
Milk is often consumed as a beverage, but it is important to realize that cow's milk proteins can be found throughout the food supply. Foods Commonly Containing Cow's Milk/Cow's Milk Protein listed in Table 1.
Label reading
Food labelling guidance, regulations, and laws differ world-wide and health care professionals should at a minimum give information regarding national labelling laws when educating families. The success of safely avoiding food allergens also depends on the availability and implementation of strict food labelling laws in the country. Milk is considered a major allergen in most countries and is mandated to be fully disclosed on pre-packed food and in the United Kingdom and European Union only, in restaurant foods, foods pre-packed for direct sale, and not pre-packed. Milk should be listed on the ingredient list or in the CONTAINS statement, where CONTAINS statements are allowed (see Table 2). However, the majority of low- and middle-income countries are yet to introduce such regulatory laws despite the rising prevalence of food allergy, especially CMA.13 See Table 3 for further information on labelling information in Europe/United Kingdom, Australia, United States, and Canada.13,14
Table 2.
How to read a food label and foods to avoid
| |
|---|---|
|
|
|
|---|
|
Table 3.
Cow's milk allergen labelling laws in the US, EU, Canada and Australia/New Zealand (Adapted from Durban et al.)b
| US | EU | Canada | Aust/NZ | ||
|---|---|---|---|---|---|
| Milk covered by mandated labelling laws | ✓ | ✓ | ✓ | ✓ | |
| Labelling statements allowed | “Contains" | ✓ | ✓ | ✓ | |
| Allergen emphasized in ingredient list | ✓ | ✓ | ✓ | ✓ | |
| Precautionary allergen labelling | Voluntary | ✓ | ✓ | ✓ | ✓ |
| Regulated | |||||
| Type of food labeled | Prepacked | ✓ | ✓ | ✓ | ✓ |
| Prepacked for direct sale | ✓ | ||||
| Non-prepacked | ✓a | ||||
| Foods covered under allergen labelling laws | Over the counter or prescription drugs | ||||
| Cosmetics and beauty products | ✓ | ||||
| Restaurant foods | ✓ | ||||
| Exemptions | Milk (casein) products used as fining agents in cider and wines | ✓ | |||
| Whey used in distillates for spirits | ✓ | ||||
| Lactitol | ✓ | ||||
Allergen information must be supplied to the consumer, but labelling is not required.
Durban R, Groetch M, Meyer R et al. Dietary Management of Food Allergy. Immunol Allergy Clin North Am 2021; 41(2):233–270. (In eng). https://doi.org/10.1016/j.iac.2021.01.009.
Allowing or avoiding precautionary advisory labelling (PAL) is a crucial discussion to have during a food allergy consultation and may vary between IgE and non-IgE mediated allergies.15 It is important that individuals understand that one cannot “risk stratify” based on the term used; for example, foods containing a “may contain” warning are not more likely to be contaminated with milk than foods carrying a warning, “produced in the same facility”.16 Other than in South Africa and Switzerland, PAL is voluntary and not regulated. PAL is however prohibited in Japan and Argentina. One of the most difficult discussions with patients is to explain that the absence of PAL does not guarantee that a product is not contaminated. Many families get anxious with this knowledge. The likelihood of foods being contaminated with milk also differs between countries and products.17, 18, 19 It is important to inform families that most larger manufacturers have PAL policies and consumers can check the manufacturer website for PAL policy information.
Practical tips on label reading include: 1) read the ingredient list and CONTAINS statement, where used, each and every time an item is purchased as ingredients may change, 2) do not rely on front-of-package information such as “vegan” or “milk free”, as this is not covered by allergen regulation laws in most countries, and 3) allergen-free labelling may help guide consumers with food allergy toward safe products; however an allergen-free label does not negate the need for careful label reading as indicated above, as allergen-free labeling is not regulated.
Level of avoidance required
It is important that each child and adult with food allergy understand to what extent they need to avoid milk. This may include allowing those with food protein-induced enterocolitis (FPIES) to consume products with PAL and making a clinical decision about allowing these products in those diagnosed with eosinophilic esophagitis.15,20 Other individuals may need to avoid milk and its derivatives more strictly.21 Some patients with IgE mediated cow's milk allergy may be able to tolerate small amounts of milk and as the clinical practice of oral immunotherapy increases, being able to quantify certain levels of cow's milk intake will become highly important.22,23 The ability to calculate the milk protein content of food and explain these levels and food portions to patients with IgE mediated cow's milk allergy is a novel and highly interesting aspect of the role of the allergy specialist dietitian.22,24
Baked milk
A high percentage of children with IgE-mediated milk allergy tolerate milk ingredients, when subjected to extensive heating, such as in the form of a baked-goods with a grain matrix.25 In 2008, Nowak et al25 reported on 100 children with cow's milk allergy undergoing baked milk (BM) oral food challenges (OFC), and if negative, unheated liquid milk OFC. Sixty-eight participants were BM tolerant, 9 tolerated both baked and non-baked milk, and 23 reacted to BM. The majority of children, who were clinically reactive to non-baked milk, tolerated baked forms. If tolerant to baked proteins, patients with CMA display higher reactivity thresholds than the general population of children allergic to milk.26
As cow's milk is a common food allergen in young children,27 and cow's milk is a nutritionally important food and ubiquitous in the food supply, the impact of tolerance of BM ingredients is significant. Tolerating BM can result in greater dietary options, improved nutrition, and potentially decrease parental anxiety regarding accidental allergen ingestion.28 Several small studies reported that regular consumption of BM might accelerate tolerance acquisition of non-baked milk, while others found no difference. A single-center, randomized clinical trial found no difference in tolerance acquisition to non-heated milk with more rapid escalation from lower dose of more extensively BM to a higher dose, less extensively BM.29 Further research is needed.
However, following a BM diet approach may result in more proactive management of cow's milk allergy and leads to earlier introduction of non-baked milk compared to strict avoidance diet.30
For performing OFCs, many food allergy centers use a published baked muffin recipe containing 1.33 g milk protein in a wheat matrix that is baked in a 350 °F (±180 °C) oven for 30–35 min (Table 4). In addition to the application of extensive heat, the wheat/grain matrix may play a role in tolerance by reducing exposure of the proteins to the immune system in the gut although these interactions are poorly understood.31 Miceli Sopo et al evaluated the impact of the wheat matrix and found that it may not be relevant for all BM tolerant patients.32 The temperature and baking time is also not standardized in BM recipes; however, most clinical trials used temperatures ranging from 350 to 400 °F (±180–200 °C) and baking times between 20 and 35 min. This is an important point as leaving the middle/inside of baked food too moist will greatly increase its allergenicity.33 Numerous recipes with slight variations in protein content, matrix volume, and baking times and temperatures have been published.34 The use of a standardized recipe provides the health care practitioner with information regarding how much BM ingredient is tolerated. If the patient reports they eat store purchased baked goods that contain milk, it is not easy to determine the threshold level or know if they will tolerate other products, potentially with more BM ingredient. Therefore, a BM OFC using a standardized recipe is helpful.
Table 4.
Baked milk recipea.
| Baked Milk Recipe Yield: 6 muffins |
|---|
| Ingredients: |
| Dry Ingredients |
| 1 ¼ cup (155g) of all-purpose flour (wheat) |
| ½ cup (100g) sugar |
| ¼ tsp. (1.5g) salt |
| 2 tsp. (10g) baking powder |
| Wet Ingredients |
| 1 cup (240 mL) of Milk |
| 2 Tbsp. (30 mL) vegetable oil (corn, soy, canola or other tolerated vegetable oil) |
| 1 tsp. (5 mL) vanilla extract |
| 1 Egga (50g) or 1 ½ tsp. (4g) powdered egg replacer if child is allergic to egg |
| aDO NOT use egg if allergic to egg |
| 1. Preheat oven to 350° F (±180 °C). This step may take 30–45 min. Bake muffins only in an oven that is completely preheated to 350° F (±180 °C). |
| 2. Line a muffin pan with 6 muffin liners. |
| 3. Stir together the liquid ingredients until well combined: milk, canola oil, vanilla extract, egg or egg replacer (although the egg replacer is a dry ingredient, please add at this step). Set aside. |
| 4. In a separate mixing bowl, mix together the dry ingredients (flour, sugar, salt, baking powder). |
| 5. Add liquids ingredients to dry ingredients all at once and gently stir with a wooden spoon (about 15–20 light strokes) until wet and dry ingredients are just combined. Do not over-stir. Some small lumps may remain. |
| 6. Divide the batter into the six prepared muffin liners. |
| 7. Bake 30–35 min or until golden brown and firm to the touch. Cool completely before serving. |
Leonard SA, Caubet JC, Kim JS, Groetch M, Nowak-Wegrzyn A. Baked milk- and egg-containing diet in the management of milk and egg allergy. J Allergy Clin Immunol Pract 2015; 3:13–23; quiz 4.
Although the majority of children will tolerate BM, there remains a smaller percentage who will not tolerate these ingredients and several studies report anaphylaxis upon oral food challenge.34 These children are more likely to have higher casein specific IgE antibodies;33 however there are no consistent predictive values for skin prick tests or food specific IgE levels to determine which patient will react to BM.35 As a result, and because severe allergic reactions including anaphylaxis are possible, physician-supervised oral food challenge to BM is usually recommended.36 Table 5 summarizes factors against and in favor of home introduction using so called “milk ladder”.
Table 5.
Factors in favor and against home introduction of milk
| Factor | Milk ladder not recommended | Milk ladder might be considered for gradual home introduction |
|---|---|---|
| Age | 5 years and older | Younger than 5 years |
| IgE-CMA | Prior anaphylaxis | No prior anaphylaxis |
| Non-IgE CMA | Severe FPIES with documented low blood pressure, fluid resuscitation, hospitalization | Food protein-induced allergic proctocolitis, food protein-induced enteropathy |
| Eliciting dose | Low previous reaction threshold to baked or no-baked CM, reactions to trace amounts or cross-contamination | High previous reaction threshold to baked or non-baked CM |
| Reactivity to baked milk | Reactions to baked milk | Ingestion of small amount of baked milk without symptoms |
| Asthma | Poor control | Optimal control |
| Laboratory tests | High and or increasing SPT wheal or serum CM-specific IgE levels | Small and or decreasing SPT wheal or serum CM-specific IgE levels |
| Adherence to chronic therapies, e.g., for asthma, eczema | Poor/sub-optimal | Adequate |
| Shared decisions | Caregivers apprehensive about the ladder approach, language or comprehension barrier | Caregivers seeking/accepting ladder approach, no language or comprehension barriers |
Recent reports highlighted an alternative approach of a careful home introduction of BM products in younger children (less than 3 years old) with milder phenotype defined as no history of anaphylaxis or any wheezing (unrelated to food ingestion) and skin prick test (with commercial extract) wheal less than 8 mm for milk.37 The authors reported a positive experience with low rate of mild allergic reactions during home introduction. These protocols might be considered in select cases, especially in the settings with limited access to allergy specialists’ services, for example, due to geographic location or restrictions due to pandemic.38
After a successful BM challenge, detailed instructions on how to safely incorporate BM ingredients is required. Table 6 lists detailed instructions that were used successfully during clinical research trials.25
Table 6.
Instructions after a baked milk challengea.
| Include | Avoid | Unknown | |
|---|---|---|---|
| Volume of milk protein | The amount of baked milk allowed is dependent on the amount tolerated in the oral food challenge.The recipe provided in Table 4 provides 1.33g baked milk protein per serving. | More baked milk than tolerated in the oral food challenge. | The amount of baked milk protein tolerated may also depend on other factors such as the degree of baking (time/temperature), the size of the finished product, and the volume of wheat matrix. |
| Grain matrix | Based on the recipe provided, the ratio should be no more than 1 cup of milk per 1 ¼ cups flour with a yield of 6 servings unless the patient has tolerated less matrix on a prior oral food challenge. | Please continue to use milk-free chocolate chips as chocolate chips will melt during baking but not mix into a grain matrix.Items such baked macaroni and cheese, and custards, will not have a grain matrix. | It is unknown how a different grain matrix (such as gluten-free flour) or less grain matrix will affect the immune response. The cooking time, temperature, and size of finished product may also affect the immune response to the food product in addition to the grain matrix. |
| Time/Temperature | All items must be baked in the oven to a dry crumb texture, must be cooked throughout and not be wet, gummy or soggy in the middle.The recipe provided requires a full size muffin to be baked at 350° for 30 min. Other recipes have been published with varying times/temperatures | Continue to avoid any unbaked milk item that is cooked but not baked like pudding, stovetop macaroni and cheese, or heated milk. | Smaller baked items such as mini muffins/crackers/cookies will bake for a shorter time. Ensure they are baked thoroughly to a dry crumb texture. It is unknown if less baking time will be change allergenicity. |
| Store-bought baked goods | Commercial baked products with milk ingredient listed as the third ingredient or further down the list of ingredients.This approach has been used successfully in multiple clinical trials. | Any baked item that also has an unbaked milk ingredient such as a frosted cupcake or an iced cookie or a filled pastry or a cracker with a topically applied flavoring after the item has been baked.Any item that has a milk ingredient as the first or second ingredient. | It is unlikely to find a commercial baked good, such as bread, cookies, crackers, muffins that have more milk protein than the recipe provided, when that ingredient is listed as the third ingredient or further down the list of ingredients. |
Leonard SA, Caubet JC, Kim JS, Groetch M, Nowak-Wegrzyn A. Baked milk- and egg-containing diet in the management of milk and egg allergy. J Allergy Clin Immunol Pract 2015; 3:13–23; quiz 4.
Identify and treat acute severe reactions (anaphylaxis)
There is no reliable in-vitro test able to predict severe reactions in individuals with CMA. STAT6 (signal transducer and activation of transcription 6) gene variants have been associated with more severe allergic reactions during both peanut and cow's milk OFC.39 Significant association has been found between anaphylaxis during the OFC with cow's milk and the sIgE levels for caseins, as well as with higher basophil reactivity.40 There is however, no reliable in-vitro test able to predict the occurrence and the severity of in-vivo reactions.
Not only should families be advised on food avoidance, but they also need advice on the steps to take on inadvertent exposure. Severe reactions should be identified and managed according to the consensus on DEfinition of Food Allergy SEverity (DEFASE).41
Human milk
The World Health Organization (WHO) recommends exclusive breastfeeding for the first 6 months of life. B-lactoglobulin has been detected in human milk of up to 95% of mothers consuming cow's milk.42 Yet, the amounts secreted in human milk are very small in the region of nanogram per milliliter. The amounts present in human milk vary widely, irrespective of the amounts consumed by the mother and the timing of consumption.43 Timing of detecting of milk proteins is around 1–2 h from consumption for milk.44 For most infants with cow's milk allergy, there will be no need to restrict the maternal diet. Maternal avoidance of cow's milk during breastfeeding may be required in some infants with non-IgE mediated cow's milk allergy; in around 5% of infants of FPIES);45 between 18 and 50% of infants with proctocolitis46,47 and the number of children with eosinophilic esophagitis (EoE) who react to milk via human milk is not clear.45 The need for maternal avoidance of cow's milk in children with IgE mediated cow's milk allergy, needs to be assessed on a basis.45,47, 48, 49 If a maternal dietary restriction is required, a dietetic assessment is required to ensure the maternal diet is nutritionally sound and the necessary supplementation provided when required.47
When infants are not breastfed or human milk is not sufficient, cow's milk infant formulas provide the mainstay of nutrition.50 For infants, children and adults with CMA, a milk alternative should be provided.
Specialized formulas
For infants fed with formula, a substitute formula is recommended. Substitute formulas are divided into soy infant formula (not hydrolyzed), milk-based extensively hydrolyzed formulas, rice-based extensive and partially hydrolyzed infant formulas and amino acid-based formulas. Extensively hydrolyzed cow's milk formulas51 are the result of enzymatic hydrolysis and ultrafiltration of cow's milk. Amino acid based formulas are based on pure amino acids and therefore peptide free.52 Special hypoallergenic formulas with an adjusted nutrient profile are available for children over 12 months and in some cases, may even be used in teenagers and adults.
The claim of “hypoallergenicity” is regulated in most countries. Hypoallergenic formulas sold in the European Union must comply to European Food Safety Authority (EFSA) regulations53 and in the United States to Food and Drug Administration (FDA) regulations.54 The definition of hypoallergenic formulas are based on 2 historical definitions. Host et al stated that the regulations of the European Union for labelling infant formulas as having reduced allergenicity (or antigenicity), are based arbitrarily on a content of immunoreactive protein of <1% of total nitrogen containing substances, but there is no evidence that such a threshold of immunogenic protein would ensure a reduced clinical allergenicity.55 The American Academy of Pediatrics (AAP) defines a hypoallergenic formula as a formula that is tolerated by 90% of individuals with cow's milk allergy with a 95% confidence when given in prospective randomized, double-blind, placebo-controlled trials.56
The macro and micronutrient content of substitute formulas for CMA must comply with both EFSA and FDA regulations, which implies limited differences between formulas based on these ingredients. Infants therefore consuming sufficient volume of any of these specialist CMA formulas will achieve their requirements. There are differences with the addition of cow's milk protein free lactose, prebiotics, human milk oligosaccharides, probiotics and synbiotics in formulas, aimed at supporting the microbiome to more closely align with an infant who has been breastfed and to induce tolerance.57, 58, 59, 60, 61 A recent systematic review concluded that there is currently insufficient evidence to choose one cow's milk based extensively hydrolyzed formula over another.62 Healthcare professionals may consider the lactose content, the osmolality and the presence of thickening agents (pre-thickened hypoallergenic formulas exist in some countries) in these formulas, in particular in children with gastrointestinal symptoms including diarrhea and vomiting.63, 64, 65 For amino acid based formulas, which are advised for severe CMA, no specific recommendation can also be made to choose one formula above another based on novel ingredients with the current available evidence.65,66
Hydrolyzed infant formula can be either partially or extensively hydrolyzed. Although rice is rich in essential amino acids, it lacks some amino acids that are present in human milk. For this reason and to ensure nutritional safety and growth in infants allergic to cow's milk, hydrolyzed rice protein infant formulas are supplemented with lysine, threonine, tryptophan and, similar to other hypoallergenic formulas, they also meet the micronutrient requirements for infant formulas.67 There are currently no studies assessing the bone mineral density of long-term use of hydrolyzed rice infant formula; however, these are data that are also not available for many other formulas used in CMA. Future research in this area is welcome, in particular as reduced bone mineral density has been reported in CMA.68,69
In terms of concerns about the inorganic arsenic content of these formulas, a recent study reassures the arsenic content is within safe limits.70 However, it is advised that arsenic content should be declared on these formulas. The main characteristics of formulas suitable for the treatment of cow's milk allergy are reported in Table 7.
Table 7.
Suitable infant formula substitutes for the management of cow's milk allergy.
| Milk alternative | Characteristics |
|---|---|
| Soy infant formula | Soy proteins not similar to cow's milk proteins |
| Extensively hydrolyzed cow's milk based formulas | Based on milk proteins which are hydrolyzed |
| Amino acid/Elemental formulas (AAF) | Amino acids are derived from non-milk sources |
| Rice hydrolysates (partially or extensively hydrolyzed options) | Rice based hydrolysates |
Partially hydrolyzed formulas based on cow's milk are not recommended for the management of cow's milk allergy.
Soy infant formula
A further option for the management of cow's milk allergy, is the use of soy infant formula. And infant formula milk contains completely different proteins than those derived from cow's milk. Soy-based infant formulas are well tolerated in the majority of infants with IgE mediated CMA, but data from some countries points towards more children with non-IgE mediated CMA also reacting to soy.65,71,72 Current soy infant formulas are enriched with amino acids (methionine, taurine and carnitine), iron, zinc, calcium, phosphorus.52 There are however concerns regarding the use of soy infant formulas and the potential hormonal effects on the reproductive system, due to the isoflavones present in soy proteins.73 The negative influence of isoflavones, demonstrated in some animals models, has not been evidenced with the same relevance in humans. Only children with congenital hypothyroidism can have problems and require remodulation of thyroid hormone replacement doses. However, this does not mean that the potential harmful effects of isoflavones can be fully excluded.74 On the other hand, it is acknowledged that soy has a significantly better taste, which may affect acceptance of formula and carries less of a financial burden for families in particular in resource poor countries.75,76 This is reflected in the newly published guidelines of the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) that suggest that soy infant formulas can be considered for certain economical, cultural and palatability reasons.
The 2023 DRACMA guidelines make the following conditional recommendations.
-
1)
Extensively hydrolyzed (milk) formula or a hydrolyzed infant formula can be used as the first option for managing infants with IgE and non-IgE-mediated CMA if breastfeeding is not possible or available
-
2)
An amino-acid formula can be a second option
-
3)
A soy infant formula would be regarded as the last option
-
4)
Formulas without a probiotic or an extensively hydrolyzed (milk) formula containing Lacticaseibacillus rhamnosus (formerly Lactobacillus rhamnosus) GG can be used for infants with either IgE or non-IgE-mediated CMA
The issued recommendations are labeled as “conditional” following GRADE approach due to the very low certainty about the health effects based on the available evidence.
Other mammalian milks
The use of other mammalian milks is not recommended for the management of cow's milk allergy due to a high level of cross-reactivity and nutritional concerns.
Alternative plant-based beverages
Allergists, pediatricians and pediatric gastroenterologists are increasingly seeing caregivers who choose to feed their infants and young children plant-based beverages over cow's milk for reasons such as, medical conditions (allergies), cultural dietary preferences, or health related perceptions such as a possible beneficial effect on the gut microbiome.77 However, plant-based beverages are nutritionally inadequate to support normal growth and development compared to infant formulas or human milk, particularly in children <1 year of age. Adverse effects from inappropriate use of plant-based beverages can lead to poor growth, malnutrition, electrolyte disorders, kidney stones, and severe nutrient deficiencies including iron deficiency anemia, rickets, and scurvy. It is however difficult to tell if this is a direct association or indirectly associated with milk avoidance.78, 79, 80, 81
The North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) Nutrition Committee, recently published a document77 stating that “In young children beyond the first year of life requiring a dairy-free diet, commercial formula may be a preferable alternative to cow's milk, when such formula constitutes a substantial source of otherwise absent or reduced nutrients (e.g., protein, calcium, vitamin D) in the child's restricted diet.” They further emphasized that inadequate nutritional intake can adversely affect a child's nutritional status, growth, and development and that milk intake plays a particular important role in a child's overall diet. The working group letter concluded that alternative plant-based beverages should provide a comparable nutritional content to conventional cow's milk to prevent adverse nutritional effects, with a particular focus on protein content. They recommended that growth studies and bone mineralization studies of young children fed plant-based beverages are needed, similar to those performed to assess the nutritional effect of infant formulas.
Plant-based beverages include those made from soy, coconut, almond, rice, oat, hazelnut, cashew, walnut, pea, sesame, hemp, tigernut, and quinoa (Table 8). These beverages differ in terms of nutrition composition and characteristics. Availability of these beverages also differ internationally but the majority can be ordered on-line. A simple guide to buying these plant-based beverages is listed in Table 7. It is important to be aware of the cost of alternative beverages and compare their nutrient composition against that of cow milk, particularly in terms of protein, energy, calcium, vitamin B12, Vitamin D, and iodine. Fat content is also important in children under the age of 2 years. In addition, protein quality of these plant based beverages needs to be considered, as the amino acid profile may not be similar to cow's milk. Bioavailability of nutrients added to plant based beverages may also be less than nutrients naturally occurring in cow's milk.77
Table 8.
Plant based beverages and food alternatives available in the US and online per 100 ml of plant based beverage.
| Milk Type | Calories (per 100 mls) | Fat (g) | Protein (g) | Carbohydrates (g) | Dietary Fiber (g) | Added sugar (g) | Calcium (mg) | Vitamin D (mcg) | Potassium (mg) | Vitamin B12 (mcg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Oat milk | 34–68 | 0.6–4 | 0.8–3.4 | 4.6–10.5 | 0–0.8 | 0–7.2 | 51.1–194.1 | 0.8–1.9 | 56.1–173 | 0–1 |
| Soy milk | 30–55 | 1.5–2.5 | 3–5.1 | 1.3–7.6 | 0–1.3 | 0–2.1 | 16.9–189.9 | 0–2.1 | 0–181.4 | 0–1.3 |
| Almond milk | 15–55 | 1–4.6 | 0.4–2.1 | 0.4–3.4 | 0–0.4 | 0–3 | 3–189.9 | 0–2.1 | 0–71.7 | 0 |
| Hazelnut milk | 13–69 | 1.3–3.8 | 0–0.8 | 0.4–13.9 | 0–0.4 | 0–5.5 | 0–51.9 | 0–0.8 | 0–44.3 | 0 |
| Cashew milk | 11–55 | 0.8–4.2 | 0–1.7 | 0.4–3.4 | 0 | 0–2.1 | 6.3–189.9 | 0–1.1 | 0–63.3 | 0–1.3 |
| Walnut milk | 17–51 | 1.5–4.6 | 0.4–1.3 | 0.4–2.1 | 0–0.4 | 0–1.7 | 10.1–189.9 | 0–2.1 | 32.5–54.9 | 0 |
| Pea milk | 30–59 | 1.9 | 3.4 | 0–7.2 | 0–0.4 | 0 | 194.5–198.3 | 2.5 | 189.5–189.9 | 1.1 |
| Coconut milk | 19–46 | 1.9–4.6 | 0–0.4 | 0.4–3.8 | 0–0.4 | 0–3 | 0–194.1 | 2.5 | 0–71.7 | 0–1.3 |
| Sesame milk | 38–68 | 2.1–3.8 | 1.7–3.4 | 0.8–8.4 | 0–1.3 | 0 | 32.9–164.6 | 2.5 | 0–80.2 | 0 |
| Hemp milk | 21–59 | 2.1–3.4 | 0.8–1.7 | 0.4–8 | 0–0.4 | 0–5.1 | 111–164.6 | 2.5 | 0–61.2 | 0–0.4 |
| Rice milk | 30–51 | 1.1 | 0–0.4 | 4.6–9.7 | 0 | 0 | 12.7–164.6 | 2.5 | 0–16.9 | 0–0.3 |
| Tigernut (Chufa) milk | 56–84 | 2.1–2.5 | 0.4 | 9.7–13.5 | 0 | 0–13.1 | 0 | 2.5 | 0 | 0 |
| Quinoa milk | 29 | 1.3 | 0.4 | 3.4 | 0 | 0 | 0 | 2.5 | 0 | 0 |
The quality of the protein and absorption is different form infant formulas or cow's milk.
For toddlers who are eating well, children and adults, a suitable plant based beverage may be considered.82 These beverages should ideally only be used in children under 2 years of age following a dietary assessment, however when access to alternative formulas are limited, the health care provider can provide counseling to improve the diet using the following recommendations.
Factors to consider that may indicate a toddler is ready to transition to a plant-based beverage are the child:83
-
•
Is at least one year of age
-
•
Eats a varied solid food diet with a variety of foods from each food group;
-
•
Gets at least 2/3 of their energy from the varied solid food diet;
-
•
Consumes no more than 2 servings/day (1 serving = 8 ounces/240 mls of milk substitute day or yogurt substitute):
-
•
Eats age-appropriate textures
-
•
Gets enough protein and fat and micronutrients in the diet from the solid foods and the available milk substitute
-
•
Has no feeding difficulties that may reduce food variety
-
•
Has no known micronutrient deficiencies; AND
-
•
Has no religious/cultural dietary requirements that reduces the variety of foods consumed
This guide also highlights when a toddler should not be transitioned to a plant based beverage when a substitute formula is available. Plant based beverages should also not be used as a main drink in children under 1 year of age. It is important to consider that some of the plant-based beverages are based on high allergenic ingredients and should not be recommended to those with food allergies to any of the ingredients.
Alternative foods
Families need to be instructed on finding suitable nutritious foods and how to prepare suitable meals at home.11 Alternative sources of foods rich in important nutrients and free from cow's milk can be found in Table 9.
Table 9.
Food sources of important nutrients excluded during cow's milk avoidance
| Calcium | calcium-fortified plant based beverages including soy and products made there-of, calcium-set tofu, canned sardine or salmon with bones, fortified breakfast cereals, mineral water rich in calcium |
| Vitamin D | Vitamin D fortified foods (in particular, milk and plant-based beverages but also breakfast cereals), Fatty fish, cod liver oil, UV light exposed mushrooms, egg yolk |
| Iodine | Seaweed, fish, egg, enriched grains, iodized salt |
| Protein | Meat, fish, poultry, eggs, nuts, seeds, legumes |
| Fat | Vegetable oils, fatty fish, meats, nuts, seeds |
| Vitamin B12 | Animal products and fortified foods (breakfast cereals, fortified beverages, fortified nutritional yeast) |
Lifestyle factors
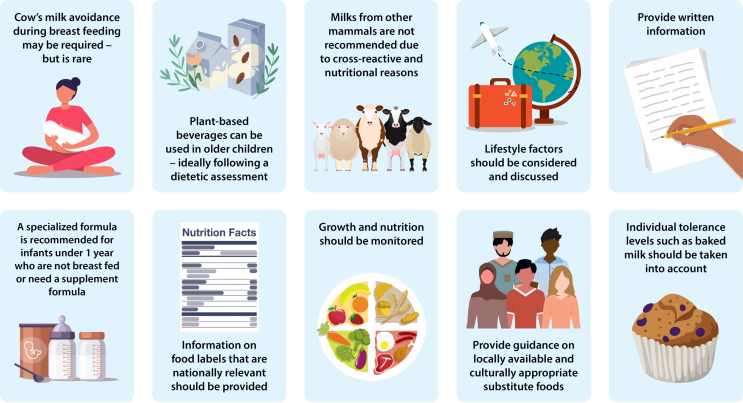
When eating outside of the home whether it be at a friend/family's home, restaurant, on vacation or at school, it is important that families contact the food provider before-hand to establish ingredients of foods and discuss risk of cross contact. In restaurants, it may be helpful to speak to the chef, but some chefs may also be insufficiently informed too.84 There is always the option to bring along allergen-free foods. Chef cards are helpful, particularly when there is a language barrier. Understanding local labeling laws is important as some cover freshly prepared foods such as in the European Union/United Kingdom. Lastly, provide tips with suitable foods and recipes (Fig. 1).
Fig. 1.
CMA and dietary management tips. Dietary management of cow's milk allergy involves food elimination, choosing appropriate alternatives, monitoring nutritional intake and status, and taking lifestyle factors into account. (Images were legally obtained through Adobe Stock and should not be extracted for reuse.)
Nutritional aspects of management of CMA
Growth
Growth faltering (GF) is included as part of the clinical presenting features of cow's milk allergy in current guidelines.8,67,85,86 Although, it is assumed that GF presents together with other IgE or non-IgE mediated symptoms, a recent study has highlighted the importance of considering CMA in children who present with GF as primary symptom, where other diagnoses have been ruled out.87 Whilst, there is no debate on the inclusion of growth assessment as part of the allergy focused history,88 it is clear from published studies, that growth continues to be a concern in children with CMA also after the diagnosis. Isolauri et al highlighted ongoing poor height growth in a study of 100 children with CMA, who were managed with a cow's milk elimination diet and appropriate milk replacements. The study from 1998 has been followed by several other studies, indicating in particular short stature as a problem in CMA,90,91 which continues in those with persistent CMA into young adulthood.92,93 Cow's milk contributes important nutrients for growth and bone mineralization and the milk proteins have been shown to have a positive correlation with serum IGF-1.94 Although most research has focused on nutrient deficiencies, including energy, protein, calcium and vitamin D, as the most plausible explanation for poor growth in CMA,92,93 other hypotheses exist. Already in 1998, Isolauri et al89 theorized low-grade ongoing gastrointestinal inflammation (due to inadvertent exposure to cow's milk or ongoing reactions to an extensively hydrolyzed formula or other allergens) as a possible factor and since then Beck et al95 has also highlighted atopic co-morbidities, in particular atopic eczema, as being associated with poor growth. The most recent DRACMA guidelines3 supported by other publications66,96,97 have found limited evidence of low certainty, that children with IgE mediated CMA who were fed amino acid-based formula may have an improved growth pattern when compared to extensively hydrolyzed (milk) formula. It should however be noted that the included studies are old, and the formulations of formulas have changed and these studies did not aim to assess failure to thrive and therefore catch-up growth. Further research is therefore needed, to answer the question about whether certain formulas better support catch-up growth. Compared to soy infant formula extensively hydrolyzed (milk) formula may favor weight gain but there is no difference on length growth. There was no difference when comparing extensively hydrolyzed (milk) formula to hydrolyzed rice infant formula or hydrolyzed rice infant formula to soy infant formula. In terms of non-IgE mediated CMA, the DRACMA guidelines suggest that compared to amino acid-based formulas, there may be reduced length growth seen with extensively hydrolyzed (milk) formula based, however this study included only patients with IgE mediated CMA, so the certainty of evidence is very low and further research is required. No difference was found when comparing extensively hydrolyzed (milk) formula to hydrolyzed infant formula or hydrolyzed rice infant formula to soy infant formula.
Most publications have focused on growth faltering; however, obesity should also be considered in CMA. Data from Meyer et al90 found that 8% of the surveyed children with food allergy were obese. In this context, food quality is also an important consideration in CMA. A recent study has highlighted that the pressure-to-eat behaviour towards food allergic children, drives unhealthy food choices and the need to make children eat more of their free-from foods.98
Micronutrient deficiencies
Calcium and vitamin D deficiency are most commonly described in cow's milk allergy, but studies have also highlighted concerns about iodine, iron and zinc and in theory children on any elimination diets are at a higher risk of nutritional deficiencies.99, 100, 101, 102, 103, 104 Many of these micronutrients are co-factors in growth (eg, zinc), bone mineralization (eg, calcium and vitamin D) and cognitive development (eg, iodine and iron). Whilst many countries have guidelines in place for vitamin D supplementation and there is great awareness of calcium deficiencies in CMA, other micronutrients are often forgotten and difficult to predict with a diet history alone and targeted nutritional bloods may be required.105,106 Concerns have also been raised in those with persistent CMA on sufficient calcium supplementation that still have sub-optimal bone mineralization,69,81 and have highlighted other nutrients in cow's milk, including phosphate and magnesium that may aid bioavailability of the calcium and also the role of medication (i.e. proton pump inhibitors and inhaled steroids). These factors also need to be considered when advising carers of and patients with CMA.
Feeding difficulties
Feeding difficulties are commonly reported in food allergic patients, in particular those with non-IgE mediated allergies, where cow's milk is a common allergen.107, 108, 109 Although vomiting, pain and discomfort may lead to alteration in sensory perception, impacting on food intake,110 studies in IgE mediated egg allergy mice-models have also observed increased food aversion and anxiety related to food consumption.111,112 Parental modelling is also critical in the positive relationship with food from early on.113 However, in CMA, this can be disrupted because of dietary elimination and increased child and parental anxiety. Food allergy associated feeding difficulties can limit the variety of accepted foods, including important nutritional meal replacements, which in turn can impact on growth and vitamin and mineral deficiencies.114 Management of feeding difficulties includes encouraging caregivers to provide age-appropriate textures and serving sizes, relaxed mealtimes without distraction and neutral caregiver responses, self-feeding and food exploration, and frequent presentation of a variety of foods.108 Early recognition and referral to feeding specialists, which may include multiple disciplines, may help prevent or limit the nutritional risks associated with feeding difficulties.
It is clear that multiple factors are involved in the development of nutritional disorders, including growth faltering, vitamin/mineral deficiencies and feeding difficulties in children with CMA.99
Whilst, they may present as individual nutritional disorders, growth is closely linked to micronutrient adequacy and both are linked to optimal dietary intake that can be affected by feeding difficulties and also parental food choices. It is therefore important in children with CMA, to not only assess growth and dietary intake, but perform targeted bloods where required, and ask about mealtimes and eating patterns.
conclusion
Educating families regarding cow's milk avoidance goes to the core of personalized nutrition. Cow's milk allergy management advice depends on complex aspects such as the range of allergens involved and the effect of baking on the different allergens, which impact the level of avoidance required, and is unique to milk and egg allergy. Cow's milk formula forms a staple for infants not breast fed or sufficiently breast fed. Cow's milk and products made of cow's milk form a staple for young children. Exclusion can have far reaching nutritional and growth complications. The management of cow's milk allergy is further complicated by having to choose a suitable hypoallergenic supplement or plant-based beverage. The future of cow's milk allergy is however exciting with progress being made on understanding gut microbial difference in infants with and without cow's milk allergy, epigenetic modifications, and factors associated with prevention and tolerance induction. Management of cow's milk allergy is therefore an evolving field and requires all health care professionals to be aware of and informed about the latest guidance such as the WAO DRACMA guidelines. Health care professionals world-wide should be encouraged to stay updated on the latest research and guidelines.
Abbreviations
AAP, American Academy of Pediatrics; BM, Baked milk; CMA, Cow's milk allergy; DRACMA, Diagnosis and Rationale for Action against Cow's Milk Allergy; EFSA, European Food Safety Authority; ESPGHAN, European Society for Pediatric Gastroenterology Hepatology and Nutrition; FDA, Food and Drug Administration; GF, Growth faltering; NASPGHAN, North American Society for Pediatric Gastroenterology; Hepatology; and Nutrition; EoE, Eosinophilic esophagitis; FPIES: food protein induced enterocolitis; OFC: oral food challenges; PAL: precautionary advisory labelling.
Members of the WAO DRACMA Guideline Group
Ignacio J. Ansotegui, MD, PhD (Department of Allergy& Immunology, Hospital Quironsalud Bizkaia, Erandio, Bilbao, Spain); Stefania Arasi, MD, PhD (Translational Research in Pediatric Specialities Area, Division of Allergy, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy); Amal H. Assa’ad, MD (Division of Allergy and Immunology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA); Sami L. Bahna, MD, DrPH (Allergy/Immunology Section, Louisiana State University Health Sciences Center, Shreveport, LA, USA); Roberto Berni Canani, MD, PhD (Department of Translational Medical Science, University of Naples Federico II, Naples, Italy); Antonio Bognanni, MD (Clinical Epidemiology and Research Center, CERC, Humanitas University & Humanitas Research Hospital, Pieve Emanuele, Milano, Italy; Department of Health Research Methods, Evidence & Impact, McMaster University, Hamilton, Ontario, Canada; Department of Medicine, Evidence in Allergy Group, McMaster University, Hamilton, Ontario, Canada); Martin Bozzola, MD (Department of Pediatrics, Pediatric Allergy/Immunology Section, British Hospital, Buenos Aires, Argentina); Jan Brozek, MD, PhD (Department of Medicine, Division of _ Clinical Immunology and Allergy, Department of Clinical Epidemiology& Biostatistics, McMaster University Health Sciences Centre, Hamilton, ON, Canada); Derek K. Chu, MD, PhD (Department of Medicine, Division of Clinical Immunology and Allergy; Department of Clinical Epidemiology& Biostatistics, McMaster University Health Sciences Centre, Hamilton, ON, Canada); Lamia Dahdah, MD (Translational Research in Pediatric Specialities Area, Division of Allergy, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy); Christophe Dupont, MD, PhD (Paris Descartes University, Pediatric Gastroenterology, Necker Hospital, Paris, Clinique Marcel Sembat, Boulogne-Billancourt, France); Piotr Dziechciarz, MD, PhD, Medical University of Warsaw, Warsaw, Poland; Motohiro Ebisawa, MD, PhD (Clinical Research Center for Allergy and Rheumatology, National Hospital Organization Sagamihara National Hospital, Kanagawa, Japan); Alessandro Fiocchi, MD (Translational Research in Pediatric Specialities Area, Division of Allergy, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy); Ramon Targino Firmino MD (Faculty of Medical Sciences of Campina Grande, UNIFACISA University Centre, Campina Grande, Paraiba, Brazil); Elena Galli, MD, PhD (Pediatric Allergy Unit, Research Center, San PietroFatebenefratelli Hospital, Rome, Italy); Rose Kamenwa, MD (Department of Pediatrics and Child Health, Aga Khan University Hospital, Nairobi, Kenya); Gideon Lack, MBBCh (Department of Women and Children’s Health/Peter Gorer Department of Immunobiology, School of Life Course Sciences, Faculty of Life Sciences& Medicine, King’s College London, UK; Evelina London Children’s Hospital, Guy’s and St Thomas’ Hospital NHS Foundation Trust, London, UK), Haiqi Li, MD (Pediatric Division Department of Primary Child Care, Children’s Hospital, Chongqing Medical University, Chongqing, China); Alberto Martelli, MD (Italian Society of Pediatric Allergy and Immunology, Milano, Italy); Anna H. Nowak-Wegrzyn, MD, PhD (Department of Pediatrics, New York University Langone Health, New York, NY, USA; Department of Pediatrics, Gastroenterology and Nutrition, Collegium Medicum, University of Warmia and Mazury, Olsztyn, Poland); Nikolaos G. Papadopoulos, MD, PhD (Allergy Unit, 2nd Pediatric Clinic, University of Athens, Athens, Greece; Division of Infection, Immunity& Respiratory Medicine, University of Manchester, UK); Ruby Pawankar, MD, PhD (Department of Pediatrics, Nippon Medical School, Bunkyo-Ku, Tokyo, Japan); Maria Said, RN (Allergy& Anaphylaxis Australia (A&AA), Castle Hills, New South Wales, Australia); Mario Sánchez-Borges MD (Department of Allergy and Clinical Immunology, Centro Médico-Docente La Trinidad Caracas, Venezuela) - posthumos; Holger J. Schünemann, MD, MSc, PhD (Department of Health Research Methods, Evidence and Impact (HEI), McMaster University, Hamilton, ON, Canada, and Cochrane Canada and McMaster GRADE Centre, Hamilton, ON, Canada); Raanan Shamir, MD, PhD (Institute of Gastroenterology, Nutrition and Liver Disease, Schneider Children’s Medical Center, Petach-Tikva, Israel; Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel); Jonathan M. Spergel, MD, PhD (Division of Allergy and Immunology, Department of Pediatrics, The Children’s Hospital of Philadelphia, Perelman School of Medicine at University of Pennsylvania, Philadelphia, PA, USA), Hania Szajewska, MD (The Medical University of Warsaw - Department of Paediatrics, Warsaw, Poland); Luigi Terracciano, MD (Italian NHS and Italian Society of Social and Preventive Pediatrics, Milano, Italy); Yvan Vandenplas, MD, PhD (Department of Pediatrics, UZ Brussel, Vrije Universiteit Brussel, Brussels, Belgium); Carina Venter, PhD, RD (Section of Allergy& Immunology, University of Colorado Denver School of Medicine, Children’s Hospital Colorado, Aurora, CO, USA); Amena Warner, RN, SN (PG Dip) (Allergy UK, Planwell House, Sidcup, Kent, UK); Susan Waserman, MD, MSc (Division of Clinical Immunology and Allergy, Department of Medicine, McMaster University, Hamilton, ON, Canada); Gary W. K. Wong, MD (Department of Paediatrics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China).
Funding
The WAO DRACMA guideline series is a project of the World Allergy Organization and the McMaster University GRADE Centre.
Author contribution
Each author was assigned to write a section of the paper. CV drafted the first combined version of the manuscript. SA composed the tables in the paper. All authors approved the final version of the manuscript.
Ethics approval
Not applicable.
Consent for publication
All authors approved the final version and its submission.
Informed consent
Not applicable.
Declaration of competing interest
CV reports grants from Reckitt Benckiser, grants from Food Allergy Research and Education, grants from National Peanut Board, during the conduct of the study; and personal fees from Reckitt Benckiser, Nestle Nutrition Institute, Danone, Abbott Nutrition, Else Nutrition, and Before Brands, outside the submitted work.
RM reports speaker honoraria and advisory panel consultancy outside of the submitted work for Nutricia/Danone, Abbott Nutrition, Nestle Clinical Nutrition, Reckitt Benckiser and Else Nutrition.
MG reports royalties from UpToDate and Academy of Nutrition and Dietetics and consulting fees from FARE.
AF reports speaker honoraria and advisory panel consultancy outside the submitted work for Nutricia, Abbott, Danone, Stallergenes, DBV, Novartis; and funded research (Institution) from Sanofi, Novartis, Ferrero, DBV, GSK, Astrazeneca, Hipp GmBDH, Humana SpA.
Footnotes
Full list of author information is available at the end of the article
Contributor Information
Carina Venter, Email: Carina.Venter@childrenscolorado.org.
DRACMA Panel:
Ignacio Ansotegui, Stefania Arasi, Sami L. Bahna, Roberto Berni Canani, Martin Bozzola, Jan Brozek, Derek Chu, Lamia Dahdah, Christophe Dupont, Motohiro Ebisawa, Ramon T. Firmino, Elena Galli, Gideon Lack, Haiqi Li, Alberto Martelli, Nikolas G. Papadopoulos, Maria Said, Mario Sánchez-Borges, Holger Schunemann, Raanan Shamir, Jonathan Spergel, Hania Szajewska, Luigi Terracciano, Yvan Vandenplas, Susan Waserman, Amena Warner, and Gary W.K. Wong
Competing Interests Statement - Members of the WAO DRACMA Guideline Group
S Arasi, S Bahna, Bognanni, J Brozek, D Chu, L Dahdah, Piotr Dziechciarz, E Galli, R Kamenwa, H Li, A Martelli, R Pawankar, H Schunemann, R Targino, L Terracciano, and A Warner have no conflicts to disclose. Relationships reported related to the submitted work: IJ Anstotegui – Abbott, Amgen, Astra Zeneca, Bayer, Bial, Faes Farma, Hikma, Menarini, Merck, Mundipharma, Roxall, Sanofi, Stallergenes, UCB. A Assa’ad – Aimmune Therapeutics, DBV Technologies, Astella, ABBVIE, Novartis, Sanofi, FARE, NIH and an intellectual property patent licensed to Hoth. M Bozzola – Danone. C Dupont – Nestle Health Science, Nestle France, Nutricia, Novalac, Sodilac, Abbott, Danone, and stock ownership at DBV Technologies. M Ebisawa – DBV Technologies, Mylan, ARS Pharmaceuticals, Novartis. A Fiocchi – Abbott, Danone. G Lack – FARE, National Peanut Board (NPB), The Davis Foundation, Action Medical Research, UK Food Standards Agency, Medical Research Council, DBV Technologies, Mission Mighty Me, Novartis, Sanofi-Genyzme, Regeneron, ALK-Abello, Lurie Children’s Hospital. A Nowak-Wegrzyn – Nestle, Nutricia, Novartis, Gerber, Aimmune. N Papadopoulos – Novartis, Nutricia, HAL Allergy, Menarini/ Faes Farma, Sanofi, Mylan/Meda, Biomay, AstraZeneca, GSK, MSD, ASIT Biotech, Boehringer Ingelheim, Gerolymatos International SA, Capricare. M Said – Nestle, Nutricia, Abbott, Bayer for Anaphylaxis Australia. J Spergel – DBV Technologies, Regeneron, Sanofi, and Aimmune. H Szajewska – Arla, Ausnutria, BioGaia, Biocodex, Cargill, Danone, Dicofarm, Else Nutrition, Hipp, Nestlé, Nestle Nutrition Institute NNI, Nutricia, Mead Johnson, and Novalac. Board member of the International Scientific Association for Probiotics and Prebiotics (ISAPP), an unpaid and voluntary role. Y Vandenplas – Abbott Nutrition, Alba Health, Arla, Ausnutria, Biogaia, Biocodex, By Heart, CHR Hansen, Danone, ELSE Nutrition, Friesland Campina, Hero, Hypocrata, Nestle Health Science, Nestle Nutrition Institute, Nutricia, Mead Johnson Nutrition, Orafti, Phacobel, Phathom Pharmaceuticals, Pileje, Sanulac, Sari Husada, United Pharmaceuticals (Novalac), Wyeth, Yakult. C Venter – Reckitt Benckiser, Nestle Nutrition Institute, Danone, Abbott Nutrition, Else Nutrition, and Before Brands, DBV Technologies. S Waserman – Aimmune, Pfizer, Kaleo, Bausch Health, GSK, AZ, Sanofi, CSL Behring, Leo, AbbVie, Takeda, Medexus Pharma, MiravoHealth, BioCryst, ALK, Novartis. President to Canadian Allergy Asthma and Immunology Foundation, BOD Asthma Canada, CHAEN. Medical Advisor to Food Allergy Canada, and, Bausch, Kaleoconsultant for epinephrine autoinjectors. GWK Wong – Nestle, Danone. R Shamir – Abbott, Else, and Nestlé. A Warner – Nutricia/ Danone, Abbott, Reckitt Benckiser/Mead Johnson, Nutricia/ Danone for Allergy UK
References
- 1.Zheng J., Wittouck S., Salvetti E., et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 2.National Academies of Sciences E, Medicine, Health, Medicine D, Food, Nutrition B . In: Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy. Oria M.P., Stallings V.A., editors. National Academies Press (US), Copyright 2017 by the National Academy of Sciences. All rights reserved; Washington (DC): 2016. [PubMed] [Google Scholar]
- 3.Bognanni A., Fiocchi A., Arasi S., et al. World allergy organization (WAO) diagnosis and Rationale for action against cow's milk allergy (DRACMA) guideline update 2013; XII; recommendations on milk formula supplements with and without probiotics for infants and toddlers with CMA. World Allergy Organization Journal. 2024;17(4) doi: 10.1016/j.waojou.2024.100888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess K. In: Muehlhoff E., Bennett A., McMahon D., editors. vol. 67. 2014. Milk and dairy products in human nutrition (2013) (Food and Agriculture Organisation of the United Nations (FAO)). Rome. E-ISBN: 978-92-5-107864-8 (PDF). Available on web-site (publications-sales@fao.org). International Journal of Dairy Technology. [Google Scholar]
- 5.D'Auria E., Abrahams M., Zuccotti G.V., Venter C. Personalized nutrition approach in food allergy: is it prime time yet? Nutrients. 2019;11(2) doi: 10.3390/nu11020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyce J.A., Assa'ad A., Burks A.W., et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roca M., Rodriguez Varela A., Donat E., et al. Fecal calprotectin and eosinophil-derived neurotoxin in healthy children between 0 and 12 years. J Pediatr Gastroenterol Nutr. 2017;65(4):394–398. doi: 10.1097/MPG.0000000000001542. [DOI] [PubMed] [Google Scholar]
- 8.Arvola T., Ruuska T., Keränen J., Hyöty H., Salminen S., Isolauri E. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics. 2006;117(4):e760–e768. doi: 10.1542/peds.2005-1069. [DOI] [PubMed] [Google Scholar]
- 9.Molnár K., Pintér P., Győrffy H., et al. Characteristics of allergic colitis in breast-fed infants in the absence of cow's milk allergy. World J Gastroenterol. 2013;19(24):3824–3830. doi: 10.3748/wjg.v19.i24.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waserman S., Bahna S.L., Arasi S., et al. World Allergy Organization Journal; 2024. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines Update – IV Clinical Presentations: IgE-Mediated & Non IgE-Mediated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venter C., Groetch M., Netting M., Meyer R. A patient-specific approach to develop an exclusion diet to manage food allergy in infants and children. Clin Exp Allergy : journal of the British Society for Allergy and Clinical Immunology. 2018;48(2):121–137. doi: 10.1111/cea.13087. [DOI] [PubMed] [Google Scholar]
- 12.Meyer R., Venter C., Bognanni A., et al. World allergy organization (WAO) diagnosis and Rationale for action against cow's milk allergy (DRACMA) guideline update - VII - milk elimination and reintroduction in the diagnostic process of cow's milk allergy. The World Allergy Organization journal. 2023;16(7) doi: 10.1016/j.waojou.2023.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen K.J., Turner P.J., Pawankar R., et al. Precautionary labelling of foods for allergen content: are we ready for a global framework? The World Allergy Organization journal. 2014;7(1):10. doi: 10.1186/1939-4551-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Vieille S., Hourihane J.O., Baumert J.L. Precautionary allergen labeling: what advice is available for health care professionals, allergists, and allergic consumers? J Allergy Clin Immunol Pract. 2023;11(4):977–985. doi: 10.1016/j.jaip.2022.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Nowak-Węgrzyn A., Chehade M., Groetch M.E., et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy. Asthma & Immunology. The Journal of allergy and clinical immunology. 2017;139(4) doi: 10.1016/j.jaci.2016.12.966. 1111-26.e4. [DOI] [PubMed] [Google Scholar]
- 16.Ford L.S., Taylor S.L., Pacenza R., Niemann L.M., Lambrecht D.M., Sicherer S.H. Food allergen advisory labeling and product contamination with egg, milk, and peanut. J Allergy Clin Immunol. 2010;126(2):384–385. doi: 10.1016/j.jaci.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Remington B.C., Baumert J.L., Blom W.M., Houben G.F., Taylor S.L., Kruizinga A.G. Unintended allergens in precautionary labelled and unlabelled products pose significant risks to UK allergic consumers. Allergy. 2015;70(7):813–819. doi: 10.1111/all.12625. [DOI] [PubMed] [Google Scholar]
- 18.Robertson O.N., Hourihane J.O., Remington B.C., Baumert J.L., Taylor S.L. Survey of peanut levels in selected Irish food products bearing peanut allergen advisory labels. Food Addit Contam Part A, Chemistry, analysis, control, exposure & risk assessment. 2013;30(9):1467–1472. doi: 10.1080/19440049.2013.804953. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd M., Loke P., Mack D.P., et al. Varying approaches to management of IgE-mediated food allergy in children around the world. J Allergy Clin Immunol Pract. 2023;11(4) doi: 10.1016/j.jaip.2023.01.049. 1010-27.e6. [DOI] [PubMed] [Google Scholar]
- 20.Groetch M., Venter C., Skypala I., et al. Dietary therapy and nutrition management of eosinophilic esophagitis: a work group report of the American Academy of allergy, asthma, and immunology. J Allergy Clin Immunol Pract. 2017;5(2) doi: 10.1016/j.jaip.2016.12.026. 312-24.e29. [DOI] [PubMed] [Google Scholar]
- 21.Turner P.J., Baumert J.L., Beyer K., et al. Can we identify patients at risk of life-threatening allergic reactions to food? Allergy. 2016;71(9):1241–1255. doi: 10.1111/all.12924. [DOI] [PubMed] [Google Scholar]
- 22.Polloni L., Toniolo A., Lazzarotto F., et al. Nutritional behavior and attitudes in food allergic children and their mothers. Clin Transl Allergy. 2013;3(1):41. doi: 10.1186/2045-7022-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leroux H., Pernice S., Dufresne É., et al. Impact of a dietitian-led counseling program to support transition to whole foods during oral immunotherapy. J Allergy Clin Immunol Pract. 2020;8(6) doi: 10.1016/j.jaip.2020.02.014. 2107-9.e3. [DOI] [PubMed] [Google Scholar]
- 24.Groetch M., Mudd K., Woch M., et al. Retail food equivalents for post-oral immunotherapy dosing in the omalizumab as monotherapy and as adjunct therapy to multi-allergen oral immunotherapy in food-allergic children and adults (OUtMATCH) clinical trial. J Allergy Clin Immunol Pract. 2023;11(2):572. doi: 10.1016/j.jaip.2022.10.022. 80.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak-Wegrzyn A., Bloom K.A., Sicherer S.H., et al. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008;122(2) doi: 10.1016/j.jaci.2008.05.043. 342-347, 7.e1-2. [DOI] [PubMed] [Google Scholar]
- 26.Valluzzi R.L., Riccardi C., Arasi S., et al. Cow's milk and egg protein threshold dose distributions in children tolerant to beef, baked milk, and baked egg. Allergy. 2022;77(10):3052–3060. doi: 10.1111/all.15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sicherer S.H., Warren C.M., Dant C., Gupta R.S., Nadeau K.C. Food allergy from infancy through adulthood. J Allergy Clin Immunol Pract. 2020;8(6):1854–1864. doi: 10.1016/j.jaip.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upton J., Nowak-Wegrzyn A. The impact of baked egg and baked milk diets on IgE- and non-IgE-mediated allergy. Clin Rev Allergy Immunol. 2018;55(2):118–138. doi: 10.1007/s12016-018-8669-0. [DOI] [PubMed] [Google Scholar]
- 29.Nowak-Węgrzyn A., Lawson K., Masilamani M., Kattan J., Bahnson H.T., Sampson H.A. Increased tolerance to less extensively heat-denatured (baked) milk products in milk-allergic children. J Allergy Clin Immunol Pract. 2018;6(2) doi: 10.1016/j.jaip.2017.10.021. 486-95.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J.S., Nowak-Węgrzyn A., Sicherer S.H., Noone S., Moshier E.L., Sampson H.A. Dietary baked milk accelerates the resolution of cow's milk allergy in children. J Allergy Clin Immunol. 2011;128(1) doi: 10.1016/j.jaci.2011.04.036. 125-31.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowak-Wegrzyn A., Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009;9(3):234–237. doi: 10.1097/ACI.0b013e32832b88e7. [DOI] [PubMed] [Google Scholar]
- 32.Miceli Sopo S., Greco M., Monaco S., et al. Matrix effect on baked milk tolerance in children with IgE cow milk allergy. Allergol Immunopathol. 2016;44(6):517–523. doi: 10.1016/j.aller.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Hindley J.P., Oliver M.A., Thorpe C., Cullinane A., Wuenschmann S., Chapman M.D. Bos d 11 in baked milk poses a risk for adverse reactions in milk-allergic patients. Clin Exp Allergy : journal of the British Society for Allergy and Clinical Immunology. 2021;51(1):132–140. doi: 10.1111/cea.13774. [DOI] [PubMed] [Google Scholar]
- 34.De Boer R., Cartledge N., Lazenby S., et al. Specific IgE as the best predictor of the outcome of challenges to baked milk and baked egg. J Allergy Clin Immunol Pract. 2020;8(4):1459. doi: 10.1016/j.jaip.2019.10.039. 61.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bird J.A., Leonard S., Groetch M., et al. Conducting an oral food challenge: an update to the 2009 adverse reactions to foods committee work group report. J Allergy Clin Immunol Pract. 2020;8(1):75–90.e17. doi: 10.1016/j.jaip.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 36.Mehr S., Turner P.J., Joshi P., Wong M., Campbell D.E. Safety and clinical predictors of reacting to extensively heated cow's milk challenge in cow's milk-allergic children. Ann Allergy Asthma Immunol : official publication of the American College of Allergy, Asthma, & Immunology. 2014;113(4):425–429. doi: 10.1016/j.anai.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Ball H.B., Luyt D. Home-based cow's milk reintroduction using a milk ladder in children less than 3 years old with IgE-mediated cow's milk allergy. Clin Exp Allergy : journal of the British Society for Allergy and Clinical Immunology. 2019;49(6):911–920. doi: 10.1111/cea.13366. [DOI] [PubMed] [Google Scholar]
- 38.Mack D.P., Chan E.S., Shaker M., et al. Novel approaches to food allergy management during COVID-19 inspire long-term change. J Allergy Clin Immunol Pract. 2020;8(9):2851–2857. doi: 10.1016/j.jaip.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagida N., Sato S., Nagakura K.I., Asaumi T., Ebisawa M. Oral food challenge using different target doses and time intervals between doses. Curr Opin Allergy Clin Immunol. 2018;18(3):222–227. doi: 10.1097/ACI.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 40.van Ginkel C.D., Pettersson M.E., Dubois A.E.J., Koppelman G.H. Association of STAT6 gene variants with food allergy diagnosed by double-blind placebo-controlled food challenges. Allergy. 2018;73(6):1337–1341. doi: 10.1111/all.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arasi S., Nurmatov U., Dunn-Galvin A., et al. Consensus on DEfinition of Food Allergy SEverity (DEFASE) an integrated mixed methods systematic review. The World Allergy Organization journal. 2021;14(3) doi: 10.1016/j.waojou.2020.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Høst A., Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy. 1990;45(8):587–596. doi: 10.1111/j.1398-9995.1990.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 43.Järvinen K.M., Martin H., Oyoshi M.K. Immunomodulatory effects of breast milk on food allergy. Ann Allergy Asthma Immunol : official publication of the American College of Allergy, Asthma, & Immunology. 2019;123(2):133–143. doi: 10.1016/j.anai.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorva R., Mäkinen-Kiljunen S., Juntunen-Backman K. Beta-lactoglobulin secretion in human milk varies widely after cow's milk ingestion in mothers of infants with cow's milk allergy. J Allergy Clin Immunol. 1994;93(4):787–792. doi: 10.1016/0091-6749(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 45.Meyer R., Chebar Lozinsky A., Fleischer D.M., et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants-An EAACI Position Paper. Allergy. 2020;75(1):14–32. doi: 10.1111/all.13947. [DOI] [PubMed] [Google Scholar]
- 46.Martin V.M., Virkud Y.V., Seay H., et al. Prospective assessment of pediatrician-diagnosed food protein-induced allergic proctocolitis by gross or occult blood. J Allergy Clin Immunol Pract. 2020;8(5):1692. doi: 10.1016/j.jaip.2019.12.029. 9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McWilliam V., Netting M.J., Volders E., Palmer D.J. World allergy organization (WAO) diagnosis and Rationale for action against cow's milk allergy (DRACMA) guidelines update - X - breastfeeding a baby with cow's milk allergy. The World Allergy Organization journal. 2023;16(11) doi: 10.1016/j.waojou.2023.100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munblit D., Perkin M.R., Palmer D.J., Allen K.J., Boyle R.J. Assessment of evidence about common infant symptoms and cow's milk allergy. JAMA Pediatr. 2020;174(6):599–608. doi: 10.1001/jamapediatrics.2020.0153. [DOI] [PubMed] [Google Scholar]
- 49.Netting M.J., Allen K.J. Reconciling breast-feeding and early food introduction guidelines in the prevention and management of food allergy. J Allergy Clin Immunol. 2019;144(2):397–400.e1. doi: 10.1016/j.jaci.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Agriculture UDo. Dietary Guidelines for Americans: DGA 2020 - 2025.
- 51.Asai Y., Yanishevsky Y., Clarke A., et al. Rate, triggers, severity and management of anaphylaxis in adults treated in a Canadian emergency department. Int Arch Allergy Immunol. 2014;164(3):246–252. doi: 10.1159/000365631. [DOI] [PubMed] [Google Scholar]
- 52.Fiocchi A., Dahda L., Dupont C., Campoy C., Fierro V., Nieto A. Cow's milk allergy: towards an update of DRACMA guidelines. The World Allergy Organization journal. 2016;9(1):35. doi: 10.1186/s40413-016-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allergies Turck D., Bresson J.-L., Burlingame B., Dean T., et al. EFSA Panel of Dietetic Products, Nutrition and Allergies Scientific and technical guidance for the preparation and presentation of an application for authorisation of an infant and/or follow-on formula manufactured from protein hydrolysates. 2017;15(5) doi: 10.2903/j.efsa.2017.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.(FDA) UFaDa. Infant Formula Guidance Documents & Regulatory Information.
- 55.Høst A., Koletzko B., Dreborg S., et al. Dietary products used in infants for treatment and prevention of food allergy. Joint statement of the European society for paediatric allergology and clinical immunology (ESPACI) committee on hypoallergenic formulas and the European society for paediatric gastroenterology, Hepatology and nutrition (ESPGHAN) committee on nutrition. Arch Dis Child. 1999;81(1):80–84. doi: 10.1136/adc.81.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Co Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106(2):346–349. [PubMed] [Google Scholar]
- 57.Chatchatee P., Nowak-Wegrzyn A., Lange L., et al. Tolerance development in cow's milk-allergic infants receiving amino acid-based formula: a randomized controlled trial. J Allergy Clin Immunol. 2022;149(2) doi: 10.1016/j.jaci.2021.06.025. 650-8.e5. [DOI] [PubMed] [Google Scholar]
- 58.Nocerino R., Di Costanzo M., Bedogni G., et al. Dietary treatment with extensively hydrolyzed casein formula containing the probiotic Lactobacillus rhamnosus GG prevents the occurrence of functional gastrointestinal disorders in children with cow's milk allergy. J Pediatr. 2019;213:137. doi: 10.1016/j.jpeds.2019.06.004. 42.e2. [DOI] [PubMed] [Google Scholar]
- 59.Berni Canani R., Nocerino R., Terrin G., et al. Effect of Lactobacillus GG on tolerance acquisition in infants with cow's milk allergy: a randomized trial. J Allergy Clin Immunol. 2012;129(2):580–582. doi: 10.1016/j.jaci.2011.10.004. 2.e1-5. [DOI] [PubMed] [Google Scholar]
- 60.Candy D.C.A., Van Ampting M.T.J., Oude Nijhuis M.M., et al. A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants. Pediatr Res. 2018;83(3):677–686. doi: 10.1038/pr.2017.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niggemann B., von Berg A., Bollrath C., et al. Safety and efficacy of a new extensively hydrolyzed formula for infants with cow's milk protein allergy. Pediatr Allergy Immunol : official publication of the European Society of Pediatric Allergy and Immunology. 2008;19(4):348–354. doi: 10.1111/j.1399-3038.2007.00653.x. [DOI] [PubMed] [Google Scholar]
- 62.Stróżyk A., Horvath A., Meyer R., Szajewska H. Efficacy and safety of hydrolyzed formulas for cow's milk allergy management: a systematic review of randomized controlled trials. Clin Exp Allergy : journal of the British Society for Allergy and Clinical Immunology. 2020;50(7):766–779. doi: 10.1111/cea.13669. [DOI] [PubMed] [Google Scholar]
- 63.Rossetti D., Cucchiara S., Morace A., Leter B., Oliva S. Hypoallergenicity of a thickened hydrolyzed formula in children with cow's milk allergy. World journal of clinical cases. 2019;7(16):2256–2268. doi: 10.12998/wjcc.v7.i16.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salvia G., De Vizia B., Manguso F., et al. Effect of intragastric volume and osmolality on mechanisms of gastroesophageal reflux in children with gastroesophageal reflux disease. Am J Gastroenterol. 2001;96(6):1725–1732. doi: 10.1111/j.1572-0241.2001.03865.x. [DOI] [PubMed] [Google Scholar]
- 65.Vandenplas Y., Broekaert I., Domellöf M., et al. An ESPGHAN position paper on the diagnosis, management and prevention of cow’s milk allergy. J Pediatr Gastroenterol Nutr. 2023 Jul 26 doi: 10.1097/MPG.0000000000003897. [DOI] [PubMed] [Google Scholar]
- 66.Meyer R., Groetch M., Venter C. When should infants with cow's milk protein allergy use an amino acid formula? A practical guide. J Allergy Clin Immunol Pract. 2018;6(2):383–399. doi: 10.1016/j.jaip.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Fiocchi A., Brozek J., Schünemann H., et al. World allergy organization (WAO) diagnosis and Rationale for action against cow's milk allergy (DRACMA) guidelines. Pediatr Allergy Immunol : official publication of the European Society of Pediatric Allergy and Immunology. 2010;21(Suppl 21):1–125. doi: 10.1111/j.1399-3038.2010.01068.x. [DOI] [PubMed] [Google Scholar]
- 68.Jensen V.B., Jørgensen I.M., Rasmussen K.B., Mølgaard C., Prahl P. Bone mineral status in children with cow milk allergy. Pediatr Allergy Immunol : official publication of the European Society of Pediatric Allergy and Immunology. 2004;15(6):562–565. doi: 10.1111/j.1399-3038.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 69.Mailhot G., Perrone V., Alos N., et al. Cow's milk allergy and bone mineral density in prepubertal children. Pediatrics. 2016;137(5) doi: 10.1542/peds.2015-1742. [DOI] [PubMed] [Google Scholar]
- 70.Meyer R., Carey M.P., Turner P.J., Meharg A.A. Low inorganic arsenic in hydrolysed rice formula used for cow's milk protein allergy. Pediatr Allergy Immunol : official publication of the European Society of Pediatric Allergy and Immunology. 2018;29(5):561–563. doi: 10.1111/pai.12913. [DOI] [PubMed] [Google Scholar]
- 71.Agostoni C., Axelsson I., Goulet O., et al. Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2006;42(4):352–361. doi: 10.1097/01.mpg.0000189358.38427.cd. [DOI] [PubMed] [Google Scholar]
- 72.Meyer R., Fleming C., Dominguez-Ortega G., et al. Manifestations of food protein induced gastrointestinal allergies presenting to a single tertiary paediatric gastroenterology unit. The World Allergy Organization journal. 2013;6(1):13. doi: 10.1186/1939-4551-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DoH U. Advice Issued on Soya- Based Infant Formulas.
- 74.Testa I., Salvatori C., Di Cara G., et al. Soy-based infant formula: are phyto-oestrogens still in doubt? Front Nutr. 2018;5:110. doi: 10.3389/fnut.2018.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pedrosa M., Pascual C.Y., Larco J.I., Esteban M.M. Palatability of hydrolysates and Other substitution formulas for cow's milk-allergic children: a comparative study of taste, smell, and texture evaluated by healthy volunteers. J Invest Allergol Clin Immunol. 2006;16(6):351–356. [PubMed] [Google Scholar]
- 76.Vandenplas Y., Castrellon P.G., Rivas R., et al. Safety of soya-based infant formulas in children. Br J Nutr. 2014;111(8):1340–1360. doi: 10.1017/S0007114513003942. [DOI] [PubMed] [Google Scholar]
- 77.Merritt R.J., Fleet S.E., Fifi A., et al. North American society for pediatric gastroenterology, Hepatology, and nutrition position paper: plant-based milks. J Pediatr Gastroenterol Nutr. 2020;71(2):276–281. doi: 10.1097/MPG.0000000000002799. [DOI] [PubMed] [Google Scholar]
- 78.Agostoni C., Terracciano L., Varin E., Fiocchi A. The nutritional value of protein-hydrolyzed formulae. Crit Rev Food Sci Nutr. 2016;56(1):65–69. doi: 10.1080/10408398.2012.713047. [DOI] [PubMed] [Google Scholar]
- 79.Meyer R., De Koker C., Dziubak R., et al. The impact of the elimination diet on growth and nutrient intake in children with food protein induced gastrointestinal allergies. Clin Transl Allergy. 2016;6:25. doi: 10.1186/s13601-016-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maslin K., Oliver E.M., Scally K.S., et al. Nutritional adequacy of a cows' milk exclusion diet in infancy. Clin Transl Allergy. 2016;6:20. doi: 10.1186/s13601-016-0109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nachshon L., Goldberg M.R., Schwartz N., et al. Decreased bone mineral density in young adult IgE-mediated cow's milk-allergic patients. J Allergy Clin Immunol. 2014;134(5) doi: 10.1016/j.jaci.2014.06.026. 1108-13.e3. [DOI] [PubMed] [Google Scholar]
- 82.Verduci E., D'Elios S., Cerrato L., et al. Cow's milk substitutes for children: nutritional aspects of milk from different mammalian species, special formula and plant-based beverages. Nutrients. 2019;11(8) doi: 10.3390/nu11081739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Groetch M., Venter C. Nutritional management of food allergies. Journal of Food Allergy. 2020;2:131–141. doi: 10.2500/jfa.2020.2.200032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bailey S., Albardiaz R., Frew A.J., Smith H. Restaurant staff's knowledge of anaphylaxis and dietary care of people with allergies. Clin Exp Allergy : journal of the British Society for Allergy and Clinical Immunology. 2011;41(5):713–717. doi: 10.1111/j.1365-2222.2011.03748.x. [DOI] [PubMed] [Google Scholar]
- 85.Koletzko S., Niggemann B., Arato A., et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221–229. doi: 10.1097/MPG.0b013e31825c9482. [DOI] [PubMed] [Google Scholar]
- 86.Muraro A., de Silva D., Halken S., et al. Managing food allergy: GA(2)LEN guideline 2022. The World Allergy Organization journal. 2022;15(9) doi: 10.1016/j.waojou.2022.100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diaferio L., Caimmi D., Verga M.C., et al. May failure to thrive in infants Be a clinical marker for the early diagnosis of cow's milk allergy? Nutrients. 2020;12(2) doi: 10.3390/nu12020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Skypala I.J., Venter C., Meyer R., et al. The development of a standardised diet history tool to support the diagnosis of food allergy. Clin Transl Allergy. 2015;5:7. doi: 10.1186/s13601-015-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isolauri E., Sütas Y., Salo M.K., Isosomppi R., Kaila M. Elimination diet in cow's milk allergy: risk for impaired growth in young children. J Pediatr. 1998;132(6):1004–1009. doi: 10.1016/s0022-3476(98)70399-3. [DOI] [PubMed] [Google Scholar]
- 90.Meyer R., Wright K., Vieira M.C., et al. International survey on growth indices and impacting factors in children with food allergies. J Hum Nutr Diet : the official journal of the British Dietetic Association. 2019;32(2):175–184. doi: 10.1111/jhn.12610. [DOI] [PubMed] [Google Scholar]
- 91.Meyer R., De Koker C., Dziubak R., et al. Malnutrition in children with food allergies in the UK. J Hum Nutr Diet : the official journal of the British Dietetic Association. 2014;27(3):227–235. doi: 10.1111/jhn.12149. [DOI] [PubMed] [Google Scholar]
- 92.Robbins K.A., Wood R.A., Keet C.A. Persistent cow's milk allergy is associated with decreased childhood growth: a longitudinal study. J Allergy Clin Immunol. 2020;145(2) doi: 10.1016/j.jaci.2019.10.028. 713-6.e4. [DOI] [PubMed] [Google Scholar]
- 93.Sinai T., Goldberg M.R., Nachshon L., et al. Reduced final height and inadequate nutritional intake in cow's milk-allergic young adults. J Allergy Clin Immunol Pract. 2019;7(2):509–515. doi: 10.1016/j.jaip.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 94.Grenov B., Michaelsen K.F. Growth components of cow's milk: emphasis on effects in undernourished children. Food Nutr Bull. 2018;39(2_suppl):S45–S53. doi: 10.1177/0379572118772766. [DOI] [PubMed] [Google Scholar]
- 95.Beck C., Koplin J., Dharmage S., et al. Persistent food allergy and food allergy coexistent with eczema is associated with reduced growth in the first 4 Years of life. J Allergy Clin Immunol Pract. 2016;4(2) doi: 10.1016/j.jaip.2015.08.009. 248-56.e3. [DOI] [PubMed] [Google Scholar]
- 96.Niggemann B., Binder C., Dupont C., Hadji S., Arvola T., Isolauri E. Prospective, controlled, multi-center study on the effect of an amino-acid-based formula in infants with cow's milk allergy/intolerance and atopic dermatitis. Pediatr Allergy Immunol : official publication of the European Society of Pediatric Allergy and Immunology. 2001;12(2):78–82. doi: 10.1034/j.1399-3038.2001.012002078.x. [DOI] [PubMed] [Google Scholar]
- 97.Agostoni C., Fiocchi A., Riva E., et al. Growth of infants with IgE-mediated cow's milk allergy fed different formulas in the complementary feeding period. Pediatr Allergy Immunol : official publication of the European Society of Pediatric Allergy and Immunology. 2007;18(7):599–606. doi: 10.1111/j.1399-3038.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 98.Mahmoud A.B., Hack-Polay D., Fuxman L., Naquiallah D., Grigoriou N. Trick or treat? – when children with childhood food allergies lead parents into unhealthy food choices. BMC Publ Health. 2020;20(1):1453. doi: 10.1186/s12889-020-09556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meyer R. Nutritional disorders resulting from food allergy in children. Pediatr Allergy Immunol : official publication of the European Society of Pediatric Allergy and Immunology. 2018;29(7):689–704. doi: 10.1111/pai.12960. [DOI] [PubMed] [Google Scholar]
- 100.Yu J.W., Pekeles G., Legault L., McCusker C.T. Milk allergy and vitamin D deficiency rickets: a common disorder associated with an uncommon disease. Ann Allergy Asthma Immunol : official publication of the American College of Allergy, Asthma, & Immunology. 2006;96(4):615–619. doi: 10.1016/S1081-1206(10)63558-2. [DOI] [PubMed] [Google Scholar]
- 101.Thomassen R.A., Kvammen J.A., Eskerud M.B., Júlíusson P.B., Henriksen C., Rugtveit J. Iodine status and growth in 0-2-year-old infants with cow's milk protein allergy. J Pediatr Gastroenterol Nutr. 2017;64(5):806–811. doi: 10.1097/MPG.0000000000001434. [DOI] [PubMed] [Google Scholar]
- 102.Ojuawo A., Lindley K.J., Milla P.J. Serum zinc, selenium and copper concentration in children with allergic colitis. East Afr Med J. 1996;73(4):236–238. [PubMed] [Google Scholar]
- 103.Lai F.P., Yang Y.J. The prevalence and characteristics of cow's milk protein allergy in infants and young children with iron deficiency anemia. Pediatrics and neonatology. 2018;59(1):48–52. doi: 10.1016/j.pedneo.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 104.Solomon J., Kamalammal R., Sait M.Y., Lohith H. Cow's milk protein allergy mimicking acrodermatitis enteropathica. J Clin Diagn Res : J Clin Diagn Res. 2014;8(3):160–161. doi: 10.7860/JCDR/2014/7925.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meyer R., De Koker C., Dziubak R., et al. A practical approach to vitamin and mineral supplementation in food allergic children. Clin Transl Allergy. 2015;5:11. doi: 10.1186/s13601-015-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meyer R., De Koker C., Dziubak R., Godwin H., Dominguez-Ortega G., Shah N. Dietary elimination of children with food protein induced gastrointestinal allergy - micronutrient adequacy with and without a hypoallergenic formula? Clin Transl Allergy. 2014;4(1):31. doi: 10.1186/2045-7022-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mukkada V.A., Haas A., Maune N.C., et al. Feeding dysfunction in children with eosinophilic gastrointestinal diseases. Pediatrics. 2010;126(3):e672–e677. doi: 10.1542/peds.2009-2227. [DOI] [PubMed] [Google Scholar]
- 108.Chehade M., Meyer R., Beauregard A. Feeding difficulties in children with non-IgE-mediated food allergic gastrointestinal disorders. Ann Allergy Asthma Immunol : official publication of the American College of Allergy, Asthma, & Immunology. 2019;122(6):603–609. doi: 10.1016/j.anai.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meyer R., Rommel N., Van Oudenhove L., Fleming C., Dziubak R., Shah N. Feeding difficulties in children with food protein-induced gastrointestinal allergies. 2014;29(10):1764–1769. doi: 10.1111/jgh.12593. [DOI] [PubMed] [Google Scholar]
- 110.Zangen T., Ciarla C., Zangen S., et al. Gastrointestinal motility and sensory abnormalities may contribute to food refusal in medically fragile toddlers. J Pediatr Gastroenterol Nutr. 2003;37(3):287–293. doi: 10.1097/00005176-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 111.Mirotti L., Mucida D., de Sá-Rocha L.C., Costa-Pinto F.A., Russo M. Food aversion: a critical balance between allergen-specific IgE levels and taste preference. Brain Behav Immun. 2010;24(3):370–375. doi: 10.1016/j.bbi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 112.Costa-Pinto F.A., Basso A.S., Britto L.R., Malucelli B.E., Russo M. Avoidance behavior and neural correlates of allergen exposure in a murine model of asthma. Brain Behav Immun. 2005;19(1):52–60. doi: 10.1016/j.bbi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 113.Lopez N.V., Schembre S., Belcher B.R., et al. Parenting styles, food-related parenting practices, and children's healthy eating: a mediation analysis to examine relationships between parenting and child diet. Appetite. 2018;128:205–213. doi: 10.1016/j.appet.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maslin K., Dean T., Arshad S.H., Venter C. Fussy eating and feeding difficulties in infants and toddlers consuming a cows' milk exclusion diet. Pediatr Allergy Immunol : official publication of the European Society of Pediatric Allergy and Immunology. 2015;26(6):503–508. doi: 10.1111/pai.12427. [DOI] [PubMed] [Google Scholar]