Abstract
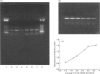
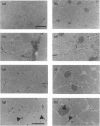
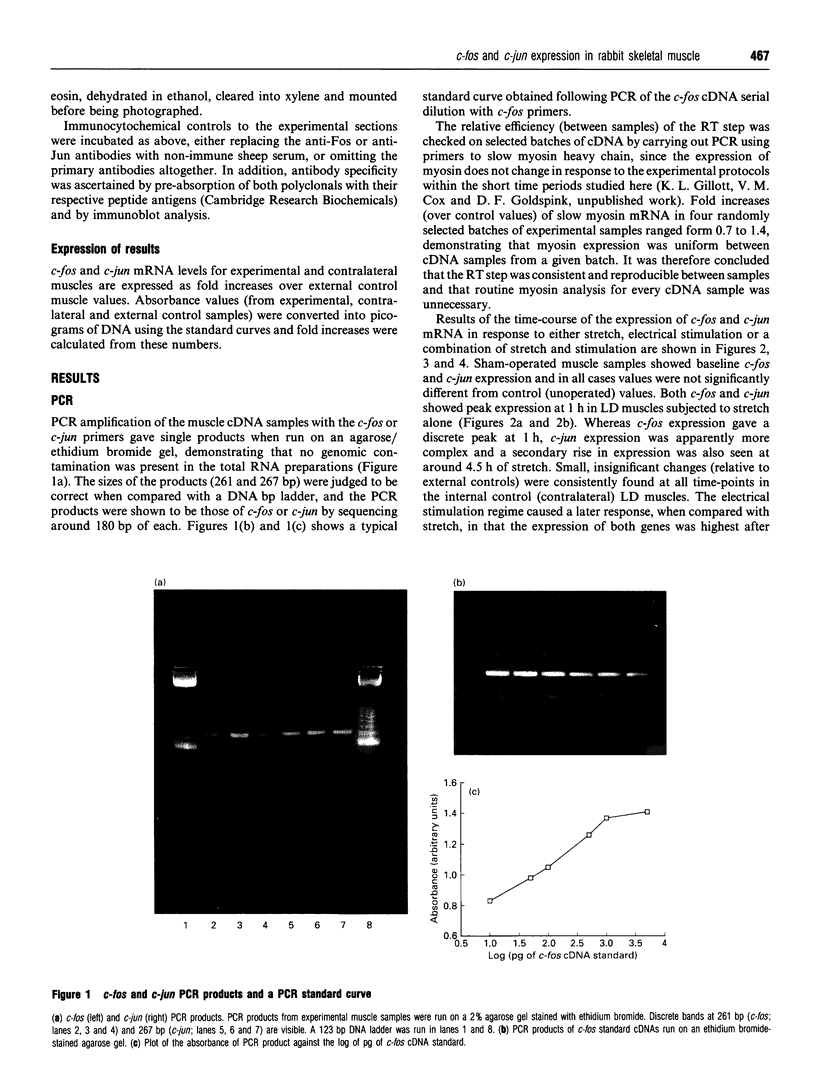
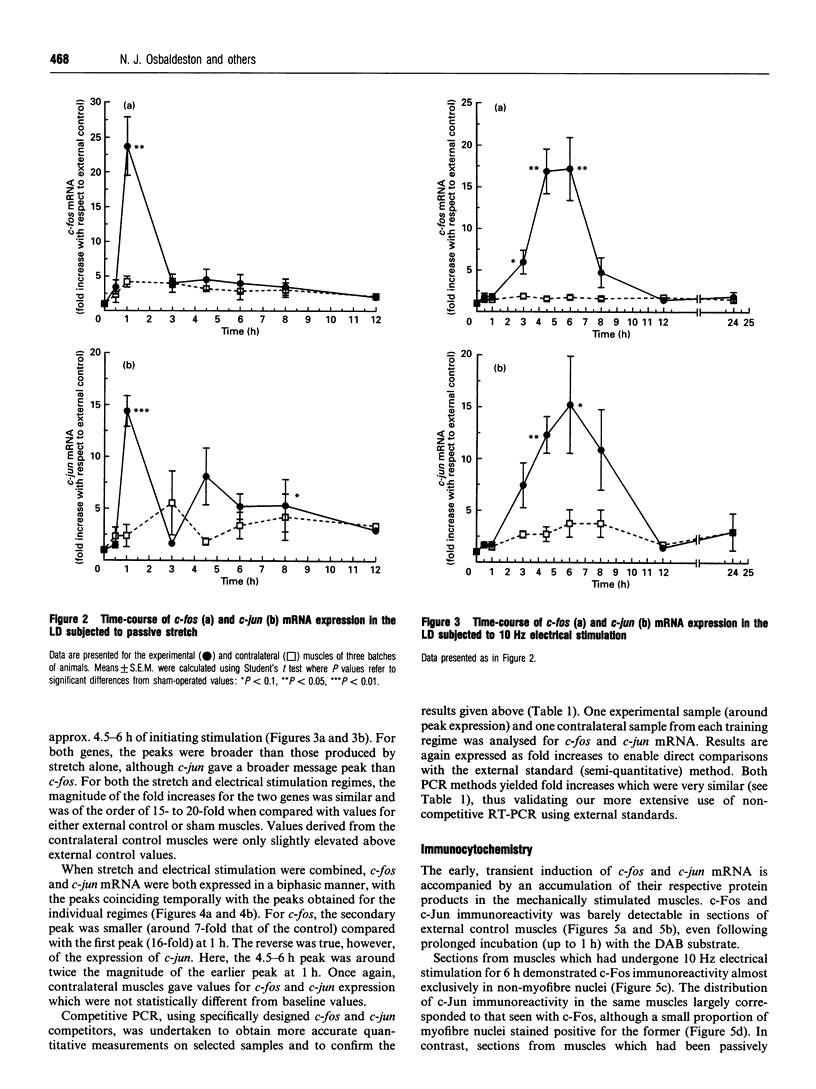
The levels of c-fos and c-jun mRNA were measured by reverse transcription PCR in the rabbit latissimus dorsi muscle following three separate training regimes, i.e. passive stretch, 10 Hz electrical stimulation or a combination of the two. Both c-fos and c-jun mRNA expression peaked at around 1 h after imposing stretch and at around 4.5-6 h after the initiation of electrical stimulation. The combined stretch/electrical stimulation regime induced biphasic expression of both c-fos and c-jun mRNA, with peaks coinciding temporally with those for the individual regimes. Immunostaining with anti-Fos and anti-Jun antibodies revealed the accumulation of these proteins in both myofibre and interstitial cell nuclei following passive stretch. In contrast, following electrical stimulation the localization of immunoreactive c-Fos and c-Jun proteins was predominantly in interstitial cell nuclei. c-Fos and c-Jun immunoreactivity was also clearly colocalized in a proportion of myonuclei from stretched muscle. These findings suggest that the rapid induction of c-fos and c-jun is an early event in response to mechanical stretch and might trigger [via activator protein-1 (AP-1) transcriptional factors] events leading to muscle fibre hypertrophy. However, the involvement of AP-1 in inducing the phenotypic changes in muscle fibres as a result of electrical stimulation appears less clear.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Bauters C., Moalic J. M., Bercovici J., Mouas C., Emanoil-Ravier R., Schiaffino S., Swynghedauw B. Coronary flow as a determinant of c-myc and c-fos proto-oncogene expression in an isolated adult rat heart. J Mol Cell Cardiol. 1988 Feb;20(2):97–101. doi: 10.1016/s0022-2828(88)80023-3. [DOI] [PubMed] [Google Scholar]
- Brownson C., Isenberg H., Brown W., Salmons S., Edwards Y. Changes in skeletal muscle gene transcription induced by chronic stimulation. Muscle Nerve. 1988 Nov;11(11):1183–1189. doi: 10.1002/mus.880111113. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Curran T., Gordon M. B., Rubino K. L., Sambucetti L. C. Isolation and characterization of the c-fos(rat) cDNA and analysis of post-translational modification in vitro. Oncogene. 1987;2(1):79–84. [PubMed] [Google Scholar]
- Eppley Z. A., Kim J., Russell B. A myogenic regulatory gene, qmf1, is expressed by adult myonuclei after injury. Am J Physiol. 1993 Aug;265(2 Pt 1):C397–C405. doi: 10.1152/ajpcell.1993.265.2.C397. [DOI] [PubMed] [Google Scholar]
- Gillott K. L., Cox V. M., Wright H., Eaves L. A., Williams P. E., Goldspink D. F. The fibre type composition of the rabbit latissimus dorsi muscle. J Anat. 1994 Aug;185(Pt 1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F., Cox V. M., Smith S. K., Eaves L. A., Osbaldeston N. J., Lee D. M., Mantle D. Muscle growth in response to mechanical stimuli. Am J Physiol. 1995 Feb;268(2 Pt 1):E288–E297. doi: 10.1152/ajpendo.1995.268.2.E288. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Easton J., Winterburn S. K., Williams P. E., Goldspink G. E. The role of passive stretch and repetitive electrical stimulation in preventing skeletal muscle atrophy while reprogramming gene expression to improve fatigue resistance. J Card Surg. 1991 Mar;6(1 Suppl):218–224. doi: 10.1111/jocs.1991.6.1s.218. [DOI] [PubMed] [Google Scholar]
- Hattori K., Angel P., Le Beau M. M., Karin M. Structure and chromosomal localization of the functional intronless human JUN protooncogene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9148–9152. doi: 10.1073/pnas.85.23.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh J. E., Whitelaw P. F. The role of cellular oncogenes in myogenesis and muscle cell hypertrophy. Int J Biochem. 1992 Feb;24(2):193–203. doi: 10.1016/0020-711x(92)90247-x. [DOI] [PubMed] [Google Scholar]
- Ianuzzo C. D., Hamilton N., O'Brien P. J., Desrosiers C., Chiu R. Biochemical transformation of canine skeletal muscle for use in cardiac-assist devices. J Appl Physiol (1985) 1990 Apr;68(4):1481–1485. doi: 10.1152/jappl.1990.68.4.1481. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. B., Taneja K., Singer R. H. Temporal resolution and sequential expression of muscle-specific genes revealed by in situ hybridization. Dev Biol. 1989 May;133(1):235–246. doi: 10.1016/0012-1606(89)90314-x. [DOI] [PubMed] [Google Scholar]
- Lucibello F. C., Lowag C., Neuberg M., Müller R. trans-repression of the mouse c-fos promoter: a novel mechanism of Fos-mediated trans-regulation. Cell. 1989 Dec 22;59(6):999–1007. doi: 10.1016/0092-8674(89)90756-3. [DOI] [PubMed] [Google Scholar]
- Osbaldeston N. J., Lee D. M., Cox V. M., Eaves L., Morrison J. F., Hesketh J., Goldspink D. F. The temporal expression of cellular oncogenes in mechanically stimulated muscle. Biochem Soc Trans. 1993 Nov;21(4):367S–367S. doi: 10.1042/bst021367s. [DOI] [PubMed] [Google Scholar]
- Pette D., Vrbová G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve. 1985 Oct;8(8):676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- Pollack P. S., Houser S. R., Budjak R., Goldman B. c-myc gene expression is localized to the myocyte following hemodynamic overload in vivo. J Cell Biochem. 1994 Jan;54(1):78–84. doi: 10.1002/jcb.240540109. [DOI] [PubMed] [Google Scholar]
- Ransone L. J., Verma I. M. Nuclear proto-oncogenes fos and jun. Annu Rev Cell Biol. 1990;6:539–557. doi: 10.1146/annurev.cb.06.110190.002543. [DOI] [PubMed] [Google Scholar]
- Schunkert H., Jahn L., Izumo S., Apstein C. S., Lorell B. H. Localization and regulation of c-fos and c-jun protooncogene induction by systolic wall stress in normal and hypertrophied rat hearts. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11480–11484. doi: 10.1073/pnas.88.24.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütte J., Viallet J., Nau M., Segal S., Fedorko J., Minna J. jun-B inhibits and c-fos stimulates the transforming and trans-activating activities of c-jun. Cell. 1989 Dec 22;59(6):987–997. doi: 10.1016/0092-8674(89)90755-1. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Larrick J. W. PCR MIMICS: competitive DNA fragments for use as internal standards in quantitative PCR. Biotechniques. 1993 Feb;14(2):244–249. [PubMed] [Google Scholar]
- Whitelaw P. F., Hesketh J. E. Expression of c-myc and c-fos in rat skeletal muscle. Evidence for increased levels of c-myc mRNA during hypertrophy. Biochem J. 1992 Jan 1;281(Pt 1):143–147. doi: 10.1042/bj2810143. [DOI] [PMC free article] [PubMed] [Google Scholar]