Abstract
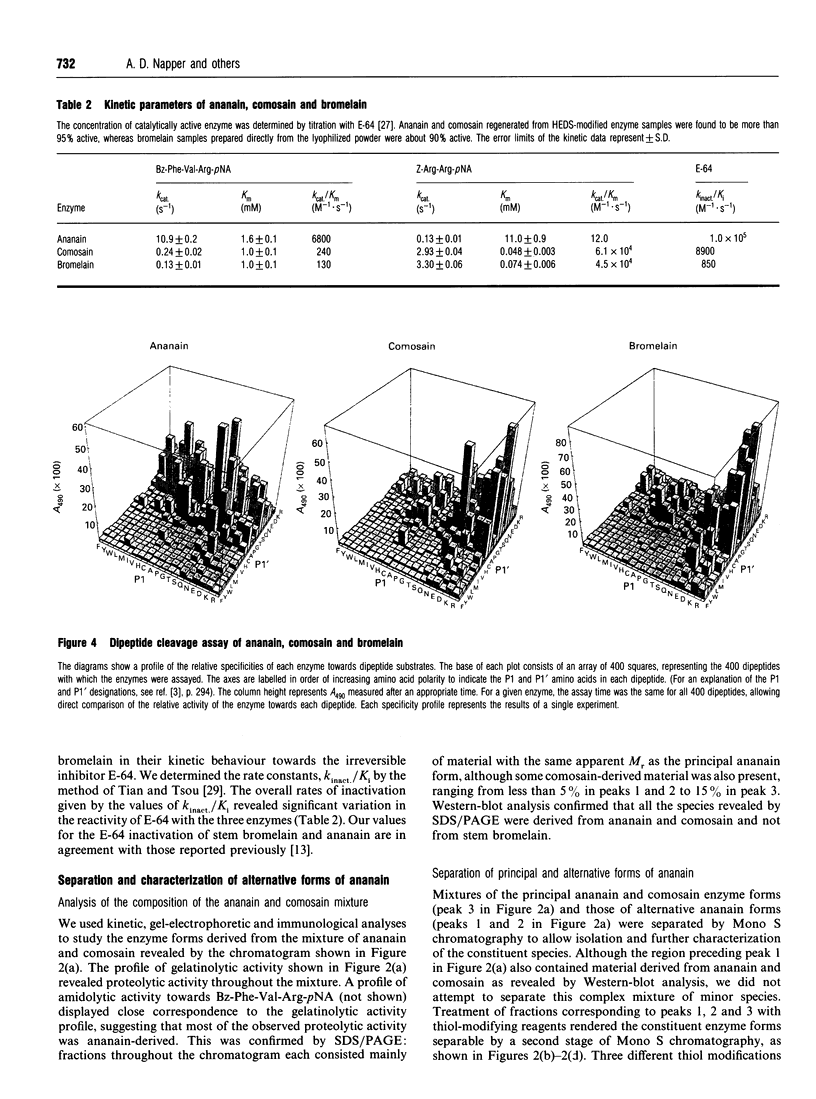
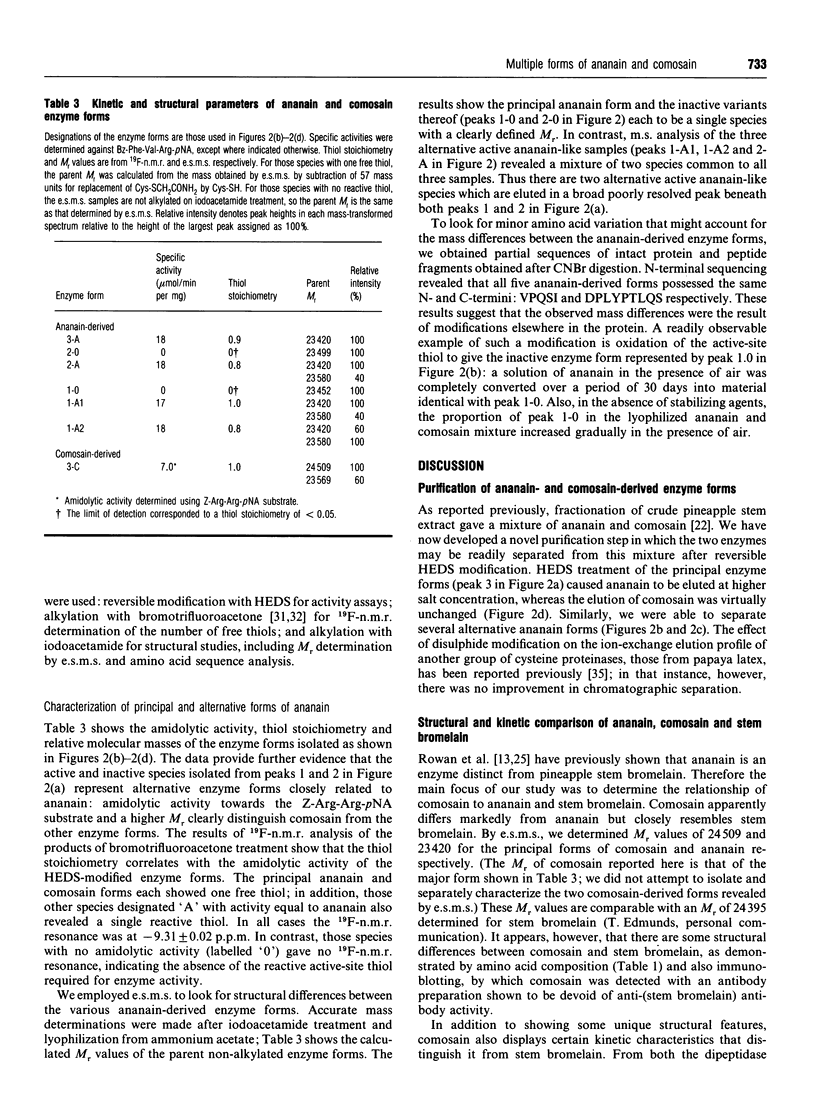
A mixture of ananain (EC 3.4.22.31) and comosain purified from crude pineapple stem extract was found to contain numerous closely related enzyme forms. Chromatographic separation of the major enzyme forms was achieved after treatment of the mixture with thiol-modifying reagents: reversible modification with 2-hydroxyethyl disulphide provided enzyme for kinetic studies, and irreversible alkylation with bromotrifluoroacetone or iodoacetamide gave enzyme for structural analyses by 19F-n.m.r. and electrospray mass spectrometry respectively. Structural and kinetic analyses revealed comosain to be closely related to stem bromelain (EC 3.4.22.32), whereas ananain differed markedly from both comosain and stem bromelain. Nevertheless, differences were seen between comosain and stem bromelain in amino acid composition and kinetic specificity towards the epoxide inhibitor E-64. Differences between five isolatable alternative forms of ananain were characterized by amidolytic activity, thiol stoichiometry and accurate mass determinations. Three of the enzyme forms displayed ananain-like amidolytic activity, whereas the other two forms were inactive. Thiol-stoichiometry determinations revealed that the active enzyme forms contained one free thiol, whereas the inactive forms lacked the reactive thiol required for enzyme activity. M.s. provided direct evidence for oxidation of the active-site thiol to the corresponding sulphinic acid.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Buttle D. J. Names and numbers of papaya proteinases. Biochem J. 1985 Jun 1;228(2):527–527. doi: 10.1042/bj2280527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall M. R., Lowe G. Co-operative ionisation of aspartic-acid-158 and histidine-159 in papain. Evidence from 19F nuclear-magnetic-resonance and fluorescence spectroscopy. Eur J Biochem. 1976 Jun 1;65(2):481–491. doi: 10.1111/j.1432-1033.1976.tb10364.x. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Salih E., McKee R., Smith H. Fresh non-fruit latex of Carica papaya contains papain, multiple forms of chymopapain A and papaya proteinase omega. Biochem J. 1985 Jun 1;228(2):525–527. doi: 10.1042/bj2280525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Barrett A. J. Chymopapain. Chromatographic purification and immunological characterization. Biochem J. 1984 Oct 1;223(1):81–88. doi: 10.1042/bj2230081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando P. M., Brown M. A., Barrett A. J. Human thimet oligopeptidase. Biochem J. 1993 Sep 1;294(Pt 2):451–457. doi: 10.1042/bj2940451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelMar E. G., Largman C., Brodrick J. W., Geokas M. C. A sensitive new substrate for chymotrypsin. Anal Biochem. 1979 Nov 1;99(2):316–320. doi: 10.1016/s0003-2697(79)80013-5. [DOI] [PubMed] [Google Scholar]
- Donald L. J., Crane B. R., Anderson D. H., Duckworth H. W. The role of cysteine 206 in allosteric inhibition of Escherichia coli citrate synthase. Studies by chemical modification, site-directed mutagenesis, and 19F NMR. J Biol Chem. 1991 Nov 5;266(31):20709–20713. [PubMed] [Google Scholar]
- Duan Y., Laursen R. A. Protease substrate specificity mapping using membrane-bound peptides. Anal Biochem. 1994 Feb 1;216(2):431–438. doi: 10.1006/abio.1994.1064. [DOI] [PubMed] [Google Scholar]
- Dubois T., Jacquet A., Schnek A. G., Looze Y. The thiol proteinases from the latex of Carica papaya L. I. Fractionation, purification and preliminary characterization. Biol Chem Hoppe Seyler. 1988 Aug;369(8):733–740. doi: 10.1515/bchm3.1988.369.2.733. [DOI] [PubMed] [Google Scholar]
- Friedenson B., Liener I. E. The active site sequence of multiple forms of ficin. Arch Biochem Biophys. 1972 Mar;149(1):169–174. doi: 10.1016/0003-9861(72)90311-6. [DOI] [PubMed] [Google Scholar]
- Kossiakoff A. A. Tertiary structure is a principal determinant to protein deamidation. Science. 1988 Apr 8;240(4849):191–194. doi: 10.1126/science.3353715. [DOI] [PubMed] [Google Scholar]
- Kóródi I., Asbóth B., Polgár L. Disulfide bond formation between the active-site thiol and one of the several free thiol groups of chymopapain. Biochemistry. 1986 Nov 4;25(22):6895–6900. doi: 10.1021/bi00370a024. [DOI] [PubMed] [Google Scholar]
- Lynn K. R. The fractionation of bromelain. Anal Biochem. 1977 Jan;77(1):33–38. doi: 10.1016/0003-2697(77)90287-1. [DOI] [PubMed] [Google Scholar]
- Ménard R., Khouri H. E., Plouffe C., Laflamme P., Dupras R., Vernet T., Tessier D. C., Thomas D. Y., Storer A. C. Importance of hydrogen-bonding interactions involving the side chain of Asp158 in the catalytic mechanism of papain. Biochemistry. 1991 Jun 4;30(22):5531–5538. doi: 10.1021/bi00236a028. [DOI] [PubMed] [Google Scholar]
- Ota S., Muta E., Katahira Y., Okamoto Y. Reinvestigation of fractionation and some properties of the proteolytically active components of stem and fruit bromelains. J Biochem. 1985 Jul;98(1):219–228. doi: 10.1093/oxfordjournals.jbchem.a135261. [DOI] [PubMed] [Google Scholar]
- Polgár L., Csoma C. Dissociation of ionizing groups in the binding cleft inversely controls the endo- and exopeptidase activities of cathepsin B. J Biol Chem. 1987 Oct 25;262(30):14448–14453. [PubMed] [Google Scholar]
- Polgár L. Isolation of highly active papaya peptidases A and B from commercial chymopapain. Biochim Biophys Acta. 1981 Apr 14;658(2):262–269. doi: 10.1016/0005-2744(81)90296-5. [DOI] [PubMed] [Google Scholar]
- Polgár L. Problems of classification of papaya latex proteinases. Biochem J. 1984 Jul 15;221(2):555–556. doi: 10.1042/bj2210555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritonja A., Rowan A. D., Buttle D. J., Rawlings N. D., Turk V., Barrett A. J. Stem bromelain: amino acid sequence and implications for weak binding of cystatin. FEBS Lett. 1989 Apr 24;247(2):419–424. doi: 10.1016/0014-5793(89)81383-3. [DOI] [PubMed] [Google Scholar]
- Rowan A. D., Buttle D. J., Barrett A. J. Ananain: a novel cysteine proteinase found in pineapple stem. Arch Biochem Biophys. 1988 Nov 15;267(1):262–270. doi: 10.1016/0003-9861(88)90031-8. [DOI] [PubMed] [Google Scholar]
- Rowan A. D., Buttle D. J., Barrett A. J. The cysteine proteinases of the pineapple plant. Biochem J. 1990 Mar 15;266(3):869–875. [PMC free article] [PubMed] [Google Scholar]
- Rowan A. D., Christopher C. W., Kelley S. F., Buttle D. J., Ehrlich H. P. Debridement of experimental full-thickness skin burns of rats with enzyme fractions derived from pineapple stem. Burns. 1990 Aug;16(4):243–246. doi: 10.1016/0305-4179(90)90132-g. [DOI] [PubMed] [Google Scholar]
- Tian W. X., Tsou C. L. Determination of the rate constant of enzyme modification by measuring the substrate reaction in the presence of the modifier. Biochemistry. 1982 Mar 2;21(5):1028–1032. doi: 10.1021/bi00534a031. [DOI] [PubMed] [Google Scholar]
- Zucker S., Buttle D. J., Nicklin M. J., Barrett A. J. The proteolytic activities of chymopapain, papain, and papaya proteinase III. Biochim Biophys Acta. 1985 Apr 5;828(2):196–204. doi: 10.1016/0167-4838(85)90057-3. [DOI] [PubMed] [Google Scholar]