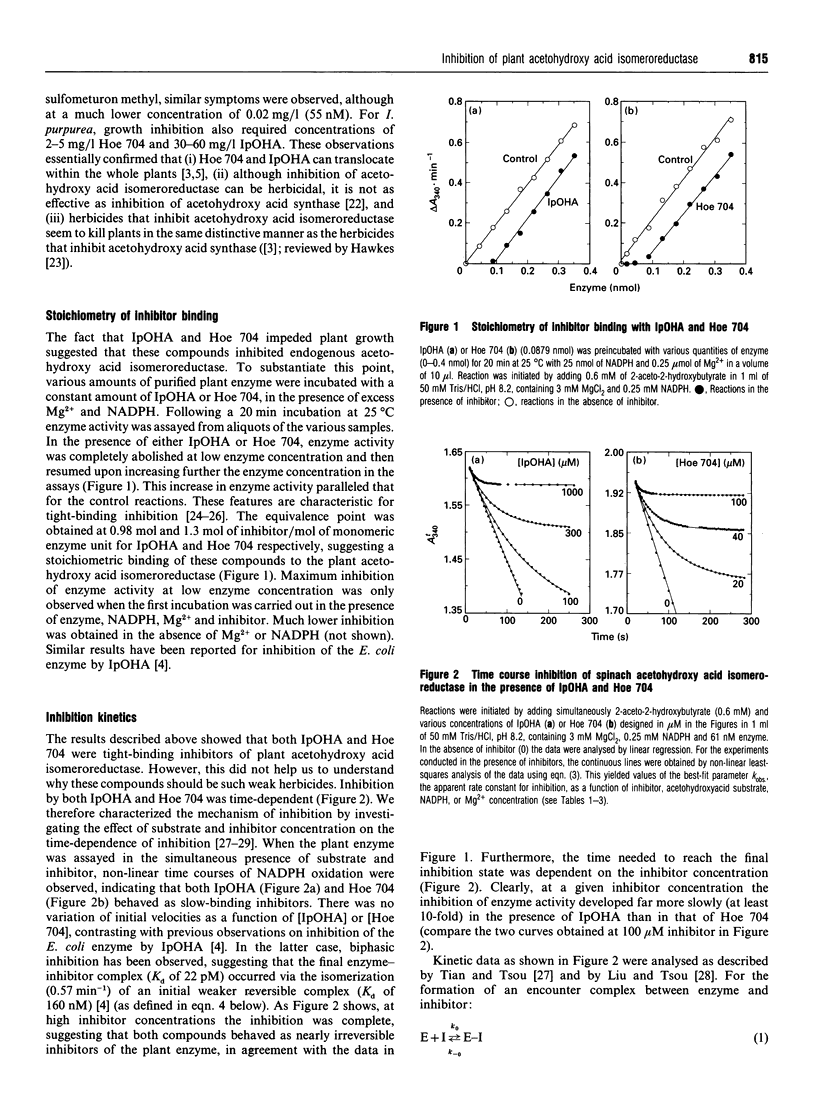
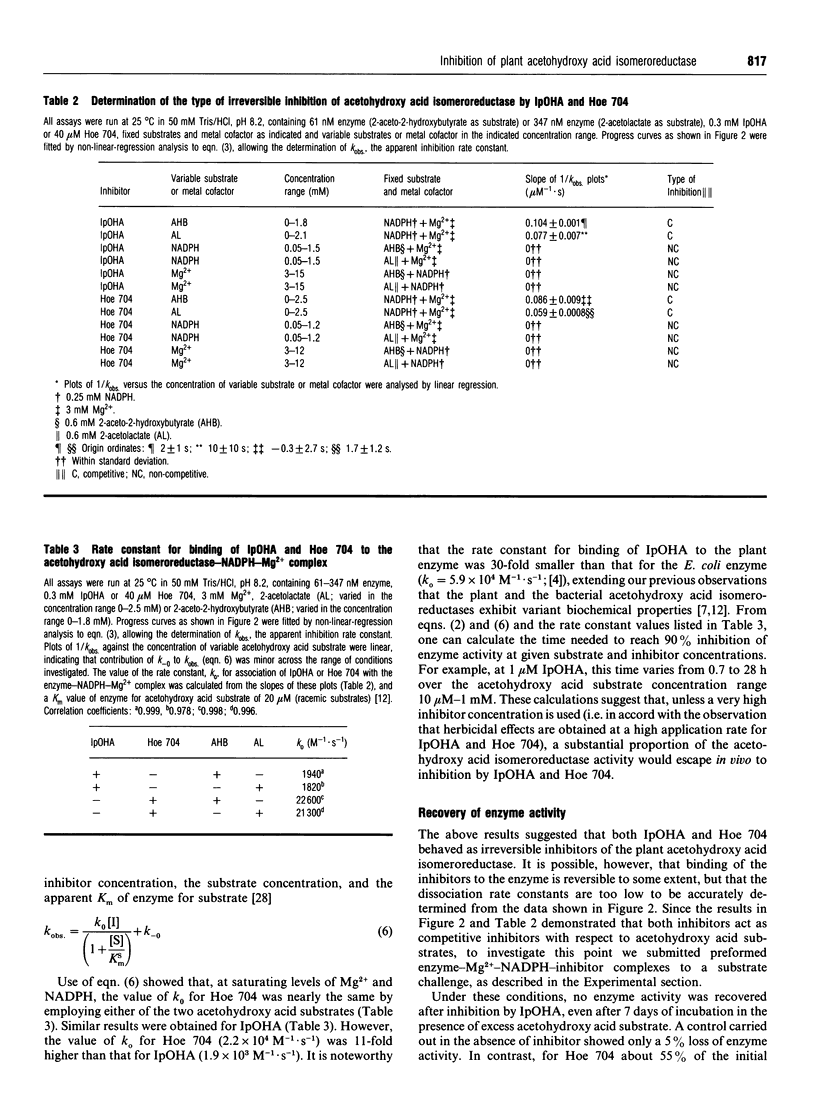
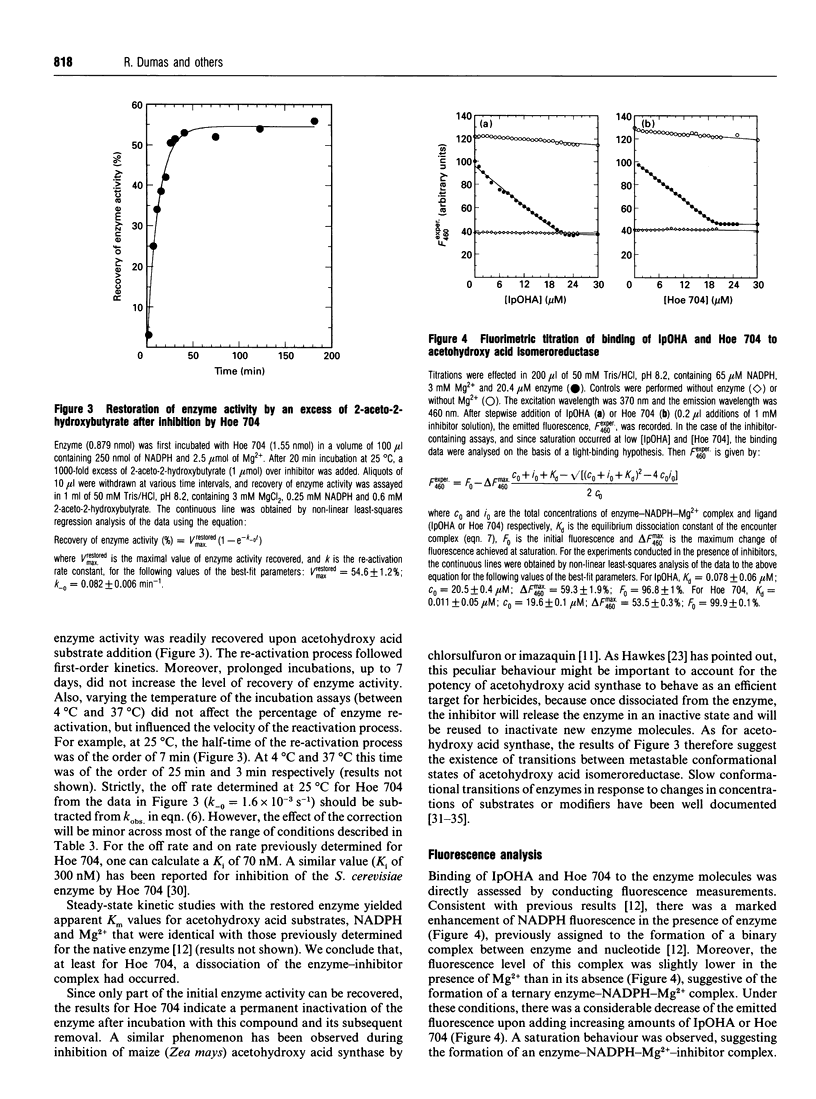
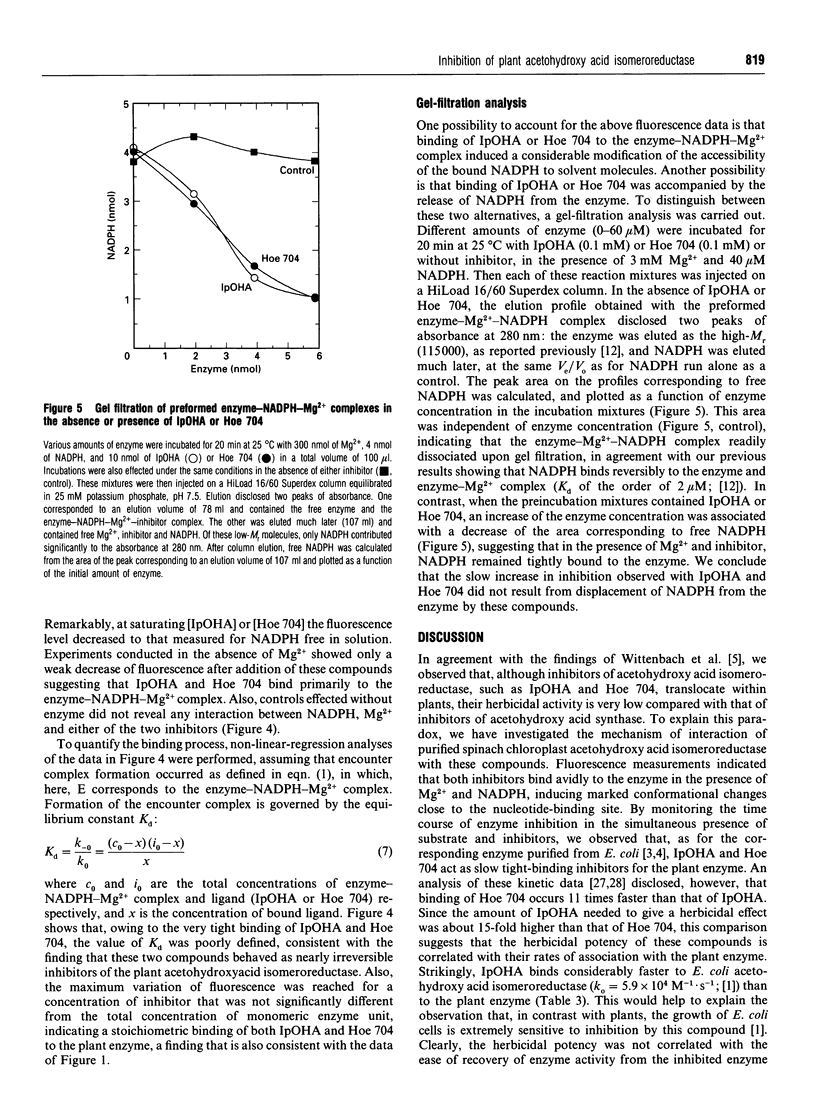
Abstract
N-Hydroxy-N-isopropyloxamate (IpOHA) is known to inhibit extremely tightly (Ki of 22 pM) the bacterial acetohydroxy acid isomeroreductase (EC 1.1.1.86) [Aulabaugh and Schloss (1990) Biochemistry 29, 2824-2830], the second enzyme of the branched-chain-amino-acid-biosynthetic pathway. Yet, although the same pathway exists in plant cells, this compound presents only very poor herbicidal action. Towards the goal of gaining a better understanding of this behaviour, we have studied the mechanism of interaction of this compound with a highly purified acetohydroxy acid isomeroreductase of plant origin, i.e. the spinach (Spinacia oleracea) chloroplast enzyme. IpOHA behaved as a nearly irreversible inhibitor of the enzyme. Encounter complex formation was very slow (association rate constant 1.9 x 10(3) M-1.s-1) and involved a single bimolecular step. Since inhibition was competitive with respect to acetohydroxy acid substrates, the time needed to achieve substantial (90%) inhibition in vitro of enzyme activity in the simultaneous presence of substrates and inhibitors was extremely long (for example of the order of hours at 1 microM IpOHA and 100 microM acetohydroxy acid substrates). Thus, under in vivo conditions, binding of the inhibitor may be so slow that it may delay considerably the time required for inhibition of the target enzyme. Simialr kinetic behaviour was observed with another reaction intermediate analogue described by Schulz, Spönemann, Köcher and Wengenmayer [(1988) FEBS Lett. 238, 375-378], 2-dimethyl-phosphinoyl-2-hydroxyacetic acid (Hoe 704), which displays a higher herbicide activity than IpOHA. The herbicidal potency of these two compounds appeared to be correlated with their rates of association with the plant acetohydroxy acid isomeroreductase, since the bimolecular rate constant for Hoe 704 (2.2 x 10(4) M-1.s-1) was higher than that for IpOHA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG F. B., WAGNER R. P. Biosynthesis of valine and isoleucine. IV. alpha-Hydroxy-beta-keto acid reductoisomerase of Salmonella. J Biol Chem. 1961 Jul;236:2027–2032. [PubMed] [Google Scholar]
- Arfin S. M., Umbarger H. E. Purification and properties of the acetohydroxy acid isomeroreductase of Salmonella typhimurium. J Biol Chem. 1969 Mar 10;244(5):1118–1127. [PubMed] [Google Scholar]
- Aulabaugh A., Schloss J. V. Oxalyl hydroxamates as reaction-intermediate analogues for ketol-acid reductoisomerase. Biochemistry. 1990 Mar 20;29(11):2824–2830. doi: 10.1021/bi00463a027. [DOI] [PubMed] [Google Scholar]
- Cha S. Tight-binding inhibitors-I. Kinetic behavior. Biochem Pharmacol. 1975 Dec 1;24(23):2177–2185. doi: 10.1016/0006-2952(75)90050-7. [DOI] [PubMed] [Google Scholar]
- Chunduru S. K., Mrachko G. T., Calvo K. C. Mechanism of ketol acid reductoisomerase--steady-state analysis and metal ion requirement. Biochemistry. 1989 Jan 24;28(2):486–493. doi: 10.1021/bi00428a012. [DOI] [PubMed] [Google Scholar]
- Curien G., Dumas R., Douce R. Nucleotide sequence and characterization of a cDNA encoding the acetohydroxy acid isomeroreductase from Arabidopsis thaliana. Plant Mol Biol. 1993 Feb;21(4):717–722. doi: 10.1007/BF00014556. [DOI] [PubMed] [Google Scholar]
- Dumas R., Curien G., DeRose R. T., Douce R. Branched-chain-amino-acid biosynthesis in plants: molecular cloning and characterization of the gene encoding acetohydroxy acid isomeroreductase (ketol-acid reductoisomerase) from Arabidopsis thaliana (thale cress). Biochem J. 1993 Sep 15;294(Pt 3):821–828. doi: 10.1042/bj2940821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas R., Job D., Ortholand J. Y., Emeric G., Greiner A., Douce R. Isolation and kinetic properties of acetohydroxy acid isomeroreductase from spinach (Spinacia oleracea) chloroplasts overexpressed in Escherichia coli. Biochem J. 1992 Dec 15;288(Pt 3):865–874. doi: 10.1042/bj2880865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas R., Joyard J., Douce R. Purification and characterization of acetohydroxyacid reductoisomerase from spinach chloroplasts. Biochem J. 1989 Sep 15;262(3):971–976. doi: 10.1042/bj2620971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas R., Lebrun M., Douce R. Isolation, characterization and sequence analysis of a full-length cDNA clone encoding acetohydroxy acid reductoisomerase from spinach chloroplasts. Biochem J. 1991 Jul 15;277(Pt 2):469–475. doi: 10.1042/bj2770469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J., Gailus V., Böger P. New aspects on inhibition of plant acetolactate synthase by chlorsulfuron and imazaquin. Plant Physiol. 1991 Apr;95(4):1144–1149. doi: 10.1104/pp.95.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- Hofler J. G., Decedue C. J., Luginbuhl G. H., Reynolds J. A., Burns R. O. The subunit structure of alpha-acetohydroxyacid isomeroreductase from Salmonella typhimurium. J Biol Chem. 1975 Feb 10;250(3):877–882. [PubMed] [Google Scholar]
- LaRossa R. A., Schloss J. V. The sulfonylurea herbicide sulfometuron methyl is an extremely potent and selective inhibitor of acetolactate synthase in Salmonella typhimurium. J Biol Chem. 1984 Jul 25;259(14):8753–8757. [PubMed] [Google Scholar]
- Liu W., Tsou C. L. Determination of rate constants for the irreversible inhibition of acetylcholine esterase by continuously monitoring the substrate reaction in the presence of the inhibitor. Biochim Biophys Acta. 1986 Mar 28;870(2):185–190. doi: 10.1016/0167-4838(86)90220-7. [DOI] [PubMed] [Google Scholar]
- Morrison J. F. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta. 1969;185(2):269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Ainslie G. R., Jr Hysteretic enzymes. Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- Ricard J., Buc J., Meunier J. C. Enzyme memory. 1. A transient kinetic study of wheat-germ hexokinase LI. Eur J Biochem. 1977 Nov 1;80(2):581–592. doi: 10.1111/j.1432-1033.1977.tb11915.x. [DOI] [PubMed] [Google Scholar]
- Ricard J., Meunier J. C., Buc J. Regulatory behavior of monomeric enzymes. 1. The mnemonical enzyme concept. Eur J Biochem. 1974 Nov 1;49(1):195–208. doi: 10.1111/j.1432-1033.1974.tb03825.x. [DOI] [PubMed] [Google Scholar]
- Schulz A., Spönemann P., Köcher H., Wengenmayer F. The herbicidally active experimental compound Hoe 704 is a potent inhibitor of the enzyme acetolactate reductoisomerase. FEBS Lett. 1988 Oct 10;238(2):375–378. doi: 10.1016/0014-5793(88)80515-5. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Shaner D. L., Anderson P. C., Stidham M. A. Imidazolinones: potent inhibitors of acetohydroxyacid synthase. Plant Physiol. 1984 Oct;76(2):545–546. doi: 10.1104/pp.76.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shematek E. M., Arfin S. M., Diven W. F. A kinetic study of -acetohydroxy acid isomeroreductase from Salmonella typhimurium. Arch Biochem Biophys. 1973 Sep;158(1):132–138. doi: 10.1016/0003-9861(73)90605-x. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Kinetic evidence for a 'mnemonical' mechanism for rat liver glucokinase. Biochem J. 1977 Jul 1;165(1):61–69. doi: 10.1042/bj1650061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W. X., Tsou C. L. Determination of the rate constant of enzyme modification by measuring the substrate reaction in the presence of the modifier. Biochemistry. 1982 Mar 2;21(5):1028–1032. doi: 10.1021/bi00534a031. [DOI] [PubMed] [Google Scholar]
- Walsh C. T. Suicide substrates, mechanism-based enzyme inactivators: recent developments. Annu Rev Biochem. 1984;53:493–535. doi: 10.1146/annurev.bi.53.070184.002425. [DOI] [PubMed] [Google Scholar]


