Abstract
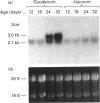
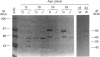
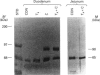
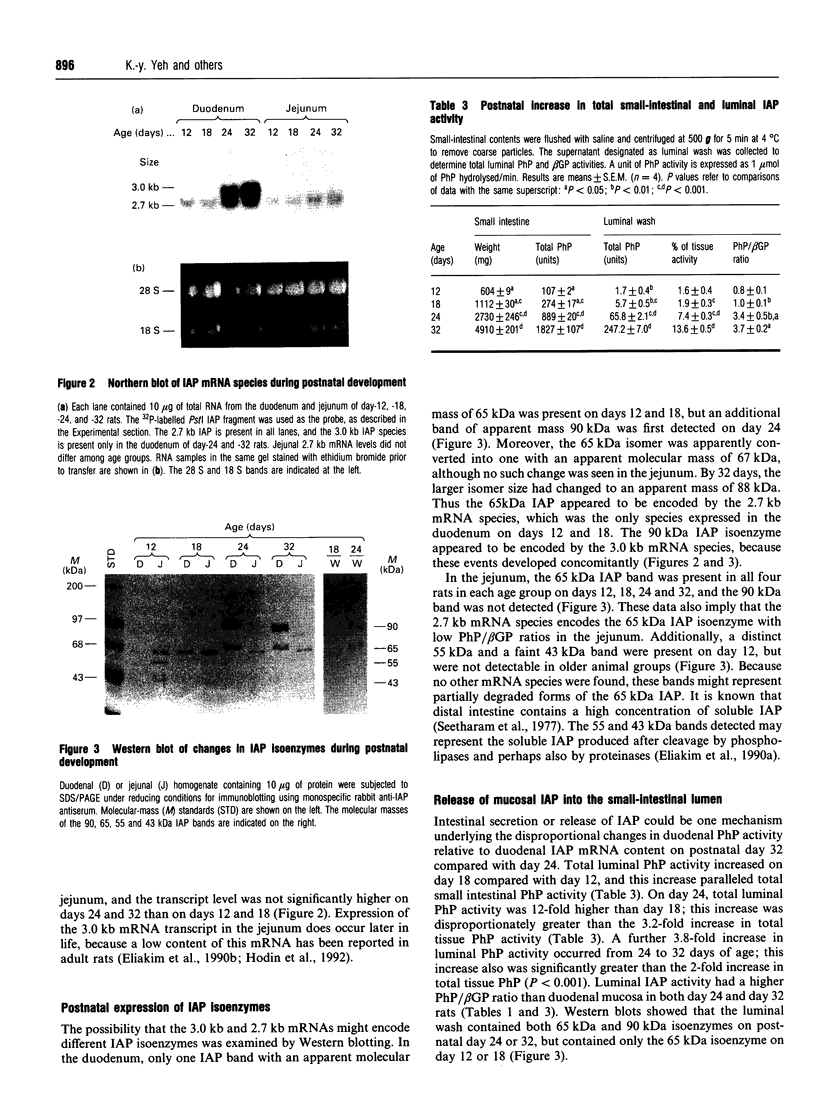
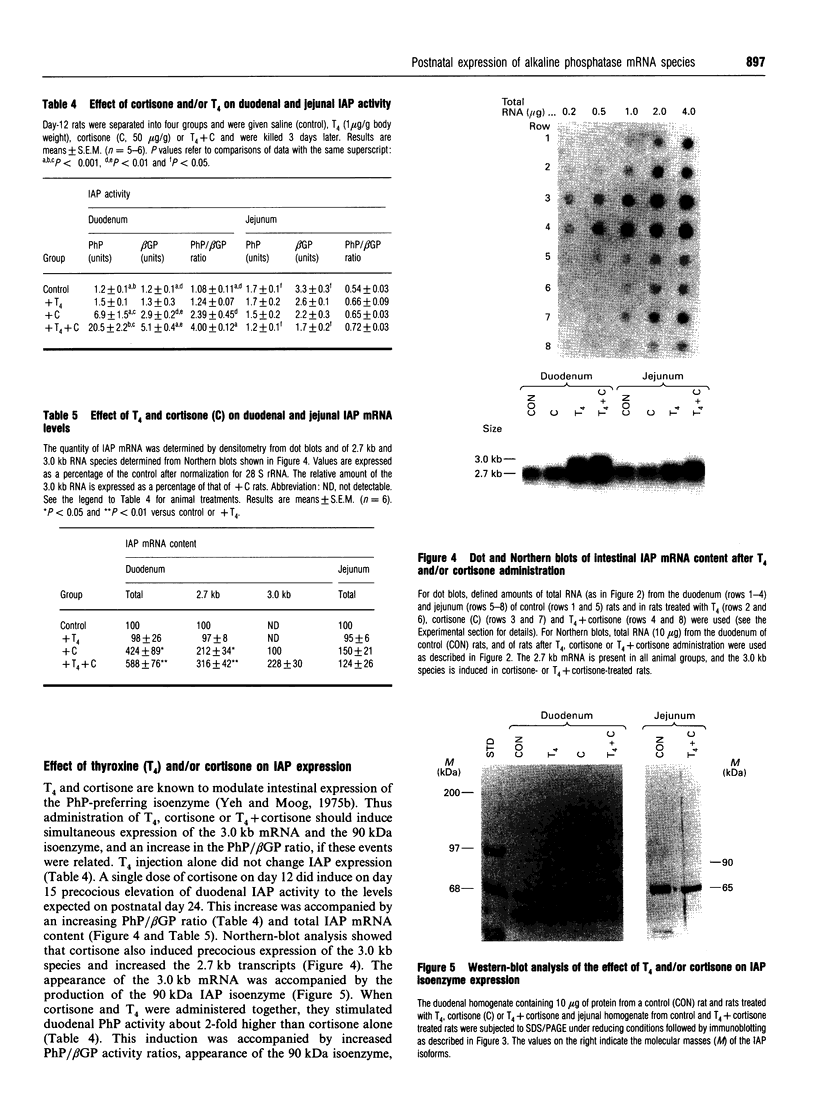
In the rat, intestinal alkaline phosphatase (IAP) activity in the duodenum, but not jejunum, increases on day 22-24 after birth and exhibits higher activity hydrolysing phenyl phosphate (PhP) than beta-glycerophosphate (beta GP) [Moog and Yeh (1973) Comp. Biochem. Physiol. 44B, 657-666]. The mechanism underlying these developmental changes remains unknown. To define possible mechanisms, we have measured IAP activity and mRNA levels, and analysed IAP mRNA species and isoenzymes on postnatal days 12, 18, 24 and 32. Duodenal IAP activity and mRNA content were identical on postnatal days 12 and 18, but were 7-fold and 3-fold higher on day 24, respectively than on day 18. The increased IAP activity exhibited a high PhP/beta GP ratio and was accompanied by initial appearance of the 3.0 kb mRNA and 90 kDa isoenzyme. On day 32, duodenal IAP activity did not increase over the levels on day 24, whereas mRNA levels doubled. The lack of enzyme increase might be related in part to increased apical release, as luminal IAP activity increased from 2% of total mucosal IAP on days 12 and 18 to 7% and 14% on days 24 and 32 respectively. In the jejunum, IAP activity decreased postnatally, but mRNA content was unaltered; only the 2.7 kb mRNA and 65 kDa IAP isoenzyme were present. Administration of cortisone or cortisone+thyroxine induced simultaneous appearance of the duodenal 3.0 kb mRNA and 90 kDa isoenzyme with an increased PhP/beta GP ratio. Thus postnatal increase in duodenal IAP activity is related to the expression of a 90 kDa PhP-preferring isoenzyme encoded by the 3.0 kb mRNA. The low-PhP/beta GP-ratio 65 kDa isoenzyme is expressed in the duodenum and in the jejunum and is encoded by the 2.7 kb mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H. Pre-translational, translational, and post-translational mechanisms in adaptation of intestinal proteins. Digestion. 1990;46 (Suppl 2):50–58. doi: 10.1159/000200367. [DOI] [PubMed] [Google Scholar]
- Beaudoin A. R., Grondin G. Shedding of vesicular material from the cell surface of eukaryotic cells: different cellular phenomena. Biochim Biophys Acta. 1991 Nov 13;1071(3):203–219. doi: 10.1016/0304-4157(91)90014-n. [DOI] [PubMed] [Google Scholar]
- Berger J., Garattini E., Hua J. C., Udenfriend S. Cloning and sequencing of human intestinal alkaline phosphatase cDNA. Proc Natl Acad Sci U S A. 1987 Feb;84(3):695–698. doi: 10.1073/pnas.84.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliakim R., Becich M. J., Green K., Alpers D. H. Both tissue and serum phospholipases release rat intestinal alkaline phosphatase. Am J Physiol. 1990 Oct;259(4 Pt 1):G618–G625. doi: 10.1152/ajpgi.1990.259.4.G618. [DOI] [PubMed] [Google Scholar]
- Eliakim R., DeSchryver-Kecskemeti K., Nogee L., Stenson W. F., Alpers D. H. Isolation and characterization of a small intestinal surfactant-like particle containing alkaline phosphatase and other digestive enzymes. J Biol Chem. 1989 Dec 5;264(34):20614–20619. [PubMed] [Google Scholar]
- Eliakim R., Mahmood A., Alpers D. H. Rat intestinal alkaline phosphatase secretion into lumen and serum is coordinately regulated. Biochim Biophys Acta. 1991 Jan 10;1091(1):1–8. doi: 10.1016/0167-4889(91)90213-h. [DOI] [PubMed] [Google Scholar]
- Eliakim R., Seetharam S., Tietze C. C., Alpers D. H. Differential regulation of mRNAs encoding for rat intestinal alkaline phosphatase. Am J Physiol. 1990 Jul;259(1 Pt 1):G93–G98. doi: 10.1152/ajpgi.1990.259.1.G93. [DOI] [PubMed] [Google Scholar]
- Engle M. J., Alpers D. H. The two mRNAs encoding rat intestinal alkaline phosphatase represent two distinct nucleotide sequences. Clin Chem. 1992 Dec;38(12):2506–2509. [PubMed] [Google Scholar]
- Etzler M. E., Moog F. Immunochemical characterization of alkaline phosphatase isozymes of the young mouse duodenum. Biochim Biophys Acta. 1968 Jan 22;154(1):150–161. doi: 10.1016/0005-2795(68)90267-5. [DOI] [PubMed] [Google Scholar]
- Freund J. N., Duluc I., Raul F. Lactase expression is controlled differently in the jejunum and ileum during development in rats. Gastroenterology. 1991 Feb;100(2):388–394. doi: 10.1016/0016-5085(91)90207-2. [DOI] [PubMed] [Google Scholar]
- Harris H. Multilocus enzyme systems and the evolution of gene expression: the alkaline phosphatases as a model example. Harvey Lect. 1980;76:95–124. [PubMed] [Google Scholar]
- Henthorn P. S., Raducha M., Edwards Y. H., Weiss M. J., Slaughter C., Lafferty M. A., Harris H. Nucleotide and amino acid sequences of human intestinal alkaline phosphatase: close homology to placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1234–1238. doi: 10.1073/pnas.84.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P. S., Raducha M., Kadesch T., Weiss M. J., Harris H. Sequence and characterization of the human intestinal alkaline phosphatase gene. J Biol Chem. 1988 Aug 25;263(24):12011–12019. [PubMed] [Google Scholar]
- Hodin R. A., Chamberlain S. M., Upton M. P. Thyroid hormone differentially regulates rat intestinal brush border enzyme gene expression. Gastroenterology. 1992 Nov;103(5):1529–1536. doi: 10.1016/0016-5085(92)91174-3. [DOI] [PubMed] [Google Scholar]
- King E. J., Armstrong A. R. A CONVENIENT METHOD FOR DETERMINING SERUM AND BILE PHOSPHATASE ACTIVITY. Can Med Assoc J. 1934 Oct;31(4):376–381. [PMC free article] [PubMed] [Google Scholar]
- Koyama I., Arai K., Sakagishi Y., Ikezawa H., Komoda T. Blood appearance of rat alkaline phosphatase originating from the duodenum in vitro. J Chromatogr. 1987 Sep 25;420(2):275–286. doi: 10.1016/0378-4347(87)80184-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowe M., Strauss A. W., Alpers R., Seetharam S., Alpers D. H. Molecular cloning and expression of a cDNA encoding the membrane-associated rat intestinal alkaline phosphatase. Biochim Biophys Acta. 1990 Feb 9;1037(2):170–177. doi: 10.1016/0167-4838(90)90164-b. [DOI] [PubMed] [Google Scholar]
- MOOG F. The functional differentiation of the small intestine. VIII. Regional differences in the alkaline phosphatases of the small intestine of the mouse from birth to one year. Dev Biol. 1961 Apr;3:153–174. doi: 10.1016/0012-1606(61)90003-3. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Manes T., Glade K., Ziomek C. A., Millán J. L. Genomic structure and comparison of mouse tissue-specific alkaline phosphatase genes. Genomics. 1990 Nov;8(3):541–554. doi: 10.1016/0888-7543(90)90042-s. [DOI] [PubMed] [Google Scholar]
- Millán J. L., Manes T. Seminoma-derived Nagao isozyme is encoded by a germ-cell alkaline phosphatase gene. Proc Natl Acad Sci U S A. 1988 May;85(9):3024–3028. doi: 10.1073/pnas.85.9.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog F. The regulation of alkaline phosphatase activity in the duodenum of the mouse from birth to maturity. J Exp Zool. 1966 Apr;161(3):353–367. doi: 10.1002/jez.1401610305. [DOI] [PubMed] [Google Scholar]
- Moog F., Yeh K. Y. Intestinal alkaline phosphatase of the rat: development and distribution of activity with phenylphosphate and -glycerophosphate. Comp Biochem Physiol B. 1973 Mar 15;44(3):657–666. doi: 10.1016/0305-0491(73)90214-9. [DOI] [PubMed] [Google Scholar]
- Seetharam B., Yeh K. Y., Moog F., Alpers D. H. Development of intestinal brush border membrane proteins in the rat. Biochim Biophys Acta. 1977 Nov 1;470(3):424–436. doi: 10.1016/0005-2736(77)90133-x. [DOI] [PubMed] [Google Scholar]
- Strom M., Krisinger J., DeLuca H. F. Isolation of a mRNA that encodes a putative intestinal alkaline phosphatase regulated by 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1991 Nov 11;1090(3):299–304. doi: 10.1016/0167-4781(91)90193-p. [DOI] [PubMed] [Google Scholar]
- Sussman N. L., Seetharam S., Blaufuss M. C., Alpers D. H. Translation of rat intestinal RNA yields two alkaline phosphatases. Biochem J. 1986 Mar 15;234(3):563–568. doi: 10.1042/bj2340563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Weiss M. J., Ray K., Henthorn P. S., Lamb B., Kadesch T., Harris H. Structure of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem. 1988 Aug 25;263(24):12002–12010. [PubMed] [Google Scholar]
- Yedlin S. T., Young G. P., Seetharam B., Seetharam S., Alpers D. H. Characterization and comparison of soluble and membranous forms of intestinal alkaline phosphatase from the suckling rat. J Biol Chem. 1981 Jun 10;256(11):5620–5626. [PubMed] [Google Scholar]
- Yeh K. Y. Cell kinetics in the small intestine of suckling rats. I. Influence of hypophysectomy. Anat Rec. 1977 May;188(1):69–76. doi: 10.1002/ar.1091880108. [DOI] [PubMed] [Google Scholar]
- Yeh K. Y., Moog F. Development of the small intestine in the hypophysectomized rat. I. Growth histology, and activity of alkaline phosphatase, maltase, and sucrase. Dev Biol. 1975 Nov;47(1):156–172. doi: 10.1016/0012-1606(75)90270-5. [DOI] [PubMed] [Google Scholar]
- Yeh K. Y., Moog F. Development of the small intestine in the hypophysectomized rat. II. Influence of cortisone, thyroxine, growth hormone, and prolactin. Dev Biol. 1975 Nov;47(1):173–184. doi: 10.1016/0012-1606(75)90271-7. [DOI] [PubMed] [Google Scholar]
- Yeh K. Y., Yeh M., Holt P. R. Thyroxine and cortisone cooperate to modulate postnatal intestinal enzyme differentiation in the rat. Am J Physiol. 1991 Mar;260(3 Pt 1):G371–G378. doi: 10.1152/ajpgi.1991.260.3.G371. [DOI] [PubMed] [Google Scholar]
- Yeh K. Y., Yeh M., Montgomery R. K., Grand R. J., Holt P. R. Cortisone and thyroxine modulate intestinal lactase and sucrase mRNA levels and activities in the suckling rat. Biochem Biophys Res Commun. 1991 Oct 15;180(1):174–180. doi: 10.1016/s0006-291x(05)81272-0. [DOI] [PubMed] [Google Scholar]