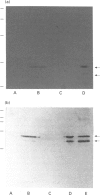
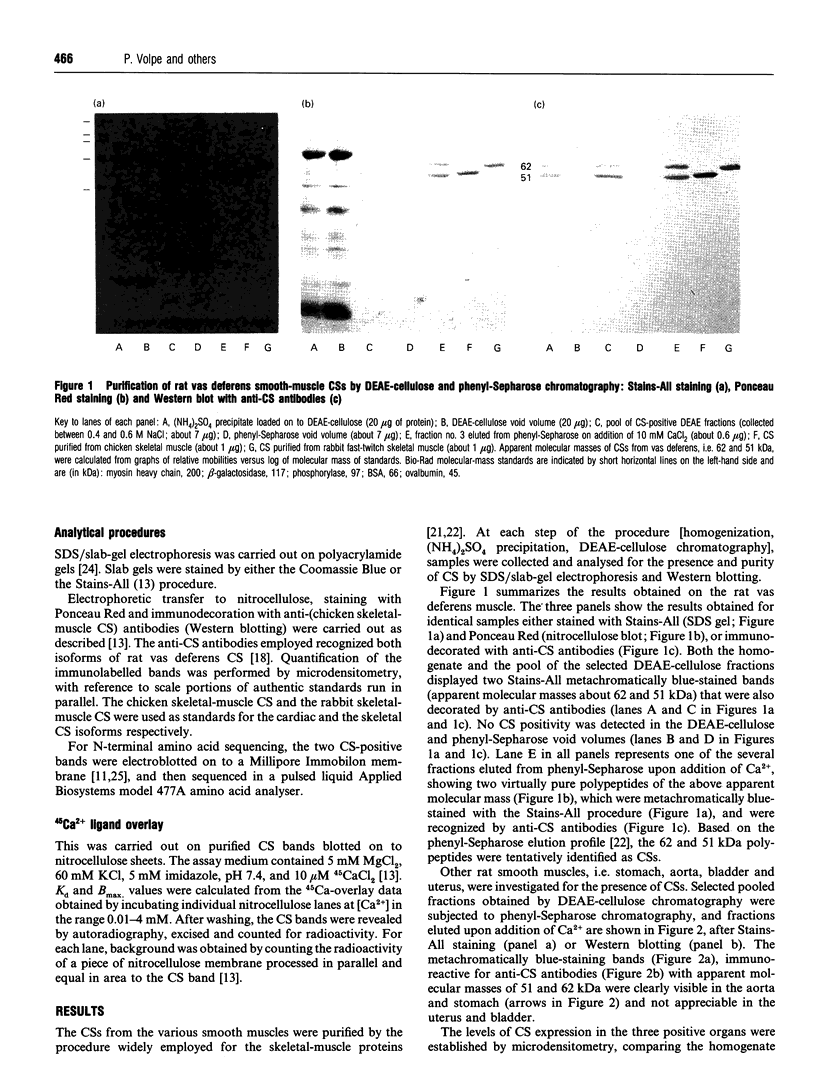
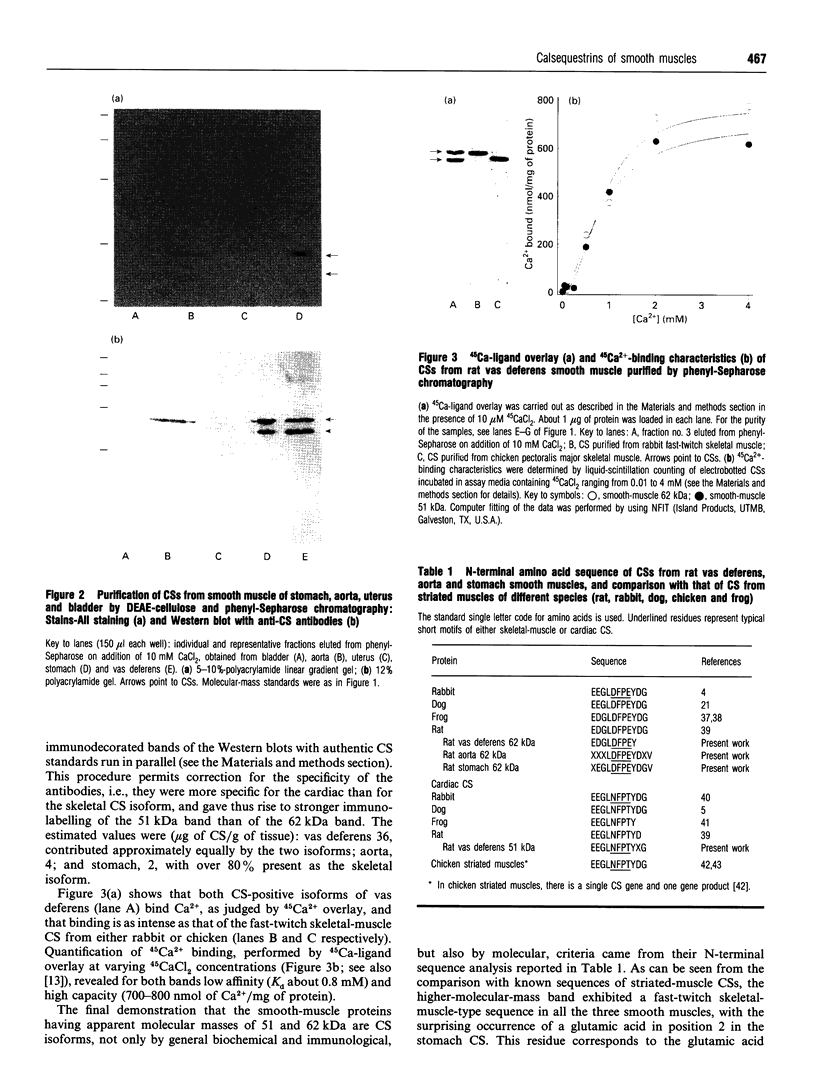
Abstract
Expression by smooth-muscle cells of calsequestrin (CS), the low-affinity/high-capacity Ca(2+)-binding protein of striated-muscle sarcoplasmic reticulum (SR), has been investigated in recent years with conflicting results. Here we report the purification and characterization from rat vas deferens of two CS isoforms, the first deemed skeletal muscle, the second cardiac type, on account of their N-terminal amino acids and other relevant biochemical and molecular properties. Compared with vas deferens, the smooth muscles from aorta and stomach, in that order, were found to express lower amounts of CS, whereas in the uterus and bladder the protein was not detectable. The ratio between the two CS isoforms was also variable, with the stomach and aorta predominantly expressing the skeletal-muscle type and the vas deferens expressing the two CSs in roughly similar amount. Because of the property of CSs to localize within the skeletal-muscle SR lumen not uniformly, but according to the distribution of their anchorage membrane proteins, the expression of the protein suggests the existence in smooth-muscle cells of discrete endoplasmic-reticulum areas specialized in the rapidly exchanging Ca2+ storage and release, and thus in the control of a variety of functions, including smooth-muscle contraction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai M., Alpert N. R., Periasamy M. Cloning and characterization of the gene encoding rabbit cardiac calsequestrin. Gene. 1991 Dec 30;109(2):275–279. doi: 10.1016/0378-1119(91)90621-h. [DOI] [PubMed] [Google Scholar]
- Arber S., Krause K. H., Caroni P. s-cyclophilin is retained intracellularly via a unique COOH-terminal sequence and colocalizes with the calcium storage protein calreticulin. J Cell Biol. 1992 Jan;116(1):113–125. doi: 10.1083/jcb.116.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biral D., Volpe P., Damiani E., Margreth A. Coexistence of two calsequestrin isoforms in rabbit slow-twitch skeletal muscle fibers. FEBS Lett. 1992 Mar 9;299(2):175–178. doi: 10.1016/0014-5793(92)80241-8. [DOI] [PubMed] [Google Scholar]
- Cala S. E., Jones L. R. Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl-sepharose. J Biol Chem. 1983 Oct 10;258(19):11932–11936. [PubMed] [Google Scholar]
- Choi E. S., Clegg D. O. Identification and developmental expression of a chicken calsequestrin homolog. Dev Biol. 1990 Nov;142(1):169–177. doi: 10.1016/0012-1606(90)90160-k. [DOI] [PubMed] [Google Scholar]
- Damiani E., Margreth A. Characterization study of the ryanodine receptor and of calsequestrin isoforms of mammalian skeletal muscles in relation to fibre types. J Muscle Res Cell Motil. 1994 Apr;15(2):86–101. doi: 10.1007/BF00130421. [DOI] [PubMed] [Google Scholar]
- Fliegel L., Burns K., MacLennan D. H., Reithmeier R. A., Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989 Dec 25;264(36):21522–21528. [PubMed] [Google Scholar]
- Fliegel L., Ohnishi M., Carpenter M. R., Khanna V. K., Reithmeier R. A., MacLennan D. H. Amino acid sequence of rabbit fast-twitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorza L., Schiaffino S., Volpe P. Inositol 1,4,5-trisphosphate receptor in heart: evidence for its concentration in Purkinje myocytes of the conduction system. J Cell Biol. 1993 Apr;121(2):345–353. doi: 10.1083/jcb.121.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata Y., Nakai J., Takeshima H., Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett. 1992 Nov 9;312(2-3):229–235. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- Jorgensen A. O., Campbell K. P. Evidence for the presence of calsequestrin in two structurally different regions of myocardial sarcoplasmic reticulum. J Cell Biol. 1984 Apr;98(4):1597–1602. doi: 10.1083/jcb.98.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A. O., McLeod A. G., Campbell K. P., Denney G. H. Evidence for the presence of calsequestrin in both peripheral and interior regions of sheep Purkinje fibers. Circ Res. 1984 Aug;55(2):267–270. doi: 10.1161/01.res.55.2.267. [DOI] [PubMed] [Google Scholar]
- Jorgensen A. O., Shen A. C., Campbell K. P., MacLennan D. H. Ultrastructural localization of calsequestrin in rat skeletal muscle by immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1983 Nov;97(5 Pt 1):1573–1581. doi: 10.1083/jcb.97.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lytton J., Nigam S. K. Intracellular calcium: molecules and pools. Curr Opin Cell Biol. 1992 Apr;4(2):220–226. doi: 10.1016/0955-0674(92)90036-c. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McLeod A. G., Shen A. C., Campbell K. P., Michalak M., Jorgensen A. O. Frog cardiac calsequestrin. Identification, characterization, and subcellular distribution in two structurally distinct regions of peripheral sarcoplasmic reticulum in frog ventricular myocardium. Circ Res. 1991 Aug;69(2):344–359. doi: 10.1161/01.res.69.2.344. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Villa A., Volpe P., Pozzan T. Cellular sites of IP3 action. Adv Second Messenger Phosphoprotein Res. 1992;26:187–208. [PubMed] [Google Scholar]
- Michalak M., Milner R. E., Burns K., Opas M. Calreticulin. Biochem J. 1992 Aug 1;285(Pt 3):681–692. doi: 10.1042/bj2850681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R. E., Baksh S., Shemanko C., Carpenter M. R., Smillie L., Vance J. E., Opas M., Michalak M. Calreticulin, and not calsequestrin, is the major calcium binding protein of smooth muscle sarcoplasmic reticulum and liver endoplasmic reticulum. J Biol Chem. 1991 Apr 15;266(11):7155–7165. [PubMed] [Google Scholar]
- Mourey R. J., Verma A., Supattapone S., Snyder S. H. Purification and characterization of the inositol 1,4,5- trisphosphate receptor protein from rat vas deferens. Biochem J. 1990 Dec 1;272(2):383–389. doi: 10.1042/bj2720383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988 Apr;7(4):913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter F., Nguyen Van P., Söling H. D. Different sorting of Lys-Asp-Glu-Leu proteins in rat liver. J Biol Chem. 1992 May 25;267(15):10631–10637. [PubMed] [Google Scholar]
- Raeymaekers L., Verbist J., Wuytack F., Plessers L., Casteels R. Expression of Ca2+ binding proteins of the sarcoplasmic reticulum of striated muscle in the endoplasmic reticulum of pig smooth muscles. Cell Calcium. 1993 Sep;14(8):581–589. doi: 10.1016/0143-4160(93)90058-e. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Danoff S. K., Schell M. J., Snyder S. H., Ullrich A. Three additional inositol 1,4,5-trisphosphate receptors: molecular cloning and differential localization in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. T., Simmerman H. K., Collins J. H., Nadal-Ginard B., Jones L. R. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J Biol Chem. 1988 Jun 25;263(18):8958–8964. [PubMed] [Google Scholar]
- Slupsky J. R., Ohnishi M., Carpenter M. R., Reithmeier R. A. Characterization of cardiac calsequestrin. Biochemistry. 1987 Oct 6;26(20):6539–6544. doi: 10.1021/bi00394a038. [DOI] [PubMed] [Google Scholar]
- Sorrentino V., Volpe P. Ryanodine receptors: how many, where and why? Trends Pharmacol Sci. 1993 Mar;14(3):98–103. doi: 10.1016/0165-6147(93)90072-r. [DOI] [PubMed] [Google Scholar]
- Takei K., Stukenbrok H., Metcalf A., Mignery G. A., Südhof T. C., Volpe P., De Camilli P. Ca2+ stores in Purkinje neurons: endoplasmic reticulum subcompartments demonstrated by the heterogeneous distribution of the InsP3 receptor, Ca(2+)-ATPase, and calsequestrin. J Neurosci. 1992 Feb;12(2):489–505. doi: 10.1523/JNEUROSCI.12-02-00489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S., De Mattei M., Landfredi M., Villa A., Green N. M., MacLennan D. H., Meldolesi J., Pozzan T. Calreticulin is a candidate for a calsequestrin-like function in Ca2(+)-storage compartments (calciosomes) of liver and brain. Biochem J. 1990 Oct 15;271(2):473–480. doi: 10.1042/bj2710473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S., Vilsen B., Chiozzi P., Andersen J. P., Zorzato F. Molecular cloning, functional expression and tissue distribution of the cDNA encoding frog skeletal muscle calsequestrin. Biochem J. 1992 May 1;283(Pt 3):767–772. doi: 10.1042/bj2830767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevesø S., Zorzato F., Chiozzi P., Melandri P., Volpe P., Pozzan T. Frog brain expresses a 60 KDa Ca2+ binding protein similar to mammalian calreticulin. Biochem Biophys Res Commun. 1991 Mar 15;175(2):444–450. doi: 10.1016/0006-291x(91)91584-y. [DOI] [PubMed] [Google Scholar]
- Villa A., Podini P., Clegg D. O., Pozzan T., Meldolesi J. Intracellular Ca2+ stores in chicken Purkinje neurons: differential distribution of the low affinity-high capacity Ca2+ binding protein, calsequestrin, of Ca2+ ATPase and of the ER lumenal protein, Bip. J Cell Biol. 1991 May;113(4):779–791. doi: 10.1083/jcb.113.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Podini P., Panzeri M. C., Söling H. D., Volpe P., Meldolesi J. The endoplasmic-sarcoplasmic reticulum of smooth muscle: immunocytochemistry of vas deferens fibers reveals specialized subcompartments differently equipped for the control of Ca2+ homeostasis. J Cell Biol. 1993 Jun;121(5):1041–1051. doi: 10.1083/jcb.121.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Alderson-Lang B. H., Madeddu L., Damiani E., Collins J. H., Margreth A. Calsequestrin, a component of the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of chicken cerebellum. Neuron. 1990 Nov;5(5):713–721. doi: 10.1016/0896-6273(90)90225-5. [DOI] [PubMed] [Google Scholar]
- Volpe P., Furlan S., Damiani E. Purification and characterization of calsequestrin from chicken cerebellum. Biochem Biophys Res Commun. 1991 Nov 27;181(1):28–35. doi: 10.1016/s0006-291x(05)81377-4. [DOI] [PubMed] [Google Scholar]
- Volpe P., Villa A., Damiani E., Sharp A. H., Podini P., Snyder S. H., Meldolesi J. Heterogeneity of microsomal Ca2+ stores in chicken Purkinje neurons. EMBO J. 1991 Nov;10(11):3183–3189. doi: 10.1002/j.1460-2075.1991.tb04880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuytack F., Raeymaekers L., Verbist J., Jones L. R., Casteels R. Smooth-muscle endoplasmic reticulum contains a cardiac-like form of calsequestrin. Biochim Biophys Acta. 1987 May 29;899(2):151–158. doi: 10.1016/0005-2736(87)90395-6. [DOI] [PubMed] [Google Scholar]
- Yazaki P. J., Salvatori S., Sabbadini R. A., Dahms A. S. Calsequestrin, an intracellular calcium-binding protein of skeletal muscle sarcoplasmic reticulum, is homologous to aspartactin, a putative laminin-binding protein of the extracellular matrix. Biochem Biophys Res Commun. 1990 Jan 30;166(2):898–903. doi: 10.1016/0006-291x(90)90895-t. [DOI] [PubMed] [Google Scholar]
- Zhang Z. D., Kwan C. Y., Daniel E. E. Subcellular-membrane characterization of [3H]ryanodine-binding sites in smooth muscle. Biochem J. 1993 Feb 15;290(Pt 1):259–266. doi: 10.1042/bj2900259. [DOI] [PMC free article] [PubMed] [Google Scholar]