Abstract
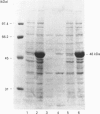
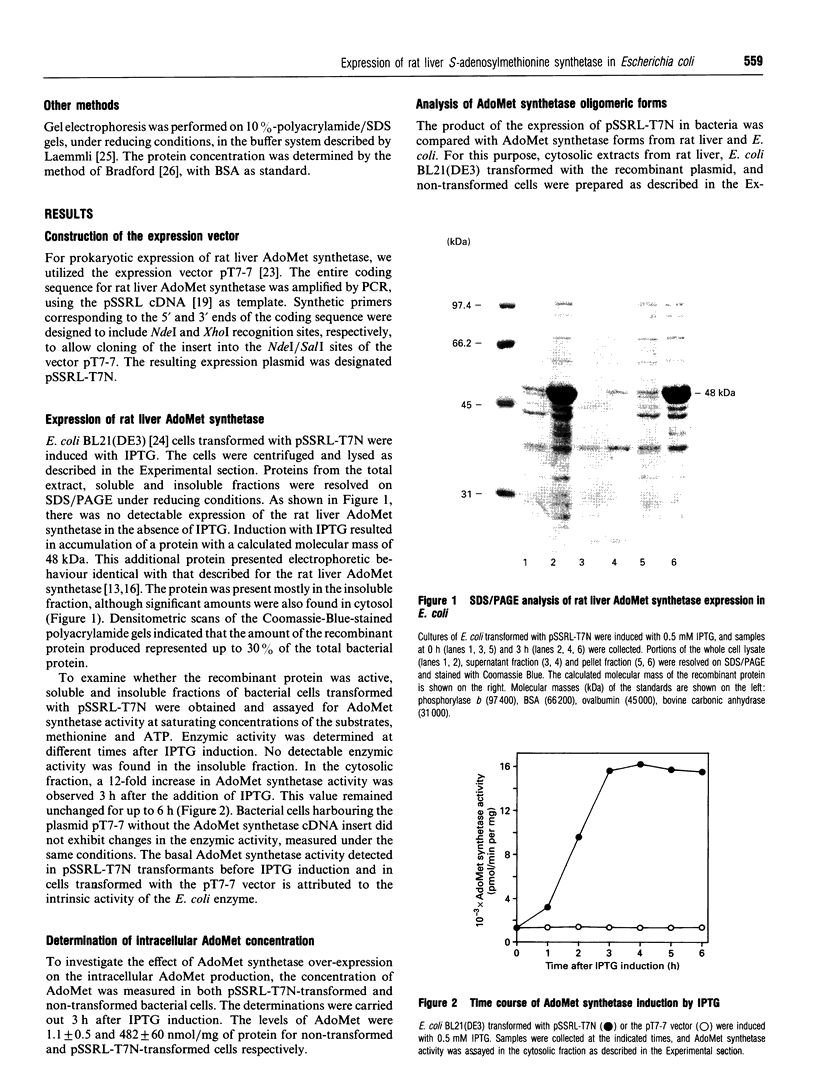
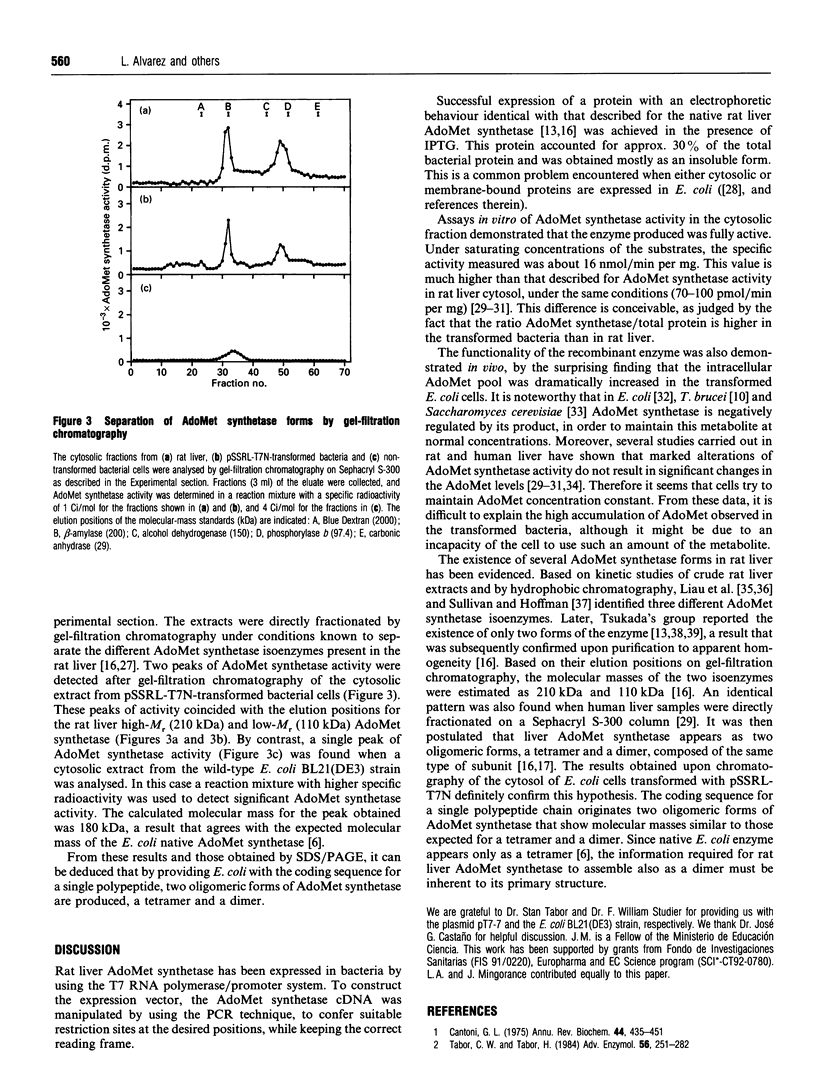
A cDNA containing the complete coding sequence for rat liver S-adenosylmethionine synthetase was cloned into the prokaryotic expression vector pT7-7 and expressed in Escherichia coli BL21(DE3). A major additional band corresponding to a protein of 48 kDa was detected on SDS/PAGE after induction with isopropyl beta-D-thiogalactopyranoside. This protein was distributed in both the soluble and insoluble fractions and accounted for approx. 30% of the total bacterial protein. The soluble enzyme was fully active, as revealed by assays in vitro of S-adenosylmethionine synthetase activity. In addition, transformed bacteria exhibited highly increased levels of intracellular S-adenosylmethionine. Two active forms of the recombinant enzyme, with apparent molecular masses of 210 kDa and 110 kDa, were detected when cytosolic extracts of the transformed cells were fractionated by gel-filtration chromatography. It is concluded that the expressed S-adenosylmethionine synthetase polypeptide assemble as tetramers and dimers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez L., Asunción M., Corrales F., Pajares M. A., Mato J. M. Analysis of the 5' non-coding region of rat liver S-adenosylmethionine synthetase mRNA and comparison of the Mr deduced from the cDNA sequence and the purified enzyme. FEBS Lett. 1991 Sep 23;290(1-2):142–146. doi: 10.1016/0014-5793(91)81245-4. [DOI] [PubMed] [Google Scholar]
- Alvarez L., Corrales F., Martín-Duce A., Mato J. M. Characterization of a full-length cDNA encoding human liver S-adenosylmethionine synthetase: tissue-specific gene expression and mRNA levels in hepatopathies. Biochem J. 1993 Jul 15;293(Pt 2):481–486. doi: 10.1042/bj2930481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cabrero C., Alemany S. Conversion of rat liver S-adenosyl-L-methionine synthetase from high-Mr form to low-Mr form by LiBr. Biochim Biophys Acta. 1988 Feb 10;952(3):277–281. doi: 10.1016/0167-4838(88)90127-6. [DOI] [PubMed] [Google Scholar]
- Cabrero C., Duce A. M., Ortiz P., Alemany S., Mato J. M. Specific loss of the high-molecular-weight form of S-adenosyl-L-methionine synthetase in human liver cirrhosis. Hepatology. 1988 Nov-Dec;8(6):1530–1534. doi: 10.1002/hep.1840080610. [DOI] [PubMed] [Google Scholar]
- Cabrero C., Puerta J., Alemany S. Purification and comparison of two forms of S-adenosyl-L-methionine synthetase from rat liver. Eur J Biochem. 1987 Dec 30;170(1-2):299–304. doi: 10.1111/j.1432-1033.1987.tb13699.x. [DOI] [PubMed] [Google Scholar]
- Cantoni G. L. Biological methylation: selected aspects. Annu Rev Biochem. 1975;44:435–451. doi: 10.1146/annurev.bi.44.070175.002251. [DOI] [PubMed] [Google Scholar]
- Chiang P. K., Cantoni G. L. Activation of methionine for transmethylation. Purification of the S-adenosylmethionine synthetase of bakers' yeast and its separation into two forms. J Biol Chem. 1977 Jul 10;252(13):4506–4513. [PubMed] [Google Scholar]
- Corrales F., Giménez A., Alvarez L., Caballería J., Pajares M. A., Andreu H., Parés A., Mato J. M., Rodés J. S-adenosylmethionine treatment prevents carbon tetrachloride-induced S-adenosylmethionine synthetase inactivation and attenuates liver injury. Hepatology. 1992 Oct;16(4):1022–1027. doi: 10.1002/hep.1840160427. [DOI] [PubMed] [Google Scholar]
- Corrales F., Ochoa P., Rivas C., Martin-Lomas M., Mato J. M., Pajares M. A. Inhibition of glutathione synthesis in the liver leads to S-adenosyl-L-methionine synthetase reduction. Hepatology. 1991 Sep;14(3):528–533. [PubMed] [Google Scholar]
- Duce A. M., Ortíz P., Cabrero C., Mato J. M. S-adenosyl-L-methionine synthetase and phospholipid methyltransferase are inhibited in human cirrhosis. Hepatology. 1988 Jan-Feb;8(1):65–68. doi: 10.1002/hep.1840080113. [DOI] [PubMed] [Google Scholar]
- Horikawa S., Ishikawa M., Ozasa H., Tsukada K. Isolation of a cDNA encoding the rat liver S-adenosylmethionine synthetase. Eur J Biochem. 1989 Oct 1;184(3):497–501. doi: 10.1111/j.1432-1033.1989.tb15042.x. [DOI] [PubMed] [Google Scholar]
- Horikawa S., Tsukada K. Molecular cloning and nucleotide sequence of cDNA encoding the human liver S-adenosylmethionine synthetase. Biochem Int. 1991 Sep;25(1):81–90. [PubMed] [Google Scholar]
- Kotb M., Kredich N. M. S-Adenosylmethionine synthetase from human lymphocytes. Purification and characterization. J Biol Chem. 1985 Apr 10;260(7):3923–3930. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liau M. C., Chang C. F., Belanger L., Grenier A. Correlation of isozyme patterns of S-adenosylmethionine synthetase with fetal stages and pathological states of the liver. Cancer Res. 1979 Jan;39(1):162–169. [PubMed] [Google Scholar]
- Liau M. C., Lin G. W., Hurlbert R. B. Partial purification and characterization of tumor and liver S-adenosylmethionine synthetases. Cancer Res. 1977 Feb;37(2):427–435. [PubMed] [Google Scholar]
- Markham G. D., DeParasis J., Gatmaitan J. The sequence of metK, the structural gene for S-adenosylmethionine synthetase in Escherichia coli. J Biol Chem. 1984 Dec 10;259(23):14505–14507. [PubMed] [Google Scholar]
- Markham G. D., Hafner E. W., Tabor C. W., Tabor H. S-Adenosylmethionine synthetase from Escherichia coli. J Biol Chem. 1980 Oct 10;255(19):9082–9092. [PubMed] [Google Scholar]
- Markham G. D., Hafner E. W., Tabor C. W., Tabor H. S-adenosylmethionine synthetase (methionine adenosyltransferase) (Escherichia coli). Methods Enzymol. 1983;94:219–222. doi: 10.1016/s0076-6879(83)94037-5. [DOI] [PubMed] [Google Scholar]
- Marston F. A. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986 Nov 15;240(1):1–12. doi: 10.1042/bj2400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto C., Suma Y., Tsukada K. The alpha-form of S-adenosylmethionine synthetase isozymes from liver is principally functional. J Biochem. 1984 Apr;95(4):1223–1226. doi: 10.1093/oxfordjournals.jbchem.a134714. [DOI] [PubMed] [Google Scholar]
- Mitsui K., Teraoka H., Tsukada K. Complete purification and immunochemical analysis of S-adenosylmethionine synthetase from bovine brain. J Biol Chem. 1988 Aug 15;263(23):11211–11216. [PubMed] [Google Scholar]
- Okada G., Teraoka H., Tsukada K. Multiple species of mammalian S-adenosylmethionine synthetase. Partial purification and characterization. Biochemistry. 1981 Feb 17;20(4):934–940. doi: 10.1021/bi00507a045. [DOI] [PubMed] [Google Scholar]
- Pajares M. A., Durán C., Corrales F., Pliego M. M., Mato J. M. Modulation of rat liver S-adenosylmethionine synthetase activity by glutathione. J Biol Chem. 1992 Sep 5;267(25):17598–17605. [PubMed] [Google Scholar]
- Peleman J., Boerjan W., Engler G., Seurinck J., Botterman J., Alliotte T., Van Montagu M., Inzé D. Strong cellular preference in the expression of a housekeeping gene of Arabidopsis thaliana encoding S-adenosylmethionine synthetase. Plant Cell. 1989 Jan;1(1):81–93. doi: 10.1105/tpc.1.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleman J., Saito K., Cottyn B., Engler G., Seurinck J., Van Montagu M., Inzé D. Structure and expression analyses of the S-adenosylmethionine synthetase gene family in Arabidopsis thaliana. Gene. 1989 Dec 14;84(2):359–369. doi: 10.1016/0378-1119(89)90510-6. [DOI] [PubMed] [Google Scholar]
- Sakata S. F., Shelly L. L., Ruppert S., Schutz G., Chou J. Y. Cloning and expression of murine S-adenosylmethionine synthetase. J Biol Chem. 1993 Jul 5;268(19):13978–13986. [PubMed] [Google Scholar]
- Satishchandran C., Taylor J. C., Markham G. D. Isozymes of S-adenosylmethionine synthetase are encoded by tandemly duplicated genes in Escherichia coli. Mol Microbiol. 1993 Aug;9(4):835–846. doi: 10.1111/j.1365-2958.1993.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Satishchandran C., Taylor J. C., Markham G. D. Novel Escherichia coli K-12 mutants impaired in S-adenosylmethionine synthesis. J Bacteriol. 1990 Aug;172(8):4489–4496. doi: 10.1128/jb.172.8.4489-4496.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sullivan D. M., Hoffman J. L. Fractionation and kinetic properties of rat liver and kidney methionine adenosyltransferase isozymes. Biochemistry. 1983 Mar 29;22(7):1636–1641. doi: 10.1021/bi00276a017. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Methionine adenosyltransferase (S-adenosylmethionine synthetase) and S-adenosylmethionine decarboxylase. Adv Enzymol Relat Areas Mol Biol. 1984;56:251–282. doi: 10.1002/9780470123027.ch4. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Rothstein R., Rosenberg N., Surdin-Kerjan Y. SAM2 encodes the second methionine S-adenosyl transferase in Saccharomyces cerevisiae: physiology and regulation of both enzymes. Mol Cell Biol. 1988 Dec;8(12):5132–5139. doi: 10.1128/mcb.8.12.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Surdin-Kerjan Y. SAM1, the structural gene for one of the S-adenosylmethionine synthetases in Saccharomyces cerevisiae. Sequence and expression. J Biol Chem. 1987 Dec 5;262(34):16704–16709. [PubMed] [Google Scholar]
- Thomas D., Surdin-Kerjan Y. The synthesis of the two S-adenosyl-methionine synthetases is differently regulated in Saccharomyces cerevisiae. Mol Gen Genet. 1991 Apr;226(1-2):224–232. doi: 10.1007/BF00273607. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Garofalo J., Goldberg B., Ciminelli M. A., Ruggiero V., Sufrin J. R., Bacchi C. J. S-adenosylmethionine synthetase in bloodstream Trypanosoma brucei. Biochim Biophys Acta. 1993 Mar 24;1181(1):68–76. doi: 10.1016/0925-4439(93)90092-f. [DOI] [PubMed] [Google Scholar]