Abstract
Background
Breathing exercises for people with chronic obstructive pulmonary disease (COPD) aim to alter respiratory muscle recruitment, improve respiratory muscle performance and reduce dyspnoea. Although some studies have reported positive short‐term physiological effects of breathing exercises in people with COPD, their effects on dyspnoea, exercise capacity and well being are unclear.
Objectives
To determine whether breathing exercises in people with COPD have beneficial effects on dyspnoea, exercise capacity and health‐related quality of life compared to no breathing exercises in people with COPD; and to determine whether there are any adverse effects of breathing exercises in people with COPD.
Search methods
The Cochrane Airways Group Specialised Register of trials and the PEDro database were searched from inception to October 2011.
Selection criteria
We included randomised parallel trials that compared breathing exercises to no breathing exercises or another intervention in people with COPD.
Data collection and analysis
Two review authors independently extracted data and assessed the risk of bias. Primary outcomes were dyspnoea, exercise capacity and health‐related quality of life; secondary outcomes were gas exchange, breathing pattern and adverse events. To determine whether effects varied according to the treatment used, we assessed each breathing technique separately.
Main results
Sixteen studies involving 1233 participants with mean forced expiratory volume in one second (FEV1) 30% to 51% predicted were included. There was a significant improvement in six‐minute walk distance after three months of yoga involving pranayama timed breathing techniques (mean difference to control 45 metres, 95% confidence interval 29 to 61 metres; two studies; 74 participants), with similar improvements in single studies of pursed lip breathing (mean 50 metres; 60 participants) and diaphragmatic breathing (mean 35 metres; 30 participants). Effects on dyspnoea and health‐related quality of life were inconsistent across trials. Addition of computerised ventilation feedback to exercise training did not provide additional improvement in dyspnoea‐related quality of life (standardised mean difference ‐0.03; 95% CI ‐0.43 to 0.49; two studies; 73 participants) and ventilation feedback alone was less effective than exercise training alone for improving exercise endurance (mean difference ‐15.4 minutes; 95% CI ‐28.1 to ‐2.7 minutes; one study; 32 participants). No significant adverse effects were reported. Few studies reported details of allocation concealment, assessor blinding or intention‐to‐treat analysis.
Authors' conclusions
Breathing exercises over four to 15 weeks improve functional exercise capacity in people with COPD compared to no intervention; however, there are no consistent effects on dyspnoea or health‐related quality of life. Outcomes were similar across all the breathing exercises examined. Treatment effects for patient‐reported outcomes may have been overestimated owing to lack of blinding. Breathing exercises may be useful to improve exercise tolerance in selected individuals with COPD who are unable to undertake exercise training; however, these data do not suggest a widespread role for breathing exercises in the comprehensive management of people with COPD.
Keywords: Humans; Breathing Exercises; Forced Expiratory Volume; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/rehabilitation; Randomized Controlled Trials as Topic; Yoga
Plain language summary
Breathing exercises for chronic obstructive pulmonary disease
People with chronic obstructive pulmonary disease (COPD) often have an altered breathing pattern and experience shortness of breath, particularly when they exercise. This review aimed to determine whether breathing exercises that are designed to retrain the breathing pattern could reduce breathlessness, increase exercise capacity and improve well being for people with COPD.
Sixteen trials with 1233 participants were included, most of whom had severe COPD. The breathing techniques studied included pursed lip breathing (breathing out slowly with the lips in a whistling position), diaphragmatic breathing (deep breathing focusing on the abdomen), pranayam yoga breathing (timed breathing with a focus on exhalation), changing the breathing pattern using computerised feedback to slow the respiratory rate and increase exhalation time, or combinations of these techniques. The study quality was generally low. Breathing exercises appeared to be safe for people with COPD. Yoga breathing, pursed lip breathing and diaphragmatic breathing improved the distance walked in six minutes by an average of 35 to 50 metres in four studies. Effects of breathing exercises on shortness of breath and well being were variable. When added to whole body exercise training, breathing exercises did not appear to have any additional benefit.
Summary of findings
Summary of findings for the main comparison. Pursed lip breathing compared to no breathing exercises for COPD.
| Pursed lip breathing compared to no breathing exercises for COPD | ||||||
| Patient or population: individuals with COPD Settings: outpatient Intervention: pursed lip breathing Comparison: no breathing exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No breathing exercises | Pursed lip breathing | |||||
|
Dyspnoea during exercise
Modified Borg Dyspnoea score Scores range from 0 to 10, with lower scores indicating less shortness of breath Follow‐up: 12 weeks |
4 units | The mean dyspnoea score during exercise in the intervention groups was 1 unit lower (2.1 lower to 0.1 higher) | ‐1.00 units (‐2.10 to 0.10 units) | 19 (1 study) | ⊕⊕⊝⊝ Lowa |
The CI crosses zero but does not rule out a small effect |
|
Dyspnoea during daily life
University of California San Diego Shortness of Breath Questionnaire Scores range from 0 to 120, with lower scores indicating less shortness of breath Follow‐up: 12 weeks |
69 units | The mean dyspnoea score during daily life in the intervention groups was 10 units lower (28.89 lower to 8.89 higher) | ‐10.00 units (‐28.99 to 8.89 units) | 19 (1 study) | ⊕⊕⊝⊝ Lowa |
The CI crosses zero but does not rule out an effect |
| Walking capacity 6‐minute walk distance (metres) Follow‐up: 8 weeks | 233 metres | The mean walking distance in the intervention groups was 50.1 metres higher (37.21m to 62.99m higher) | 10.10 metres (37.21 to 62.99 metres) | 30 (1 study) | ⊕⊕⊝⊝ Lowb |
This exceeds the minimum clinically important difference of 25 to 35 m |
|
Health‐related quality of life
Dyspnoea domain of Hiratsuka scale Scores range from 0 to 100, with lower scores indicating less shortness of breath Follow‐up: 8 to 12 weeks |
46 units | The mean quality of‐life score in the intervention groups was 12.94 units better (lower) (22.29 lower to 3.6 lower) | ‐12.94 units (‐22.29 to ‐3.60 units) | 60 (2 studies) | ⊕⊕⊝⊝ Lowb |
|
| *The basis for the assumed risk (e.g. the mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a ‐2 for risk of bias; no blinding of assessors reported, incomplete outcome data.
b‐2 for risk of bias; No details regarding sequence generation, allocation concealment, assessor blinding or intention to treat analysis.
Summary of findings 2. Diaphragmatic breathing compared to no breathing exercises for COPD.
| Diaphragmatic breathing compared to no breathing exercises for COPD | ||||||
| Patient or population: individuals with COPD Settings: outpatient Intervention: diaphragmatic breathing Comparison: no breathing exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No breathing exercises | Diaphragmatic breathing | |||||
|
Dyspnoea
Change in Medical Research Council Score
Follow‐up: 4 weeks Scores range from 1 to 5, with lower scores indicating less shortness of breath |
Decrease of 0.33 units | The mean reduction in dyspnoea score in the intervention groups was 0.27 units greater (0.76 greater to 0.22 smaller) | ‐0.27 units (‐0.76 to 0.22 units) | 30 (1 study) | ⊕⊕⊕⊝ Moderatea |
|
| Walking capacity Change in 6‐minute walk distance (metres) Follow‐up: 4 weeks | Reduction of 8 metres | The mean walking distance in the intervention groups was 34.67 metres greater (4.05 higher to 65.29 higher) | 34.67 metres (4.05 to 65.29 metre) | 30 (1 study) | ⊕⊕⊕⊝ Moderatea |
Mean change exceeds the minimal important difference of 25 to 35 m |
|
Health‐related quality of life
Change in total score of St George Respiratory Questionnaire
Follow‐up: 4 weeks Scores range from 0 to 100, with lower scores indicating better quality of life |
Increase of 0.8 units | The mean change in quality of life score in the intervention groups was 10.51 units lower (better) (17.77 lower to 3.25 lower) | ‐10.51 units (‐17.77 to ‐3.25 units) | 30 (1 study) | ⊕⊕⊕⊝ Moderatea |
Mean change exceeds the minimal important difference of 4 points |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a ‐1 for imprecision; estimates from a single study
Summary of findings 3. Yoga compared to no breathing exercises for COPD.
| Yoga compared to no breathing exercises for COPD | ||||||
| Patient or population: individuals with COPD Settings: outpatient Intervention: yoga Comparison: no breathing exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No breathing exercises | Yoga | |||||
|
Dyspnoea intensity
Modified Borg Scale at end of 6‐minute walk test
Follow‐up: 12 weeks Scores range |
3.3 units | The mean dyspnoea intensity in the intervention group was 0.5 units higher (0.99 lower to 1.99 higher) | 0.50 units (‐0.99 to 1.99 units) | 29 (1 study) | ⊕⊕⊝⊝ Lowa |
CI crosses zero; effect unlikely to be clinically significant |
|
Dyspnoea distress
Modified Borg scale at end of 6‐minute walk test Follow‐up: 12 weeks |
1.4 units | The mean dyspnoea distress in the intervention group was 0.2 units higher (0.97 lower to 1.37 higher) | 0.20 units (‐0.97 to 1.37 units) | 29 (1 study) | ⊕⊕⊝⊝ Lowa |
CI crosses zero; effect unlikely to be clinically significant |
| Walking capacity Change in 6‐minute walk distance (m) Follow‐up: 12 weeks | Reduction of 6.38 m | The mean walking distance in the intervention groups was 44.51 m higher (28.47 higher to 60.55 higher) | 44.51 metres (28.47 to 60.55 metres) | 74 (2 studies) | ⊕⊕⊕⊝ Moderateb |
Mean change exceeds the minimal important difference of 25 to 35 metres |
| Health‐related quality of life Total score for St Georges Respiratory Questionnaire Follow‐up: 12 weeks | Reduction of 1.2 units | The mean quality of life score in the intervention group was 5.3 units lower (7.82 lower to 2.78 lower) | ‐5.30 units (‐7.82 to ‐2.78 units) | 45 (1 study) | ⊕⊕⊕⊝ Moderateb |
Mean change exceeds the minimal important difference of 4 units |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a ‐2 for risk of bias; no report of method of random sequence generation, allocation concealment, assessor blinding or intention‐to‐treat analysis.
b ‐1 for risk of bias; one study reported assessor blinding; no reports of allocation concealment or intention to treat analysis.
Summary of findings 4. Pursed lip breathing compared to expiratory muscle training for COPD.
| Pursed lip breathing compared to expiratory muscle training for COPD | ||||||
| Patient or population: individuals with COPD Settings: outpatient Intervention: pursed lip breathing Comparison: expiratory muscle training | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Expiratory muscle training | Pursed lip breathing | |||||
|
Dyspnoea during exercise
Modified Borg dyspnoea score at end of 6‐minute walk test Scores range from 0 to 10, with lower scores indicating less shortness of breath Follow‐up: 12 weeks |
3.9 units | The mean dyspnoea score following 6 minutes of walking in the intervention group was 0.9 units lower (1.71 lower to 0.09 lower) | ‐0.90 units (‐1.71 to ‐0.09 units) | 17 (1 study) | ⊕⊕⊝⊝ Lowa |
CI does not cross zero; small effect |
|
Dyspnoea during daily life
University of California San Diego Shortness of Breath Questionnaire Scores range from 0 to 120, with lower scores indicating less shortness of breath Follow‐up: 12 weeks |
68 units | The mean dyspnoea score during daily life in the intervention group was 9 units lower (28.41 lower to 10.41 higher) | ‐9.00 units (‐28.41 to 10.41 units) | 17 (1 study) | ⊕⊕⊝⊝ Lowa |
CI crosses zero; mean difference does not rule out an effect |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a ‐2 for imprecision and risk of bias; results from one study and no assessor blinding reported.
Summary of findings 5. Ventilation feedback training compared to exercise training for COPD.
| Respiratory biofeedback training compared to exercise training for COPD | ||||||
| Patient or population: individuals with COPD Settings: outpatient Intervention: respiratory biofeedback training Comparison: exercise training | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exercise training | Respiratory biofeedback training | |||||
| Exercise endurance Duration of constant work rate exercise test (minutes) Follow‐up: 15 weeks | 31.5 minutes | The mean exercise endurance time in the ventilation feedback group was 15.4 minutes lower (28.1 lower to 2.7 lower) | ‐15.40 minutes (‐28.10 to ‐2.7 minutes) | 32 (1 study) | ⊕⊕⊝⊝ Lowa |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease; VF: ventilation feedback training | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a ‐2 for imprecision and risk of bias; results from one study only and no assessor blinding reported.
Summary of findings 6. Ventilation feedback training plus exercise compared to exercise alone for COPD.
| Respiratory biofeedback training plus exercise compared to exercise alone for COPD | ||||||
| Patient or population: individuals with COPD Settings: outpatient Intervention: respiratory biofeedback training plus exercise Comparison: exercise alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exercise alone | Respiratory biofeedback training plus exercise | |||||
| Exercise endurance Duration of constant work rate exercise test on treadmill Follow‐up: 15 weeks | 31.5 minutes | The mean exercise endurance time in the VF plus exercise group was 8.5 minutes higher (4.38 lower to 21.38 higher) | 8.50 minutes (‐4.38 to 21.38 minutes) | 33 (1 study) | ⊕⊕⊝⊝ Lowa |
|
| Walking capacity Change in 6‐minute walk distance (metres) Follow‐up: 4 weeks | Increase of 36.2 metres | The mean increase in walking distance in the VF plus exercise group was 12.58 metres lower (35.93 lower to 10.77 higher) | ‐12.58 metres (‐35.93 to 10.77 metres) | 40 (1 study) | ⊕⊕⊝⊝ Lowb |
CI crosses zero; unlikely to be a clinically significant effect |
|
Dyspnoea during treadmill walking
Borg scale at isotime during constant work rate treadmill test Scale ranges from 0 to 10, with lower scores indicating less shortness of breath Follow‐up: 15 weeks |
2.2 units | The mean dyspnoea score during treadmill walking in the intervention group was 0.9 units lower (2.25 lower to 0.45 higher) | ‐0.90 units (‐2.25 to 0.45 units) | 33 (1 study) | ⊕⊕⊝⊝ Lowa |
CI crosses zero; does not rule out a small effect |
|
Dyspnoea after walking
Change in Borg scale at end of 6‐minute walk test Scale ranges from 0 to 10, with lower scores indicating less shortness of breath Follow‐up: 4 weeks |
Increase of 0.3 units | The mean dyspnoea score after 6 minutes of walking in the VF plus exercise group was 0.4 units lower (1.26 lower to 0.46 higher) | ‐0.40 units (‐1.26 to 0.46 units) | 40 (1 study) | ⊕⊕⊝⊝ Lowa |
CI crosses zero; does not rule out a small effect |
|
Health‐related quality of life
Dyspnoea domain of Chronic Respiratory Disease Questionnaire Scale ranges from 1 to 7, with higher scores indicating less shortness of breath |
6 units | The mean quality of life score in the VF plus exercise groups was 0.03 standard deviations higher (better) (0.43 lower to 0.49 higher) | 0.03 units (‐0.43 to 0.49 units) | 73 (2 studies) | ⊕⊕⊕⊝ Moderateb |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease; VF: ventilation feedback training | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a ‐2 for imprecision and risk of bias; results from single study and no assessor blinding reported.
b ‐1 for risk of bias; no assessor blinding reported.
Background
Chronic obstructive pulmonary disease (COPD) is characterised by progressive airflow obstruction and lung hyperinflation owing to loss of elastic recoil and air trapping. These physiological changes are associated with an altered pattern of ventilatory muscle recruitment. The musculature of the rib cage makes an increased contribution to chest wall movement and there is increased activity of the accessory muscles of ventilation (Levine 1988; Martinez 1990). In the event of inspiratory muscle fatigue, there may be asynchrony between rib cage and abdominal movement with paradoxical abdominal indrawing during inspiration (Gilmartin 1984).
Breathing exercises aim to alter respiratory muscle recruitment in order to reduce dyspnoea, lessen hyperinflation, improve respiratory muscle performance and optimise thoraco‐abdominal motion (Gosselink 2003). A number of breathing techniques have been used in COPD, including diaphragmatic breathing (DB) (also known as breathing control or abdominal breathing); pursed lip breathing (PLB); active expiration; pranayama yoga consisting of timed breathing techniques with a focus on expiration; and ventilation feedback training, where participants aim to achieve individualised goals for respiratory rate and pattern with computerised feedback. These techniques may result in acute improvements in gas exchange and ventilation (Breslin 1992; Vitacca 1998); however, effects on important clinical outcomes such as dyspnoea, exercise capacity and health‐related quality of life have not consistently been identified (Garrod 2005; Mueller 1970; Nield 2007). Some breathing techniques may increase dyspnoea and reduce the mechanical efficiency of breathing (Gosselink 1995; Vitacca 1998). The clinical utility of breathing exercises in COPD is therefore unclear.
The impact of breathing exercises in patients with COPD may vary according to underlying physiology, the technique employed and the conditions of training. Some authors have reported that breathing exercises reduce dyspnoea in patients who are severely obstructed and hyperinflated (Bianchi 2007), while others have found no physiological predictors of response (Garrod 2005). DB, which involves active abdominal muscle recruitment, may have different effects compared to PLB that focuses on passive, prolonged expiration. Breathing exercises performed during exercise may have different clinical benefits compared to training performed only at rest (Mueller 1970). To date the most effective type of breathing exercises and the patients to whom they are suited have not been identified.
This review was conducted to summarise the results of literature evaluating the safety and efficacy of breathing exercises in people with COPD, and to determine the effects of breathing exercises on dyspnoea, exercise tolerance and health‐related quality of life in this patient group.
Objectives
To determine whether breathing exercises in people with COPD have beneficial effects on dyspnoea, exercise capacity and health‐related quality of life compared to no breathing exercises in people with COPD.
To determine whether there are any adverse effects of breathing exercises in people with COPD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials in which breathing exercises were compared to no breathing exercises or another therapy in patients with COPD were considered. Cross‐over trials were not included as the nature of the intervention required learning of a new skill that could affect subsequent comparison conditions.
Types of participants
Adults with a clinical diagnosis of COPD in a stable condition, diagnosed according to the investigators' definition, were included.
Types of interventions
We considered any type of breathing exercises, either supervised or unsupervised. Breathing exercises was defined as any technique that aimed to alter the respiratory pattern. This could be achieved with or without external devices, and either during exercise or at rest. PLB, DB, ventilation‐feedback training and yoga breathing were eligible for inclusion. As responses to different types of breathing exercises may vary, these interventions were assessed separately. The precise nature of the training (intensity, frequency, duration, type) was recorded wherever possible. Trials where breathing exercises were combined with another training intervention (e.g. relaxation) were included provided 50% or more of the training consisted of breathing exercises.
Comparisons to be examined were:
breathing exercises versus no breathing exercises;
breathing exercises versus another intervention;
breathing exercises combined with another intervention versus no breathing exercises.
Types of outcome measures
Primary outcomes
Dyspnoea: all measures of dyspnoea used were considered, measured either at rest or during exercise.
Functional or maximal exercise capacity, measured during either formal exercise tests or field exercise tests.
Health‐related quality of life: change in health‐related quality of life as measured by generic or disease‐specific quality of life instruments. All quality of life instruments used were considered.
Secondary outcomes
Gas exchange (e.g. PaO2, PaCO2).
Ventilation (e.g. minute ventilation, tidal volume).
Energy cost (e.g. oxygen consumption).
Breathing pattern (e.g. respiratory frequency, chest wall kinematics).
Adverse events.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). Trials of COPD in MEDLINE were identified using the strategy described in Appendix 1. All records added to the Specialised Register and coded as 'COPD' were searched using the following terms: breath* or "ventilation‐feedback training" or "yoga" or "chest physiotherapy" or "chest physical therapy".
In addition, we searched the Physiotherapy Evidence Database (PEDro) with the following terms: COPD AND breathing. The Register and PEDro were both searched from their inception up to October 2011 and no language restrictions were applied.
Searching other resources
Reference lists of all primary studies and review articles were reviewed for additional references. Authors of identified trials were contacted and asked to identify other published and unpublished studies. Experts in the field were also contacted.
Data collection and analysis
Selection of studies
Two review authors (AH and CH) independently coded studies identified in the literature searches for relevance by examining titles, abstract and keywords fields as follows:
INCLUDE: study categorically met all review criteria;
UNCLEAR: study appeared to meet some review criteria but insufficient information available to categorically determine relevance;
EXCLUDE: study did not categorically meet all review criteria
Two review authors used a full‐text copy of studies in categories 1 and 2 to decide on study inclusion. Disagreements were resolved by consensus. A full record of decisions was kept and simple agreement and kappa statistics calculated.
Data extraction and management
Data were extracted independently by two review authors using a prepared checklist before being entered into Review Manager (RevMan 2011) by the primary review author (AH), with random checks on accuracy. Disagreements were resolved by consensus. Data included characteristics of included studies (methods, participants, interventions, outcomes) and results of the included studies. Authors of included studies were asked to provide details of missing data where applicable.
Assessment of risk of bias in included studies
Two review authors assessed the internal validity of included studies using the approach recommended in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011) including sequence generation for randomisation, allocation concealment, blinding of participants and assessors, loss to follow‐up, completeness of outcome assessment, selective outcome reporting and other possible sources of bias). We judged each domain as high, low or unclear risk of bias and recorded our decision and rationale in a 'Risk of bias' table. Disagreements were resolved by consensus. We contacted study authors to seek clarification where quality was unclear.
Data synthesis
For continuous variables, we recorded either the mean change from baseline or the mean post‐intervention values and standard deviation (SD) for each group. The mean difference (MD) for outcomes measured with the same metrics or standardised mean difference (SMD) for outcomes measured with different metrics with 95% confidence intervals (CI) were calculated using RevMan 2011. For binary outcome measures, we recorded the number of participants with each outcome event, by allocated treated group, to allow an intention‐to‐treat analysis. The odds ratio (OR) with 95% CIs were calculated for each study. We performed a pooled quantitative analysis where trials were clinically homogeneous.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses specified a priori were:
breathing exercises during exercise: interventions delivered during exercise may have a greater effect than those delivered at rest; and
severity of lung disease: patients with more advanced disease may obtain greater benefit from breathing exercises. Participants were considered to have severe disease if forced expiratory volume in one second (FEV1) was less than 50% predicted.
However, insufficient data were available to perform these analyses. The specified subgroup analyses will be performed in future updates if more data become available.
Homogeneity of effect sizes between pooled studies was examined with the I2 statistic. In the absence of heterogeneity, the fixed‐effect model was used; otherwise a random‐effects model was used. Funnel plots were inspected to assess publication bias where appropriate.
Sensitivity analysis
Sensitivity analyses were performed where there were sufficient data, to analyse the effects of allocation concealment, assessor blinding and use of intention‐to‐treat analysis on results.
Results
Description of studies
Results of the search
The search returned 956 references, after duplicates were removed. An additional record was identified through correspondence with an author and five records were identified through handsearching of study reference lists. Eight hundred and ninety seven records were excluded based on title and abstract, with 64 records from 52 studies retrieved for full‐text review. Thirty‐six studies (38 records) were excluded after full‐text review as they did not meet the review criteria (see Characteristics of excluded studies). A total of 16 studies (26 records) were appropriate for inclusion in the review (Figure 1). Agreement was high, with review authors reaching consistent decisions for all but two studies (kappa = 0.91) with agreement achieved by discussion and consensus.
1.
Study flow diagram.
Included studies
This review included 16 randomised controlled trials (RCTs). Ten studies were published in English, one study was published in French (Lausin 2009) and five studies were published in Chinese journals (Li 2002; Sun 2003; Wu 2006; Yan 1996; Zhang 2008). Further details are provided in Characteristics of included studies. We attempted to contact the authors of 12 studies to obtain additional details regarding study design or outcomes and received responses from two authors, who provided additional data.
Population
The sample size of included studies varied from 21 to 324 participants (total 1233 participants randomised) with mean age ranging from 51 to 73 years and mean FEV1 from 30 to 51 % predicted, indicating severe to very severe disease (Rabe 2007).
Setting
Studies were conducted in China, Europe, USA, Brazil, India, Hong Kong and the UK. Most were conducted in outpatient clinics of hospitals. The studies were conducted and published over a wide time period (1965 to 2012), with 13 studies published since 2000.
Intervention
A wide variety of breathing exercises were tested, with some studies testing more than one intervention. The interventions included PLB (three studies), DB (three studies), yoga (two studies), respiratory biofeedback (two studies), respiratory muscle gymnastics (breathing exercises performed during a serious of five physical exercises including trunk rotation, bending and leg exercises, one study), deep breathing exercises with an inspiratory hold and slow expiration (Zhang 2008) and balloon inflation (one study). A number of studies examined a package of breathing exercises including PLB and respiratory muscle gymnastics (two studies) and PLB and DB (two studies). In one report the exact nature of the breathing exercises was not stated (Saunders 1965). In two studies, both utilising respiratory biofeedback, the breathing intervention was delivered during exercise training and compared to exercise training alone (Collins 2008; van Gestel 2011). One of these studies also compared respiratory biofeedback alone to exercise training alone (Collins 2008). In both studies the respiratory biofeedback was administered using a computerised system with on‐screen visual prompts for expiratory time and respiratory rate.
Sham or placebo interventions were rare, with the most common control condition being usual care (10 studies). One study used oral capsules as a placebo condition (Yan 1996). Other active comparison treatments were expiratory muscle training (EMT) (Nield 2007), inspiratory muscle training (Noseda 1987) and Tai Chi Qigong (Chan 2011). The duration of the breathing exercises interventions varied from a single session to 20 months. The number of supervised sessions ranged from zero to three times per week, with one study providing daily supervision in the first two weeks (Noseda 1987). Twelve out of 16 studies required participants to undertake daily practice of breathing exercises at home for the duration of the study; however, adherence to home practice was not reported.
Excluded studies
Reasons for exclusion were absence of a control group that did not perform breathing exercises (eight studies), cross‐over design (seven studies), breathing exercises comprising less than 50% of the intervention (seven studies), the study was not an RCT (seven studies), participants were not in a stable clinical state (three studies), intervention was not breathing exercises (two studies) and no relevant outcomes (one study). Further details are given in the Characteristics of excluded studies table.
Risk of bias in included studies
Details of the risk of bias across all studies can be seen in Figure 2 and Figure 3. The majority of studies reported few details regarding methods of randomisation, blinding and drop‐outs, which made it difficult to assess study quality accurately.
2.
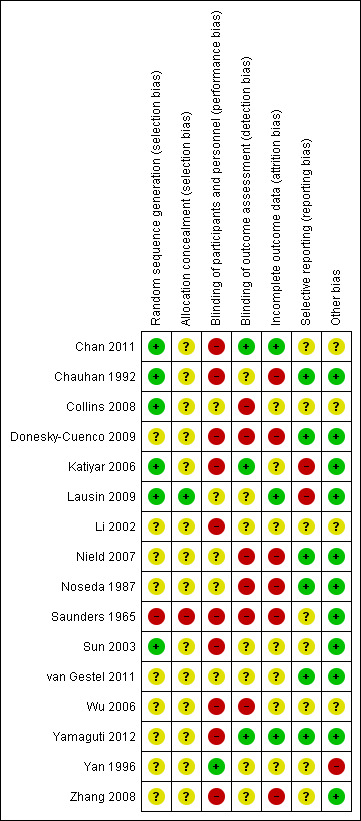
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
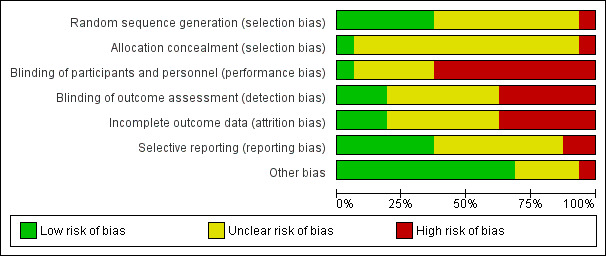
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Six studies provided sufficient detail to confirm adequate sequence generation (Chan 2011; Chauhan 1992; Collins 2008; Katiyar 2006; Lausin 2009; Sun 2003) and only one study provided evidence of allocation concealment (Lausin 2009).
Blinding
Few studies provided any details regarding blinding of participants, personnel or assessors. Due to the physical nature of the intervention, it is unlikely that participants and personnel were blinded in most studies. One study used a placebo capsule as a control intervention (Yan 1996); the remaining 15 studies did not report blinding of participants or study personnel. Blinding of assessors was seldom reported, with only three studies stating that assessors were unaware of group allocation (Chan 2011; Katiyar 2006; Yamaguti 2012). One additional study stated that assessors were blinded for respiratory function and walk test outcomes, but not for quality‐of‐life outcomes (van Gestel 2011). None of the other studies reported blinding of assessors, which may have resulted in detection bias, particularly given that many studies measured outcomes such as symptoms, health‐related quality of life and effort‐dependent exercise tests. A high risk of bias owing to inadequate blinding of outcome assessors was observed in six of the 16 studies.
Incomplete outcome data
Two studies reported an intention‐to‐treat analysis (Chan 2011; Yamaguti 2012), while another reported that an intention‐to‐treat analysis had been performed but did not report these data (Collins 2008). An additional study reported no drop‐outs (Lausin 2009). The remaining studies either did not report the number of drop‐outs, in which case the risk of bias was unclear, or performed only a per‐protocol analysis.
Selective reporting
Most studies documented findings for all pre‐specified outcomes; however, data were not always reported in a format suitable for meta‐analysis. No searches of clinical trials registers were conducted when formulating judgements for this item as most studies were conducted prior to registration requirements.
Other potential sources of bias
Some studies provided little detail regarding the breathing exercises intervention (Saunders 1965; Zhang 2008). Many studies delivered a package of multiple breathing exercises techniques and it was difficult to determine which component of the intervention package might have been be effective (Chan 2011; Li 2002; Saunders 1965; Sun 2003; Yan 1996). One study assessed outcomes at time points ranging from one month to 20 months; it was unclear whether time points of measurement were equivalent in the intervention and control groups (Yan 1996).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Breathing exercises versus no breathing exercises
Primary outcomes
Pursed lip breathing
Two studies on 49 participants examined the effects of PLB on dyspnoea (Nield 2007; Zhang 2008), using three different outcome measures. PLB, taught in once‐weekly sessions for four weeks using pulse oximetry for feedback with daily home practice, did not significantly improve dyspnoea measured on the University of California San Diego Shortness of Breath Questionnaire after four weeks (MD ‐4.00 units; 95% CI ‐20.4 to 12.4 units; Analysis 1.2) or 12 weeks (MD ‐10.00 units; 95% CI ‐28.89 to 8.89 units; Analysis 1.4), although results tended to favour the PLB group (Nield 2007). There was no effect on intensity of dyspnoea measured on the Borg scale at the end of a six‐minute walk test (6MWT) after four weeks (MD 0 units; 95% CI ‐0.76 to 0.76 units; Analysis 1.1); however, there was a trend in favour of PLB after 12 weeks (MD ‐1.00 units; 95% CI ‐2.10 to 0.10 units; Analysis 1.1) (Nield 2007). A significant effect of PLB on dyspnoea measured on the Medical Research Council (MRC) scale was evident after eight weeks of PLB performed three times per day for 15 minutes (MD ‐1.00; 95% CI ‐1.73 to ‐0.27; Analysis 1.3) (Zhang 2008).
1.2. Analysis.
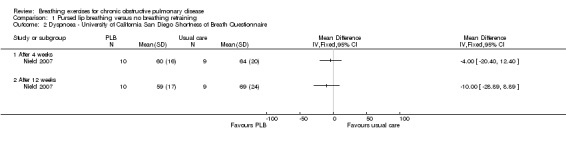
Comparison 1 Pursed lip breathing versus no breathing retraining, Outcome 2 Dyspnoea ‐ University of California San Diego Shortness of Breath Questionnaire.
1.4. Analysis.
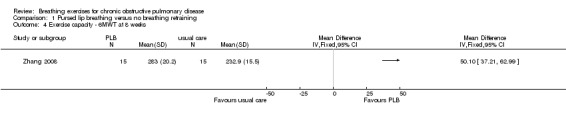
Comparison 1 Pursed lip breathing versus no breathing retraining, Outcome 4 Exercise capacity ‐ 6MWT at 8 weeks.
1.1. Analysis.
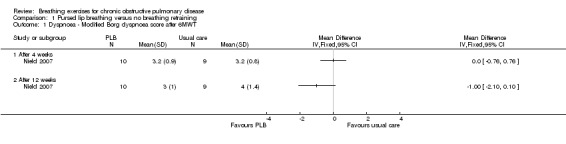
Comparison 1 Pursed lip breathing versus no breathing retraining, Outcome 1 Dyspnoea ‐ Modified Borg dyspnoea score after 6MWT.
1.3. Analysis.
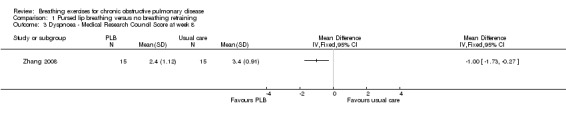
Comparison 1 Pursed lip breathing versus no breathing retraining, Outcome 3 Dyspnoea ‐ Medical Research Council Score at week 8.
In one study with 60 participants, eight weeks of PLB training improved 6MWT by a mean of 50.10 metres (95% CI 37.21 to 62.99 metres; Analysis 1.4) (Zhang 2008).
Changes in health‐related quality of life were not consistent across trials and domains. A study of 27 participants showed significant improvement in the physical function domain of the Short Form‐36 (SF‐36) after 12 weeks of PLB (mean improvement 16 units) compared to a usual care control group (mean improvement 2 units; P = 0.02) (Nield 2007); however baseline health‐related quality of life was lower in the PLB group. Two studies with a pooled total of 60 participants (Wu 2006; Zhang 2008) used the same respiratory disease‐specific quality‐of‐life instrument to measure quality of life (Hiratsuka 1993). Dyspnoea was the only domain to show a significant effect in favour of PLB following 8 to 12 weeks of training (MD ‐12.94 units; 95% CI ‐22.29 to ‐3.60 units; Analysis 1.5). There was no difference between groups for the domains of mood (MD 1.08 units; 95% CI ‐9.60 to 11.75 units), social function (MD 11.69 units; 95% CI ‐0.91 to 24.28 units), headache (MD ‐3.30 units; 95% CI ‐12.37 to 5.77 units), appetite (MD 8.42 units; 95% CI ‐5.30 to 22.15 units), well being (MD 2.16 units; 95% CI ‐19.47 to 23.79 units) or health condition (MD 7.86 units; 95% CI ‐18.88 to 34.61 units; Analysis 1.5). Changes in the housework domain favoured the control group (15.58 units; 95% CI 0.50 to 30.66 units; Analysis 1.5).
1.5. Analysis.
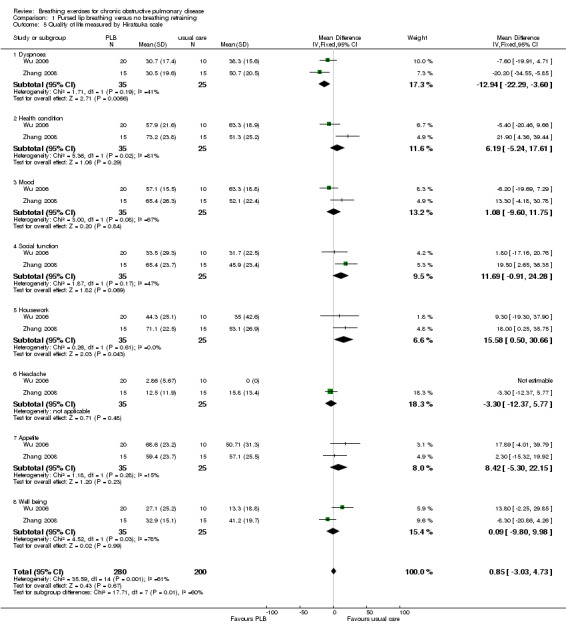
Comparison 1 Pursed lip breathing versus no breathing retraining, Outcome 5 Quality of life measured by Hiratsuka scale.
Diaphragmatic breathing
One study on 21 participants reported the effects of a single session of supervised DB on resting dyspnoea, measured using a visual analogue scale (Lausin 2009). Dyspnoea increased in the DB group after 15 minutes (1.1 units pre‐intervention to 1.4 units post‐intervention), while dyspnoea decreased in a control group who were asked to breathe normally (0.76 units pre‐intervention to 0.62 units post‐intervention). No measures of variability or significance levels were reported. Another study on 30 participants (Yamaguti 2012) reported a greater reduction in dyspnoea measured with the modified Medical Research Council Scale after four weeks of supervised DB training compared to a control group who received usual care; however, the difference was small and unlikely to be clinically significant (MD 0.27 units; 95% CI 0.22 to 0.76 units; Analysis 2.1).
2.1. Analysis.
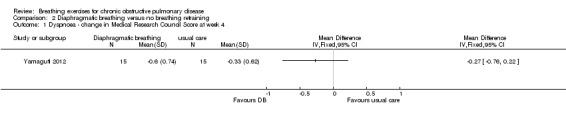
Comparison 2 Diaphragmatic breathing versus no breathing retraining, Outcome 1 Dyspnoea ‐ change in Medical Research Council Score at week 4.
After four weeks of training, the MD for change in 6MWT between participants undergoing DB training and those in a usual care control group was 34.7 metres (95% CI 4.1 to 65.3 metres; Analysis 2.2) (Yamaguti 2012).
2.2. Analysis.
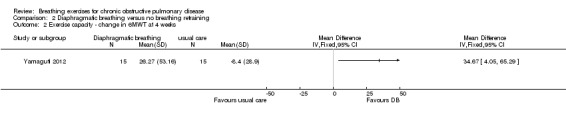
Comparison 2 Diaphragmatic breathing versus no breathing retraining, Outcome 2 Exercise capacity ‐ change in 6MWT at 4 weeks.
Reduction (improvement) in the total score of the St Georges Respiratory Questionnaire (SGRQ) was also reported after four weeks of supervised DB training, with a MD in change scores compared to the control group that exceeded the minimal important difference (MD ‐10.5 points; 95% CI ‐17.7 to ‐3.3 points; Analysis 2.3) (Yamaguti 2012).
2.3. Analysis.
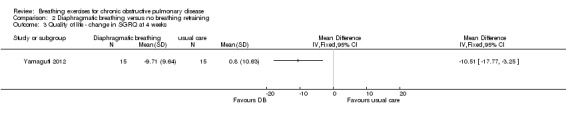
Comparison 2 Diaphragmatic breathing versus no breathing retraining, Outcome 3 Quality of life ‐ change in SGRQ at 4 weeks.
Yoga
One study on 29 participants reported the effects of 12 weeks, twice weekly supervised yoga training incorporating pranayama timed breathing on dyspnoea intensity and dyspnoea distress (Donesky‐Cuenco 2009). At the end of the intervention there was no effect of yoga on dyspnoea intensity at the end of a 6MWT (MD 0.50 units; 95% CI ‐0.99 to 1.99 units) or an incremental cycle ergometer test (MD 0.60 units; 95% CI ‐0.98 to 2.18 units). There was also no effect on dyspnoea distress at end‐exercise (6MWT: MD 0.20 units; 95% CI ‐0.97 to 1.37 units; incremental test: MD 0.50 units; 95% CI ‐1.60 to 2.60 units).
Meta‐analysis of two studies with a pooled total of 74 participants (Donesky‐Cuenco 2009; Katiyar 2006) showed a significant improvement in 6MWT after three months of yoga involving pranayama timed breathing techniques (Figure 4). Sensitivity analysis that excluded one study without assessor blinding (Donesky‐Cuenco 2009) did not reduce the size of the effect. There was no effect on peak work achieved in an incremental cycle test, although the results tended to favour the yoga group (MD 15 watts; 95% CI ‐3.16 to 33.16 watts; Analysis 3.7) (Donesky‐Cuenco 2009).
4.
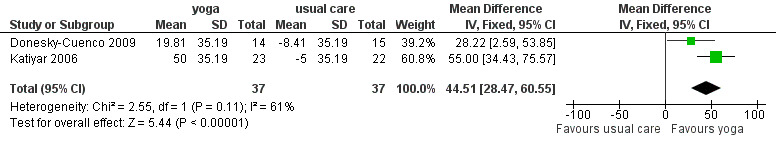
Forest plot of comparison: 3 Yoga versus no breathing exercises, outcome: 3.6 Exercise capacity ‐ change in 6MWT at 3 months.
3.7. Analysis.
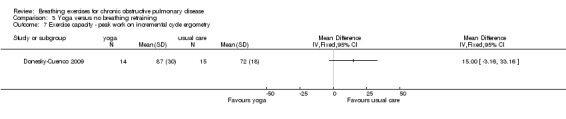
Comparison 3 Yoga versus no breathing retraining, Outcome 7 Exercise capacity ‐ peak work on incremental cycle ergometry.
Statistically significant improvements in all domains of the SGRQ were reported in participants undergoing yoga training after three months (Katiyar 2006); the reduction in score exceeded the threshold for clinically important change in all domains (Jones 1992). The MD between groups for the total score was ‐5.30 units (95% CI ‐7.82 to ‐2.78 units; Analysis 3.8). In contrast, a study of similar duration reported no difference between yoga and control groups for dyspnoea‐related quality of life measured on the Chronic Respiratory Questionnaire (CRQ) dyspnoea domain (MD 1.60 units; 95% CI ‐3.10 to 6.30 units) (Donesky‐Cuenco 2009). There were no differences in quality of life between groups for any other domain of the CRQ, or for the physical and mental component scores of the SF‐36 (Donesky‐Cuenco 2009).
3.8. Analysis.
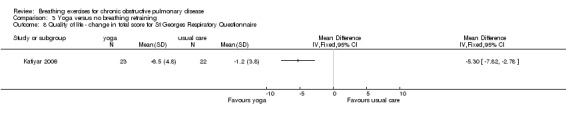
Comparison 3 Yoga versus no breathing retraining, Outcome 8 Quality of life ‐ change in total score for St Georges Respiratory Questionnaire.
Other types of breathing exercises
One study on 28 participants randomised people to receive eight weeks of balloon inflation performed 40 times per day, which resulted in a significant reduction in visual analogue score ratings for severity of breathlessness (median difference 9 units; 95% CI ‐18 to ‐1 unit) (Chauhan 1992). One study of six weeks of breathing exercises performed according to "accepted principles" (page 680) reported that "nine control subjects (20%) and 14 (42%) of those treated stated that they were less short of breath at the end of the three months" (page 681) (Saunders 1965). One study that examined the effects of eight weeks of "breathing exercises performed according to respiratory pathophysiology" (page 3966) (a quick inspiration to total lung capacity, a breath hold, followed by a slow expiration) performed three times daily reported significant improvements in MRC dyspnoea score (MD ‐1.46 units; 95% CI ‐2.19 to ‐0.73 units; Analysis 4.1) (Zhang 2008).
4.1. Analysis.
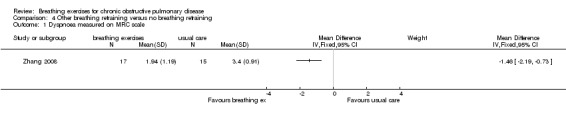
Comparison 4 Other breathing retraining versus no breathing retraining, Outcome 1 Dyspnoea measured on MRC scale.
Balloon inflation 40 times a day for eight weeks had no significant effect on 6MWT (median difference 36.5 metres in favour of control group, 95% CI ‐34 to 120 metres) (Chauhan 1992). Eight weeks of deep breathing with an inspiratory hold and a slow expiration (Zhang 2008) improved 6MWT substantially more than usual care (MD 88.2 metres; 95% 75.28 to 101.12 metres).
Daily balloon inflation for eight weeks did not improve the degree of well being measured on a visual analogue scale compared to a control group (median difference between groups 9 units; 95% CI ‐3 to 21 units) (Chauhan 1992). In contrast, a package of breathing exercises including both PLB and respiratory muscle gymnastics significantly improved disease‐specific quality of life after six months (MD ‐0.27 units; 95% CI ‐0.40 to ‐0.14 units) (Sun 2003). An eight‐week programme of deep breathing exercises with inspiratory holds (Zhang 2008) resulted in significant improvement for two out of eight domains of quality of life (dyspnoea and health condition) compared to the control group.
Limited information regarding study quality makes it difficult to assess the risk of bias affecting these findings.
Secondary outcomes
Pursed lip breathing
One study (Nield 2007) reported that the change in oxyhaemoglobin saturation (SpO2) measured by pulse oximetry between the start and the end of a 6MWT was smaller after four weeks (P = 0.003) and 12 weeks (P = 0.028) of PLB training; however, the data were not reported.
There were no significant effects on breathing frequency, inspiratory time, expiratory time or inspiratory time to expiratory time ratio after 12 weeks of PLB training; however, the data were not reported (Nield 2007). In contrast, a study of three months of PLB training (Wu 2006) reported a significant reduction in breathing frequency at rest compared to a usual care control group (mean ± SD: 25 ± 7 breaths per minute with training versus 31 ± 6.3 breaths per minute with usual care; P < 0.01).
Diaphragmatic breathing
After a single 15‐minute session there was no significant difference in SpO2 between the group that performed DB (mean 98.5%) and the group that performed normal breathing (mean 95.5%) (Lausin 2009). However, transcutaneous carbon dioxide (TcCO2) dropped from 36 mmHg to 27 mmHg in the DB group, while it remained stable in the control group (P < 0.001 for between‐group comparison) (Lausin 2009). No measures of variability were reported and it was not clear whether there were any associated adverse effects in the treatment group.
A single session of DB had no significant effect on respiratory rate; however, no group data were reported (Lausin 2009). Four weeks of DB improved diaphragmatic mobility measured by ultrasound compared to usual care, with a MD in the change in diaphragmatic displacement between groups of 6.14 mm (95% CI 3.12 to 9.16 mm) (Yamaguti 2012). Significant reductions in the amplitude of rib cage to abdominal motion were also reported during normal breathing (effect size ‐0.96) and deep breathing (effect size ‐0.69) (Yamaguti 2012).
Yoga
The only study to report on safety stated that there were no adverse clinical events associated with 12 weeks of yoga training (Donesky‐Cuenco 2009).
After three months of Pranayama yoga there was no difference in PO2 or PCO2 between the intervention and control groups; however, the data were not reported (Katiyar 2006).
Other types of breathing exercises
A study of 89 participants reported that a six‐month intervention involving PLB and respiratory muscle gymnastics (Sun 2003) significantly improved PaO2 (MD 3.00 kPa; 95% CI 2.56 to 3.35 kPa) and reduced PaCO2 (MD ‐1.60 kPa; 95% CI ‐1.84 to ‐1.36 kPa).
A study on 324 participants that compared a package of breathing exercises and respiratory muscle gymnastics to an oral placebo (Yan 1996) reported significant improvements in favour of the breathing exercises group for transdiaphragmatic pressure during normal breathing (MD 0.52 kPa; 95% CI 0.13 to 0.91 kPa) and maximal transdiaphragmatic pressure (MD 2.48 kPa; 95% CI 1.61 to 3.35 kPa). However, outcomes were measured at time points that varied between one month and 20 months; it was unclear whether the intervention and control groups had equivalent follow‐up periods
No studies reported the effects of breathing exercises on ventilation or energy cost.
Breathing exercises versus another intervention
Primary outcomes
Pursed lip breathing versus expiratory muscle training
Dyspnoea measured on the modified Borg scale at the end of a 6MWT was not different between groups after four weeks (MD ‐0.50; 95% CI ‐1.26 to 0.26; Analysis 5.1); however, there was a small but significant difference favouring the PLB group after 12 weeks (MD ‐0.90; 95% CI ‐1.71 to ‐0.09; Analysis 5.1). There were no differences between groups on the UCSD dyspnoea scale at either four weeks (MD ‐3.00 units; 95% CI ‐19.62 to 13.62 units; Analysis 5.2) or 12 weeks (MD ‐28.41; 95% CI ‐28.41 to 10.41 units; Analysis 5.2) (Nield 2007).
5.1. Analysis.
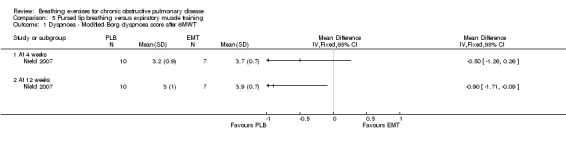
Comparison 5 Pursed lip breathing versus expiratory muscle training, Outcome 1 Dyspnoea ‐ Modified Borg dyspnoea score after 6MWT.
5.2. Analysis.
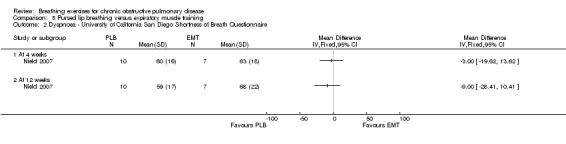
Comparison 5 Pursed lip breathing versus expiratory muscle training, Outcome 2 Dyspnoea ‐ University of California San Diego Shortness of Breath Questionnaire.
There were no studies that compared the effects of PLB and EMT on exercise capacity.
PLB training for 12 weeks improved the physical function domain of the SF‐36 by a mean of 16 units, compared to a reduction of five units in those who undertook EMT (P = 0.02); however, baseline quality of life was lower in the PLB group (Nield 2007).
Diaphragmatic breathing versus inspiratory muscle training
There were no studies comparing the effects of DB training and inspiratory muscle training on dyspnoea. However two months of unsupervised training did not change VO2peak, 12‐minute walk distance, peak work or endurance work in either intervention group (Noseda 1987; data not reported).
Ventilation feedback training versus exercise training
One study on 32 participants investigated the effects of 36 sessions of ventilation feedback training using a computerised system with visual targets for expiratory time, compared to an aerobic exercise training programme of the same duration (Collins 2008). There was no significant difference between groups in dyspnoea scores at isotime during a constant work rate treadmill test following training; however, results tended to favour the exercise training group (MD 1.10 units; 95% CI ‐0.71 to 2.91 units; Analysis 7.7). Both ventilation feedback training and exercise training resulted in clinically significant improvements in dyspnoea‐related quality of life measured on the CRQ, with a mean increase of 6 units in both groups. There was no effect of either intervention on mastery or fatigue; however, the data were not reported. Emotional function improved in the respiratory biofeedback group only; however, no between‐group comparisons were reported for this outcome (Collins 2008).
7.7. Analysis.
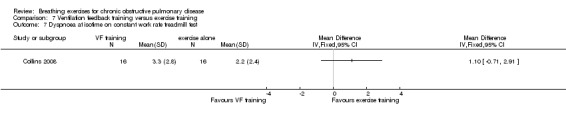
Comparison 7 Ventilation feedback training versus exercise training, Outcome 7 Dyspnoea at isotime on constant work rate treadmill test.
Exercise training was significantly more effective than ventilation feedback training for the duration of constant work rate treadmill exercise (MD ‐15.40 minutes; 95% CI ‐28.10 to ‐2.7 minutes; Analysis 7.1) (Collins 2008).
7.1. Analysis.

Comparison 7 Ventilation feedback training versus exercise training, Outcome 1 Exercise capacity ‐ duration of constant work rate exercise at 15 weeks.
Secondary outcomes
No studies comparing breathing exercises to other interventions reported on adverse events.
Pursed lip breathing versus expiratory muscle training
After 12 weeks there were no significant differences between PLB training and EMT for breathing frequency, inspiratory time, expiratory time or inspiratory time to expiratory time ratio; however, the data were not reported (Nield 2007).
Ventilation feedback training versus exercise training
Oxyhaemoglobin saturation measured at isotime on a constant work rate cycle test did not differ between participants who underwent ventilation feedback training and those who underwent exercise training (MD 0.50%; 95% CI ‐1.62 to 2.62%; Analysis 7.2) (Collins 2008). Similarly, minute ventilation at isotime on a constant work rate treadmill test did not differ between participants who underwent ventilation feedback training and those who underwent exercise training (MD ‐3.70 L/minute; 95% CI ‐11.62 to 4.22 L/minute; Analysis 7.2). Oxygen consumption (VO2) at isotime decreased by a mean of 1.7 mL/kg/minute in both groups following the intervention period. There were no differences between the ventilation feedback group and the exercise alone group for respiratory rate (MD ‐2 breaths; 95% CI ‐6.9 to 2.9 breaths; Analysis 7.4), inspiratory time (MD 0.07 seconds; 95% CI ‐0.09 to 0.23 seconds; Analysis 7.5) or expiratory time (MD 0.27 seconds; 95% CI ‐0.06 to 0.60 seconds) measured at isotime on a constant work rate treadmill test (Collins 2008).
7.2. Analysis.
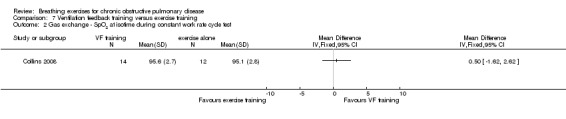
Comparison 7 Ventilation feedback training versus exercise training, Outcome 2 Gas exchange ‐ SpO2 at isotime during constant work rate cycle test.
7.4. Analysis.
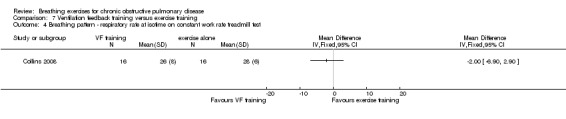
Comparison 7 Ventilation feedback training versus exercise training, Outcome 4 Breathing pattern ‐ respiratory rate at isotime on constant work rate treadmill test.
7.5. Analysis.
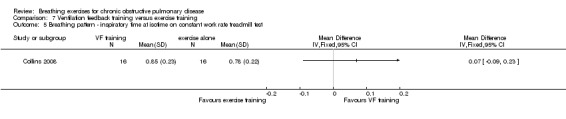
Comparison 7 Ventilation feedback training versus exercise training, Outcome 5 Breathing pattern ‐ inspiratory time at isotime on constant work rate treadmill test.
Breathing exercises combined with another intervention versus no breathing exercises
Primary outcomes
Ventilation feedback training during exercise training compared to exercise training alone
Two studies reported no additional benefit of ventilation feedback during exercise training on dyspnoea during exercise. Thirty‐six sessions of ventilation feedback training using a computerised system with visual targets for expiratory time did not reduce dyspnoea at isotime during a constant work rate treadmill test to a greater extent than exercise training alone (MD ‐0.9 units; 95% CI ‐2.25 to 0.45 units) (Collins 2008). Similarly, the changes in dyspnoea score after 6MWT from the beginning to the end of the intervention period were not different after four weeks of respiratory biofeedback training compared to exercise training (MD ‐0.40 units; 95% CI ‐1.26 to 0.46 units) (van Gestel 2011).
Two studies reported effects on exercise tolerance. Ventilation feedback training during exercise did not increase the duration of a constant work rate treadmill test more than exercise training alone, although the effect tended to favour ventilation feedback training (MD 8.50 minutes; 95% CI ‐4.38 to 21.38 minutes) (Collins 2008). Four weeks of respiratory biofeedback training using visual and acoustic signals during exercise training did not improve 6MWT, with the effect tending to favour the exercise only group (MD ‐12.58 metres, 95% CI ‐35.93 to 10 .77 metres) (van Gestel 2011).
In two studies with a combined total of 73 participants, respiratory biofeedback training had no additional effects on dyspnoea‐related quality of life measured on the CRQ compared to exercise training alone (Figure 5). No differences between groups were reported for the CRQ domains of fatigue or emotional function; however, insufficient data were available for meta‐analysis (Collins 2008; van Gestel 2011). One study reported that the mastery domain of the CRQ improved only in the group that received respiratory biofeedback training as well as exercise (mean improvement in respiratory biofeedback group of 3 units; data for exercise alone group not reported; Collins 2008); however, the other study reported no difference between groups in mastery (MD 0.17 units; 95% CI ‐0.42 to 0.77 units) (van Gestel 2011).
5.
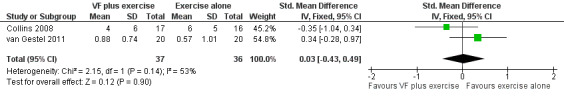
Forest plot of comparison: 6 Respiratory biofeedback training plus exercise versus exercise alone, outcome: 6.5 Quality of Life ‐ Dyspnoea domain of Chronic Respiratory Disease Questionnaire.
Pursed lip breathing, diaphragmatic breathing and nutritional supplementation versus usual care
A package of intervention including breathing exercises significantly improved the quality‐of‐life domains of activities of daily living, social function, depression and anxiety compared to the control group (Li 2002). The total quality‐of‐life score was also significantly better (lower) in the intervention group (MD ‐0.43 units; 95% CI ‐0.69 to ‐0.17 units; Analysis 9.1). Effects on dyspnoea and exercise tolerance were not reported.
9.1. Analysis.
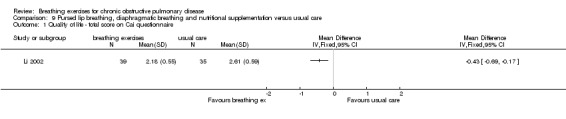
Comparison 9 Pursed lip breathing, diaphragmatic breathing and nutritional supplementation versus usual care, Outcome 1 Quality of life ‐ total score on Cai questionnaire.
Pursed lip breathing, diaphragmatic breathing and walking versus usual care
After three months, participants who were randomised to perform unsupervised walking for one hour per day while using PLB and DB had a reduction (improvement) in the symptom domain of the SGRQ compared to a usual care control group (MD ‐5.7 units; 95% CI ‐10.20 to ‐1.20 units; Analysis 11.1); however, there were no differences in the activity domain (MD 4 units; 95% CI ‐0.78 to 8.78 units), impact domain (MD ‐1.7 units; 95% CI ‐6.40 to 3.00 units) or total score (MD ‐0.60 units; 95% CI ‐4.77 to 3.57 units, Analysis 11.1 (Chan 2011). There was no difference in 6MWT compared to the usual care group (MD 0.58 metres; 95% CI ‐23.41 to 24.22 metres) and no difference in Borg dyspnoea scores at the end of the walking test. These participants received minimal training in breathing techniques, with one instruction session at baseline and refresher sessions at six weeks and three months.
11.1. Analysis.
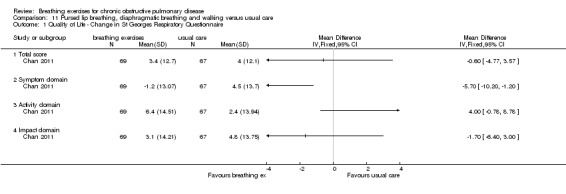
Comparison 11 Pursed lip breathing, diaphragmatic breathing and walking versus usual care, Outcome 1 Quality of Life ‐ Change in St Georges Respiratory Questionnaire.
Secondary outcomes
Respiratory biofeedback training during exercise training compared to exercise training alone
One study reported the effects of ventilation feedback training and exercise training on respiratory variables and gas exchange (Collins 2008). Addition of ventilation feedback training to exercise training had no effect on SpO2 at isotime on a constant work rate cycle test (MD 0.90%; 95% CI ‐1.19 to 2.99%) or on SpO2 after a cardiopulmonary exercise test (MD 0.42%; 95% CI ‐1.63 to 2.47%). There was a trend towards lower minute ventilation (MD ‐6.80 L/minute; 95% CI ‐14.35 to 0.75 L/minute) and a significant reduction in VO2 (MD ‐1.30 mL/kg/minute; 95% CI ‐3.38 to ‐0.78 ml/kg/minute) at isotime on a constant work rate treadmill test in participants who undertook ventilation feedback training and exercise, compared to those who did exercise only. Addition of ventilation feedback training to exercise training also reduced respiratory rate (MD ‐6 breaths; 95% CI ‐9.27 to ‐2.73 breaths) and increased expiratory time (MD 0.43 seconds; 95% CI 0.18 to 0.68 seconds) at isotime. There was no effect on inspiratory time (MD 0.08 seconds; 95% CI ‐0.06 to 0.22 seconds).
No studies examining addition of breathing exercises to other interventions reported on the occurrence of adverse events.
Discussion
Summary of main results
This review aimed to determine whether breathing exercises have clinical benefits for people with COPD who are in a stable clinical state. Results from 16 studies with 1233 participants, predominantly with severe COPD, did not demonstrate consistent effects of breathing exercises across outcomes. Most breathing techniques were effective in improving functional exercise tolerance; however, their impact on dyspnoea and health‐related quality of life was variable. Assessment of the risk of bias for included studies was difficult, owing to limited reporting of allocation concealment, assessor blinding and drop‐outs.
Training programmes in pranayama yoga, PLB and DB techniques improved 6MWD in four studies (Donesky‐Cuenco 2009; Katiyar 2006; Yamaguti 2012; Zhang 2008). The mean increase in walking distance ranged from 35 metres to 88 metres, representing improvements that are likely to be clinically significant (Holland 2010; Puhan 2008). However, gains in walking capacity were not consistently associated with improvements in dyspnoea and health‐related quality of life. These findings can be compared with the effects of pulmonary rehabilitation in COPD, a programme of exercise training and self‐management education, which consistently improves functional exercise capacity, symptoms and health‐related quality of life (Lacasse 2006). Although many pulmonary rehabilitation programmes include instruction in breathing exercises, the two studies that assessed the addition of breathing exercises to a conventional exercise training programme did not show additional benefit (Collins 2008; van Gestel 2011). Given the well‐documented benefits of pulmonary rehabilitation across a wider range of domains, this should remain the first treatment choice for the majority of individuals with COPD. However, breathing exercises may be useful to improve exercise tolerance for some individuals who are not able to undertake a pulmonary rehabilitation programme.
The safety of breathing exercises was directly addressed in only one of 16 studies (Donesky‐Cuenco 2009), which reported no adverse events associated with pranayama yoga over 12 weeks of training. Although it seems unlikely that most breathing exercises would have significant adverse consequences, one study examining the acute effects of 15 minutes of DB at rest reported increased dyspnoea, which was associated with a drop in TcCO2 from 37 mmHg to 27 mmHg (Lausin 2009). While uncorrected TcCO2 measurements cannot be substituted directly for absolute PaCO2 measurements, it is likely that this non‐invasive monitoring can detect change over short time periods with minimal drift (Berlowitz 2011). These effects are consistent with previous within‐subjects physiology studies showing that DB decreases TcCO2, reduces the mechanical efficiency of the respiratory muscles, increases work of breathing and increases dyspnoea (Gosselink 1995; Vitacca 1998). The effect of short‐term hypocapnia induced by breathing strategies is unclear, but no serious adverse consequences have been reported (Lausin 2009). In contrast to these short‐term results, one RCT of 30 participants found significant improvements in 6MWT, health‐related quality of life and dyspnoea following four weeks of training in DB, along with improved diaphragmatic mobility (Yamaguti 2012). These results should be tested in further RCTs, to confirm whether the short‐term physiological disadvantages of DB can be overcome by longer‐term intensive training programmes.
The majority of studies in this review reported outcomes of clinical relevance, including dyspnoea, health‐related quality of life and exercise tolerance. This is consistent with the aims of treatment during breathing exercises, which are to improve symptoms and enhance well being. However there were few studies that could be combined with meta‐analysis, owing to use of different outcome tools and variable reporting. Although it is unlikely that breathing exercises can impact on disease course in COPD, it is possible that effective performance of breathing exercises might enhance self efficacy, reduce anxiety and reduce hospitalisation (Benzo 2010). These outcomes, which are of importance to both people with COPD and the health system, could be considered for future studies.
A major limitation to this review was the difficulty in assessing the risk of bias for included studies. Details of allocation concealment and blinding of outcome assessment could be confirmed in two studies only. The majority of included studies did not report details regarding numbers and handling of drop‐outs. These limitations are reflected in assessments of the quality of the evidence, which ranged from very low to moderate (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6). It is possible that some treatment effects have been overestimated and results of this review must therefore be interpreted with caution.
Overall completeness and applicability of evidence
Most studies in this review predominantly included participants with severe disease. This reflects the population in whom breathing exercises would normally be applied, but results cannot be generalised to people with lesser impairment of respiratory function. Included studies addressed a wide range of breathing techniques (PLB, DB, pranayama yoga, ventilation feedback training, balloon inflation, respiratory muscle gymnastics), reflecting the diversity of clinical practice. However, the number of RCTs investigating each technique was very small, ranging from one to three studies. The small number of studies, together with the risk of bias, makes it difficult to draw firm conclusions about individual techniques. Some studies examined packages of multiple breathing techniques (Li 2002; Saunders 1965; Sun 2003; Yan 1996), which makes it difficult to establish which components might be effective. Despite this, the finding of improved functional exercise tolerance following breathing exercises was consistent across studies and techniques. Most studies that evaluated dyspnoea did so using measures of intensity such as the Borg scale or visual analogue scale. Such tools may not adequately measure the affective distress or impact of dyspnoea on daily life (Parshall 2012). Measurement instruments that assess the broader range of dyspnoea domains should be considered in future studies.
Quality of the evidence
This review included 16 studies with a pooled total of 1233 participants. Individual studies were small, ranging from 21 to 324 participants. A key methodological limitation to this body of evidence was that we could only confirm assessor blinding in two of the 16 studies. Given the nature of the interventions and outcomes investigated, involving behavioural techniques, symptom assessment and effort‐dependent exercise tests, assessor blinding is key to reducing the risk of bias. As a result of lack of assessor blinding, the quality of evidence for many outcomes was rated as low or very low (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6).
Potential biases in the review process
This review included only parallel RCTs. Cross‐over trials were not included, owing to the behavioural nature of the intervention and the potential for carryover of techniques learnt in one period to the second period. As a result, many studies investigating physiological impact of breathing exercises were not included in this review. However, the inclusion of parallel trials only allows conclusions to be drawn about clinical outcomes, which were the primary aim of this review.
Not all authors were able to be contacted to confirm details of study design or obtain additional data. This may have affected some judgements regarding risk of bias and limited the data that was included in meta‐analysis.
Agreements and disagreements with other studies or reviews
Previous reviews of the efficacy of breathing exercises in COPD have drawn variable conclusions. Two reviews concluded that the effects of breathing exercises for people with COPD were questionable or unclear (Cahalin 2002; Gigliotti 2003) and three reviews have suggested that PLB may be an effective dyspnoea management technique (Collins 2001; Dechman 2004; Facchiano 2011). Differences between reviews are likely to be related to differences in inclusion criteria and search strategy. All previous reviews have included trials of designs other than RCTs and conclusions are often based on physiological outcomes from short‐term crossover trials. Some reviews have evaluated individual breathing exercises (Cahalin 2002; Facchiano 2011) and none has specifically evaluated the effects of training programmes on exercise capacity or health‐related quality of life. This review adds to the body of knowledge by focusing on clinical outcomes from breathing exercises in COPD.
Authors' conclusions
Implications for practice.
A programme of breathing exercises lasting four to 15 weeks may improve functional exercise capacity in people with COPD compared to no treatment; however, its effects on dyspnoea and health‐related quality of life are uncertain. There is currently no evidence to suggest that breathing exercises have benefits exceeding those conveyed by a whole body exercise training programme for people with COPD. There does not appear to be significant risk from breathing exercises, although DB may result in short‐term discomfort. Individuals with COPD who have a preference not to undergo pulmonary rehabilitation, or who do not have access to a pulmonary rehabilitation programme, may choose to undertake a programme of breathing exercises.
Implications for research.
This review highlights the need for additional RCTs evaluating breathing exercises in COPD that are rigorously designed, with particular attention to blinding of assessors and intention‐to‐treat analysis. The role of DB requires particular attention, owing to discrepancies between reports of short‐term discomforts and longer‐term benefits.
Acknowledgements
Special thanks to those who gave their time to assist with translation of papers for this review: Sibel Dogan, Alireza Firooz, Toby Lasserson and Taixiang Wu.
Many thanks to Dr Shailesh Bihari and Dr Wellington Yamaguti for providing additional data for this review.
Thank you to Elizabeth Stovold for assistance with the search strategy and Emma Welsh for support throughout the review process.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Data and analyses
Comparison 1. Pursed lip breathing versus no breathing retraining.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dyspnoea ‐ Modified Borg dyspnoea score after 6MWT | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Dyspnoea ‐ University of California San Diego Shortness of Breath Questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 After 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dyspnoea ‐ Medical Research Council Score at week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Exercise capacity ‐ 6MWT at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of life measured by Hiratsuka scale | 2 | 480 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [‐3.03, 4.73] |
| 5.1 Dyspnoea | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐12.94 [‐22.29, ‐3.60] |
| 5.2 Health condition | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 6.19 [‐5.24, 17.61] |
| 5.3 Mood | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [‐9.60, 11.75] |
| 5.4 Social function | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 11.69 [‐0.91, 24.28] |
| 5.5 Housework | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 15.58 [0.50, 30.66] |
| 5.6 Headache | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐12.37, 5.77] |
| 5.7 Appetite | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 8.42 [‐5.30, 22.15] |
| 5.8 Well being | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐9.80, 9.98] |
| 6 Quality of life ‐ Total score of Cai scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.6. Analysis.
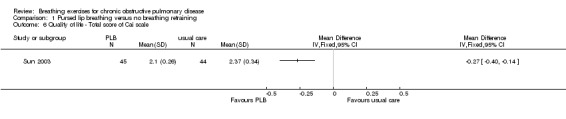
Comparison 1 Pursed lip breathing versus no breathing retraining, Outcome 6 Quality of life ‐ Total score of Cai scale.
Comparison 2. Diaphragmatic breathing versus no breathing retraining.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dyspnoea ‐ change in Medical Research Council Score at week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Exercise capacity ‐ change in 6MWT at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Quality of life ‐ change in SGRQ at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Diaphragmatic mobility | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Ratio of rib cage to abdominal motion during normal breathing | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Ratio of rib cage to abdominal motion during deep breathing | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.22, 0.04] |
2.4. Analysis.
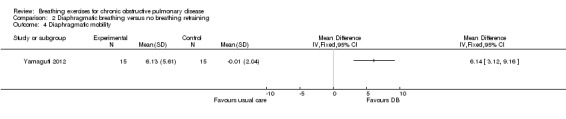
Comparison 2 Diaphragmatic breathing versus no breathing retraining, Outcome 4 Diaphragmatic mobility.
2.5. Analysis.
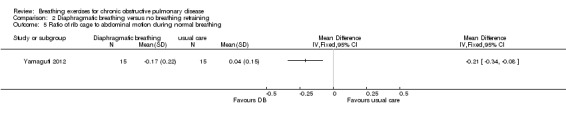
Comparison 2 Diaphragmatic breathing versus no breathing retraining, Outcome 5 Ratio of rib cage to abdominal motion during normal breathing.
2.6. Analysis.
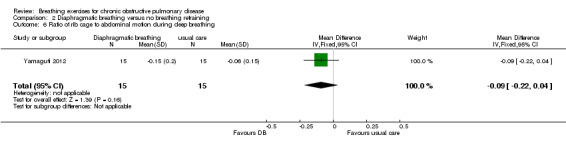
Comparison 2 Diaphragmatic breathing versus no breathing retraining, Outcome 6 Ratio of rib cage to abdominal motion during deep breathing.
Comparison 3. Yoga versus no breathing retraining.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dyspnoea intensity at end of 6MWT | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Dyspnoea intensity at end of incremental cycle ergometer test | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Dyspnoea distress at the end of 6MWT | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Dyspnoea distress at end of incremental cycle ergometer test | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Dyspnoea‐related quality of life at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Exercise capacity ‐ change in 6MWT at 3 months | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | 44.51 [28.47, 60.55] |
| 7 Exercise capacity ‐ peak work on incremental cycle ergometry | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Quality of life ‐ change in total score for St Georges Respiratory Questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
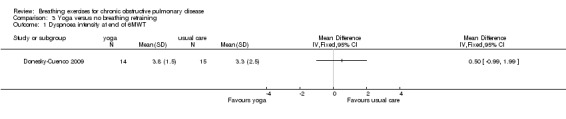
Comparison 3 Yoga versus no breathing retraining, Outcome 1 Dyspnoea intensity at end of 6MWT.
3.2. Analysis.
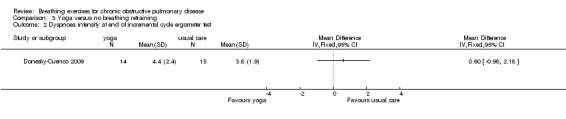
Comparison 3 Yoga versus no breathing retraining, Outcome 2 Dyspnoea intensity at end of incremental cycle ergometer test.
3.3. Analysis.
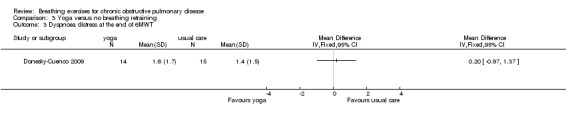
Comparison 3 Yoga versus no breathing retraining, Outcome 3 Dyspnoea distress at the end of 6MWT.
3.4. Analysis.
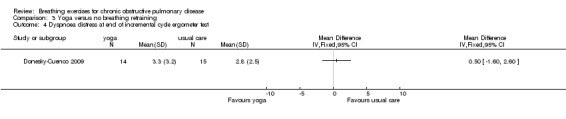
Comparison 3 Yoga versus no breathing retraining, Outcome 4 Dyspnoea distress at end of incremental cycle ergometer test.
3.5. Analysis.
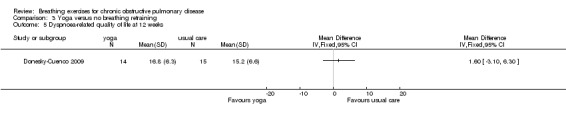
Comparison 3 Yoga versus no breathing retraining, Outcome 5 Dyspnoea‐related quality of life at 12 weeks.
3.6. Analysis.
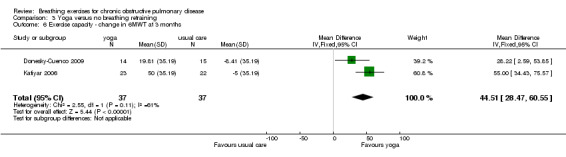
Comparison 3 Yoga versus no breathing retraining, Outcome 6 Exercise capacity ‐ change in 6MWT at 3 months.
Comparison 4. Other breathing retraining versus no breathing retraining.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dyspnoea measured on MRC scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Dyspnoea‐related quality of life at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Exercise capacity ‐ 6MWT at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Gas exchange ‐ PaO2 at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Gas exchange ‐ PCO2 at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 FEV1 after 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 FER after 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Exercise capacity ‐ 6MWT at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
4.2. Analysis.
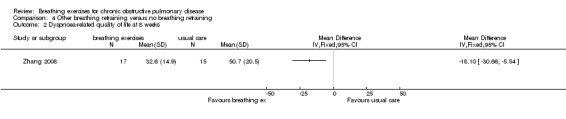
Comparison 4 Other breathing retraining versus no breathing retraining, Outcome 2 Dyspnoea‐related quality of life at 8 weeks.
4.3. Analysis.
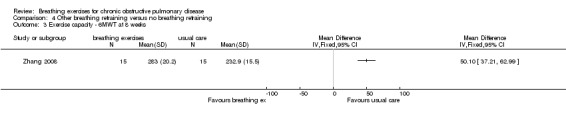
Comparison 4 Other breathing retraining versus no breathing retraining, Outcome 3 Exercise capacity ‐ 6MWT at 8 weeks.
4.4. Analysis.
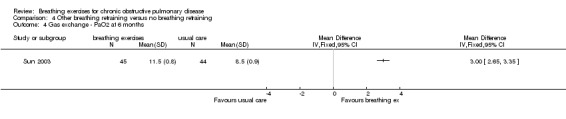
Comparison 4 Other breathing retraining versus no breathing retraining, Outcome 4 Gas exchange ‐ PaO2 at 6 months.
4.5. Analysis.
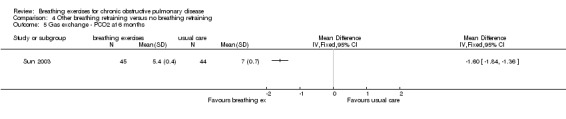
Comparison 4 Other breathing retraining versus no breathing retraining, Outcome 5 Gas exchange ‐ PCO2 at 6 months.
4.6. Analysis.
Comparison 4 Other breathing retraining versus no breathing retraining, Outcome 6 FEV1 after 6 months.
4.7. Analysis.
Comparison 4 Other breathing retraining versus no breathing retraining, Outcome 7 FER after 6 months.
4.8. Analysis.
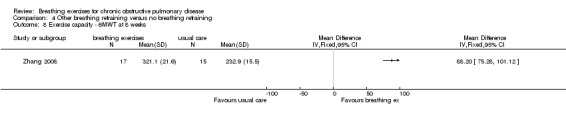
Comparison 4 Other breathing retraining versus no breathing retraining, Outcome 8 Exercise capacity ‐ 6MWT at 8 weeks.
Comparison 5. Pursed lip breathing versus expiratory muscle training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dyspnoea ‐ Modified Borg dyspnoea score after 6MWT | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Dyspnoea ‐ University of California San Diego Shortness of Breath Questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Ventilation feedback training plus exercise versus exercise alone.
6.1. Analysis.
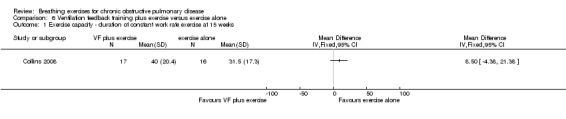
Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 1 Exercise capacity ‐ duration of constant work rate exercise at 15 weeks.
6.2. Analysis.
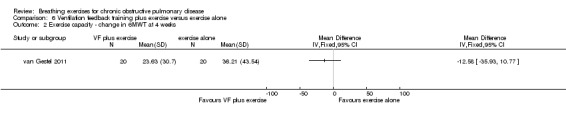
Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 2 Exercise capacity ‐ change in 6MWT at 4 weeks.
6.3. Analysis.
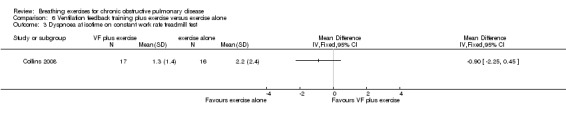
Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 3 Dyspnoea at isotime on constant work rate treadmill test.
6.4. Analysis.
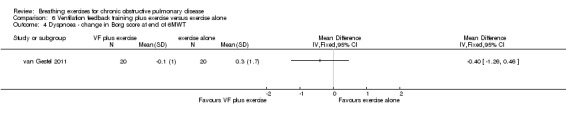
Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 4 Dyspnoea ‐ change in Borg score at end of 6MWT.
6.5. Analysis.
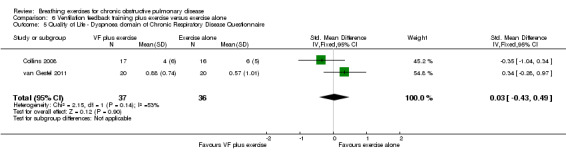
Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 5 Quality of Life ‐ Dyspnoea domain of Chronic Respiratory Disease Questionnaire.
6.6. Analysis.
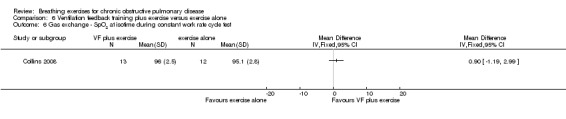
Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 6 Gas exchange ‐ SpO2 at isotime during constant work rate cycle test.
6.7. Analysis.
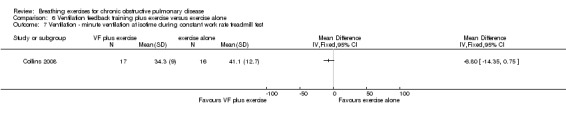
Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 7 Ventilation ‐ minute ventilation at isotime during constant work rate treadmill test.
6.8. Analysis.
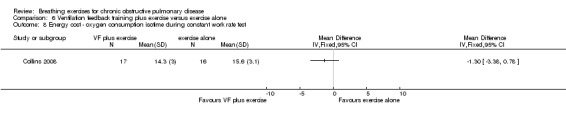
Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 8 Energy cost ‐ oxygen consumption isotime during constant work rate test.
6.9. Analysis.

Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 9 Breathing pattern ‐ respiratory rate at isotime on constant work rate treadmill test.
6.10. Analysis.
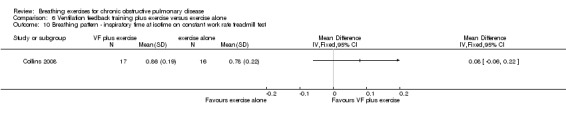
Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 10 Breathing pattern ‐ inspiratory time at isotime on constant work rate treadmill test.
6.11. Analysis.
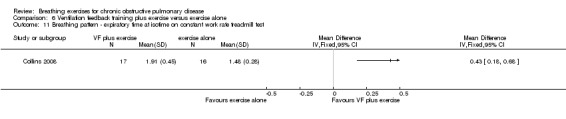
Comparison 6 Ventilation feedback training plus exercise versus exercise alone, Outcome 11 Breathing pattern ‐ expiratory time at isotime on constant work rate treadmill test.
Comparison 7. Ventilation feedback training versus exercise training.
7.3. Analysis.
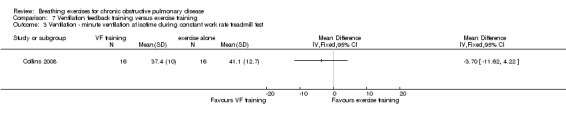
Comparison 7 Ventilation feedback training versus exercise training, Outcome 3 Ventilation ‐ minute ventilation at isotime during constant work rate treadmill test.
7.6. Analysis.
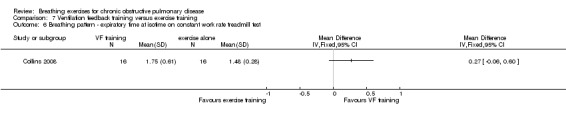
Comparison 7 Ventilation feedback training versus exercise training, Outcome 6 Breathing pattern ‐ expiratory time at isotime on constant work rate treadmill test.
Comparison 8. Diaphragmatic breathing versus inspiratory muscle training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other outcomes ‐ inspiratory muscle endurance at 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Other outcomes ‐ inspiratory muscle endurance at 2 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
8.1. Analysis.
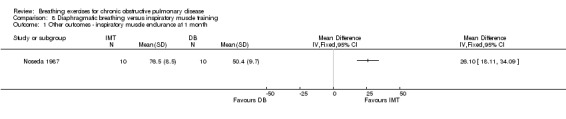
Comparison 8 Diaphragmatic breathing versus inspiratory muscle training, Outcome 1 Other outcomes ‐ inspiratory muscle endurance at 1 month.
8.2. Analysis.
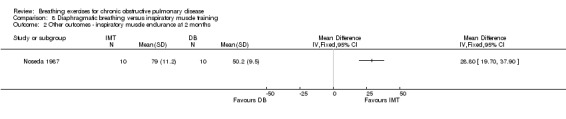
Comparison 8 Diaphragmatic breathing versus inspiratory muscle training, Outcome 2 Other outcomes ‐ inspiratory muscle endurance at 2 months.
Comparison 9. Pursed lip breathing, diaphragmatic breathing and nutritional supplementation versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life ‐ total score on Cai questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Other outcomes ‐ FEV1 after 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Other outcomes ‐ FVC after 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
9.2. Analysis.
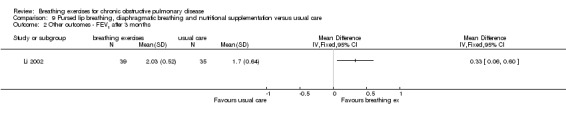
Comparison 9 Pursed lip breathing, diaphragmatic breathing and nutritional supplementation versus usual care, Outcome 2 Other outcomes ‐ FEV1 after 3 months.
9.3. Analysis.
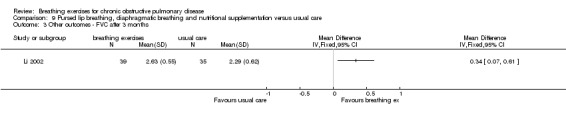
Comparison 9 Pursed lip breathing, diaphragmatic breathing and nutritional supplementation versus usual care, Outcome 3 Other outcomes ‐ FVC after 3 months.
Comparison 10. Pursed lip breathing, diaphragmatic breathing and respiratory muscle gymnastics versus no breathing retraining.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other outcomes ‐ change in inspiratory muscle strength | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Other outcomes ‐ change in expiratory muscle strength | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Other outcomes ‐ change in Pdi | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Other outcomes ‐ change in maximal Pdi | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
10.1. Analysis.
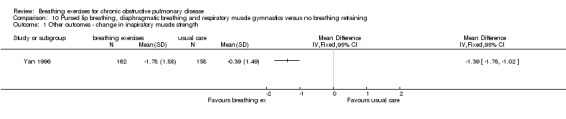
Comparison 10 Pursed lip breathing, diaphragmatic breathing and respiratory muscle gymnastics versus no breathing retraining, Outcome 1 Other outcomes ‐ change in inspiratory muscle strength.
10.2. Analysis.
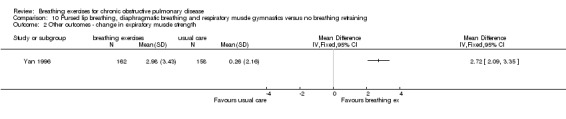
Comparison 10 Pursed lip breathing, diaphragmatic breathing and respiratory muscle gymnastics versus no breathing retraining, Outcome 2 Other outcomes ‐ change in expiratory muscle strength.
10.3. Analysis.
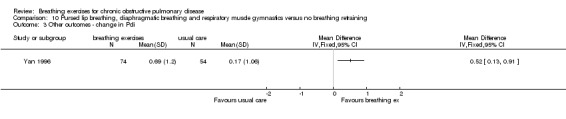
Comparison 10 Pursed lip breathing, diaphragmatic breathing and respiratory muscle gymnastics versus no breathing retraining, Outcome 3 Other outcomes ‐ change in Pdi.
10.4. Analysis.
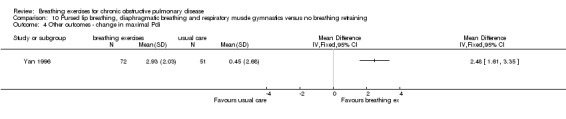
Comparison 10 Pursed lip breathing, diaphragmatic breathing and respiratory muscle gymnastics versus no breathing retraining, Outcome 4 Other outcomes ‐ change in maximal Pdi.
Comparison 11. Pursed lip breathing, diaphragmatic breathing and walking versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of Life ‐ Change in St Georges Respiratory Questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Symptom domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Activity domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Impact domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chan 2011.
| Methods | Randomised controlled trial, 3 x parallel groups, 3‐month intervention period | |
| Participants | 206 participants with mean age 73 years; 43% severe COPD, 42% moderate COPD and 15% mild COPD; independently ambulant. Excluded if severe sensory or cognitive impairment, symptomatic ischaemic heart disease or practiced TCQ within 1 year. Recruited from 5 general outpatient clinics in Hong Kong | |
| Interventions | Intervention 1: participants were taught pursed‐lip breathing and diaphragmatic breathing techniques, to be used during walking. Return demonstrations of breathing techniques by subjects were performed to ensure proper practice. They were advised to perform breathing and walking exercises for 1 hour every day for 3 months. Leaflets with pictures and instructions were given to the subjects to facilitate daily self practice. A diary was also given to the subjects so that they can record the frequency of their self practice Intervention 2: a 3‐month TCQ programme, which consisted of two 60‐minute sessions each week. The TCQ movements were performed in a slow, graceful manner; in addition, breathing, body position and mental concentration were naturally coordinated. The TCQ class was led by a qualified TCQ instructor. Subjects were instructed to self‐practice TCQ daily for 1 hour in addition to the supervised TCQ sessions. Along with TCQ pictures, a DVD was also given to each subject to facilitate daily self‐practice. A diary was also provided to each subject for recording the frequency of their self‐practice sessions Control: subjects in the control group were advised to maintain their routine activities. All subjects continued their prescribed medical treatments Subjects in the breathing exercise and control groups were encouraged to join community activities, such as Putonghua or writing classes, to ensure that all groups consistently attended weekly gatherings |
|
| Outcomes | St Georges Respiratory Questionnaire, 6MWT, Borg dyspnoea score on 6MWT at 6 weeks and 3 months | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random allocation was done using a randomizer software. Both the total number of subjects and number of groups were entered into the computer randomizer, which then generated the random assignment of subjects" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants not likely to have been blind to group allocation. May have influenced all outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The research assistants for data collection were blinded to minimize researcher bias" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis conducted |
| Selective reporting (reporting bias) | Unclear risk | Trial outcomes reported in 2 separate papers; unclear whether other outcomes were collected |
| Other bias | Unclear risk | Package of breathing exercises techniques delivered |
Chauhan 1992.
| Methods | Randomised controlled trial, 8‐week intervention period | |
| Participants | 28 participants with severe COPD, selected randomly from an outpatient clinic list over 6 months using random numbers sampling; FEV1/FVC < 0.70 and FEV1 < 1 L on at least 3 separate clinic assessments | |
| Interventions | Intervention: participants asked to inflate 1 new rubber balloon to a diameter of 20 cm, 40 times per day for 8 weeks. Intervention was not supervised Control: not specified |
|
| Outcomes | 6MWT, VAS 1‐10 scale for severity of breathlessness, VAS 1‐10 scale for well being | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Twenty eight patients were randomly recruited (by random numbers sampling from full clinic list) from an outpatient respiratory clinic over six months" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded owing to nature of intervention. No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded assessors at start of trial, not clear that assessors were blinded at the end. "At the start of the study two pulmonary technicians who were unaware of the allocation of patients assessed the baseline six minute walking distance" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis ‐ data only for the 11 patients in each group who completed the trial "Two patients were excluded from the study group as they failed to inflate balloons regularly. Compliance otherwise was good. Four patients were excluded from the control group: two were admitted to hospital (heart failure and pneumonia) and two failed to attend for repeat assessment. Eleven patients in each group therefore completed the study" |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | None evident |
Collins 2008.
| Methods | Randomised controlled trial, 3 groups, all had 36 sessions of training over a mean of 14.8 weeks | |
| Participants | 91 participants recruited from outpatient clinic; aged 40 years or more, post‐bronchodilator FEV1 < 70% predicted, FEV1/FEV < 70%, PaO 2 ≥ 56 mmHg at rest; mean SpO2 ≥ 85% at peak exercise (with or without supplemental oxygen), stable clinical condition without an exacerbation during the preceding 6 weeks, and MMSE score > 23 | |
| Interventions | All groups trained in the laboratory 3 times weekly Intervention 1: VF training alone 30 to 35 minutes of VF training at each session. The VF system consisted of a heated pneumotachometer interfaced to a computer. Goals of respiratory rate and the exhalation to inhalation ratio during feedback training were based on the breathing pattern recorded during baseline exercise stress test. Expiratory time goals were shown as targets on the computer screen. Progression of training by decrease in respiratory rate and increase in expiratory time. Included a 10‐minute period of low‐intensity exercise so that participants could experience VF during exercise Intervention 2: VF training during exercise training as described below Control: exercise training alone ‐ interval training commencing at 60% of VO2peak, increasing to 85% of VO2peak; training duration commenced at 25 minutes total and increased to 45 minutes; 18 sessions of leg cycle exercise followed by 18 sessions of treadmill exercise; light upper body strength training at all sessions |
|
| Outcomes | Treadmill constant work rate test (primary outcome); incremental treadmill test; Chronic Respiratory Disease Questionnaire; dyspnoea at isotime on constant work rate test; minute ventilation, oxygen consumption and oxyhaemoglobin saturation at isotime; respiratory rate, inspiratory time and expiratory time at isotime. Measured following 36 sessions of intervention at approximately 12 weeks | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported; could have affected both exercise test and quality of life results |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Paper states ITT analysis was performed, but summary data were not reported. Withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | Some quality of life data not reported |
| Other bias | Unclear risk | Participants continued until 36 sessions were completed, so duration of intervention may have varied between groups. Gender not reported |
Donesky‐Cuenco 2009.
| Methods | Randomised controlled trial, 3‐month intervention period | |
| Participants | 41 participants randomised, 29 completed; demographic data reported only for those who completed; 21 women, mean age 72 years in yoga group, 68 years in control group; mean FEV1 51% predicted in yoga group, 43% predicted in control group | |
| Interventions | Intervention: 12‐week yoga programme designed for COPD by panel of expert yoga instructors, primarily Iyengar yoga, twice weekly 1‐hour sessions consisting of yoga poses interspersed with timed breathing, with focus on prolonged expiration; exhalation twice as long as inspiration, no inspiratory or expiratory pauses, inhaled through the nostrils if possible and exhaled gently; stretching movements were done during exhalation. Encouraged to practice daily at home with a videotape Control group ‐ received educational pamphlet 'Living with COPD' and were offered yoga at the conclusion of the study |
|
| Outcomes | Measured at 12 weeks Safety and feasibility (yoga group) Primary efficacy outcomes: dyspnoea intensity and dyspnoea distress (modified Borg scales) during laboratory exercise – incremental cycle exercise test and 6MWT; CRQ dyspnoea domain Secondary outcomes: FEV1 and FVC; 6MWT; work load on incremental cycle exercise test; muscle strength – isokinetic muscle testing of hams and quads; depression – CESD Scale; anxiety – SSAI; quality of life (SF36 and CRQ); S‐FPI |
|
| Notes | No placebo or attention control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised but method not reported |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded owing to nature of intervention. Likely to have affected outcomes given that control group did not receive placebo or attention control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information about blinding of assessors was reported. Likely to have affected outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis ‐ no outcome data available on the considerable number of drop‐outs Drop‐outs had worse pulmonary function and higher depression scores at baseline |
| Selective reporting (reporting bias) | Low risk | All expected data reported |
| Other bias | Low risk | None evident |
Katiyar 2006.
| Methods | Randomised controlled trial with blinded assessor, 4‐week run‐in period to ensure clinical stability 3‐month intervention period | |
| Participants | 48 participants with severe COPD (FEV1 < 50% predicted) in a stable clinical state | |
| Interventions | Intervention: pranayama (yogic breathing exercises) for 30 minutes daily, in addition to usual physical activity and medications Control: usual physical activity and medications |
|
| Outcomes | FEV1, FVC, PEF, 6MWT, arterial blood gases, St Georges Respiratory Questionnaire at 12 weeks | |
| Notes | No between‐group comparisons reported; no post‐intervention measures of variability for control group reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised using computer generated randomisation into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded owing to nature of intervention. Could have affected quality of life outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All the data were collected by the same collector who was blinded to the different group" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 drop‐outs, reason unclear, no ITT analysis, small numbers of drop‐outs unlikely to have affected outcomes |
| Selective reporting (reporting bias) | High risk | Pre‐/post‐data reported for intervention group but no post‐intervention measures of variability for control group |
| Other bias | Low risk | None evident |
Lausin 2009.
| Methods | Randomised controlled trial, single treatment | |
| Participants | 21 participants with mean age 68 years, FEV1 between 30% and 80% predicted; 5 days free of acute exacerbation | |
| Interventions | Intervention ‐ single session of controlled abdominodiaphragmatic breathing. The investigator placed 1 hand on the abdomen and 1 on the rib cage during the breathing exercise. The patient was placed at a 45 degree angle. The patient was asked to breathe in through their nose and out through their mouth with pursed lips for 15 minutes Control ‐ patients received the same as above without the investigator monitoring and reinforcing the breathing technique. Patients were asked to breathe normally |
|
| Outcomes | SaO2, transcutaneous CO2, heart rate, respiratory rate, respiratory muscle strength (Pimax & Pemax), dyspnoea at rest. Measured at baseline and after 15 minutes | |
| Notes | Abstract in English reports a significant difference for respiratory rate, SpO2 and heart rate; the corresponding text in French states there was no difference. Most outcomes reported narratively without measures of variability | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants drew lots to determine which group they were allocated to |
| Allocation concealment (selection bias) | Low risk | Participants drew lots to determine which group they were allocated to |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel not blinded owing to nature of intervention. Unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. Unlikely to have influenced outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | High risk | Full data for respiratory rate and dyspnoea not reported. No between‐group comparison reported |
| Other bias | Low risk | None evident |
Li 2002.
| Methods | Randomised controlled trial, 3‐month intervention period. | |
| Participants | 74 participants with diagnosis of COPD, no baseline characteristics reported | |
| Interventions | Intervention: PLB and diaphragmatic breathing exercise ‐ 2 times per day, 10 to 15 minute per session. Daily nutrient supplement drink (which provides 8400 kJ (2000 kcal) of energy), 2 eggs and 500 mL milk Control: routine diet and normal physical activity |
|
| Outcomes | FEV1, FVC, FEV1/FVC, Quality of Life questionnaire for COPD (domains Activity of Daily Living ability, Social Function ability, Depression, Anxiety, Total score) Measured at baseline and 3 months | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Unlikely to be blinded due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. May have affected quality‐of‐life outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | All expected outcome data reported. No between‐group comparisons |
| Other bias | Unclear risk | Baseline data not provided; states "age, gender, lung function and nutritional status were similar between the two groups (p > 0.05)" |
Nield 2007.
| Methods | Randomised controlled trial, 12‐week intervention period | |
| Participants | 40 participants (38 male), FEV1/FVC < 70%, FEV1 < 80% predicted with no reversibility following bronchodilator, dyspnoea during walking, modified Borg score of 3 or more on screening 6MWT, no exacerbation in last 4 weeks | |
| Interventions | Intervention 1: PLB with a pulse oximeter provided for home use; participants were instructed to practice for 10 minutes per day in the first week, 15 minutes per day in the second week, 20 minutes per day in the third week and 25 minutes per day by the fourth week. Four weekly visits to the research laboratory for supervision. Prolonged expiration reinforced during monitoring sessions by observation of breathing pattern on a monitor. Practiced PLB during walking at each monitoring session, with cadence paced to breathing pattern. Daily diary was completed and reviewed at monitoring sessions Intervention 2: expiratory muscle training with resistive load of 4 to 20 cm H2O during exhalation. Expiratory load initially set at 10% of baseline PEmax. Duration and frequency of practice sessions and visits as for intervention 1 Control: participants received the American Lung Association health education pamphlet 'About Lungs and Lung Disease'; they visited the laboratory on the same number of occasions as the intervention subjects and received the same amount of attention during their visits |
|
| Outcomes | Measured at 4 and 12 weeks: University of San Diego Shortness of Breath Questionnaire Modified Borg at end 6MWT Human Activity Profile Physical function dimension of the Short Form 36‐item Health Survey, Version 2.0 Breathing pattern ‐ respiratory rate, inspiratory time, expiratory time, inspiratory to expiratory ratio Respiratory muscle strength |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to groups, method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding reported; unlikely that participants and personnel were blinded owing to nature of intervention. Groups received similar attention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated. Randomised after screening visit, so group allocation was known at time of baseline testing. May have affected quality of life outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data available from drop‐outs, may have resulted in overestimation of treatment effect |
| Selective reporting (reporting bias) | Low risk | All expected data were reported |
| Other bias | Low risk | None evident |
Noseda 1987.
| Methods | Randomised controlled trial, 4‐week run‐in and 8‐week intervention period | |
| Participants | 25 participants with physician diagnosed COPD, FEV1 < 60% predicted, FEV1/IVC < 55%, VEmax/FEV1x35) ≥ 80% (to ensure exercise was limited by ventilation); excluded participants who could not do a maximal exercise test | |
| Interventions | Intervention 1: breathing exercises ‐ low frequency breathing with high tidal volume and adequate abdominal motion, as well as appropriate mobilisation of the joints between ribs, spine and sternum. In supine or lateral position, 30 minutes daily. Supervised by a physiotherapist at home during the first 2 weeks Intervention 2: inspiratory muscle training ‐ PFlex 15 minutes twice a day, wearing nose clip. Initially at highest tolerated resistance, instructed to progress by 1 step each week. Unsupervised |
|
| Outcomes | Respiratory function tests, 12‐minute walk test, inspiratory muscle endurance, incremental cycle test, endurance cycle test. Measured at baseline and 8 weeks | |
| Notes | In case of illness preventing training, the duration of illness was added to the training programme for both groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unlikely that participants and personnel were blinded due to nature of intervention. Groups received similar attention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "A blinding procedure was not used, the assessors knowing in which group the patients were". May have affected effort‐dependent outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 of 25 participants dropped out, no data available for drop‐outs |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | None evident |
Saunders 1965.
| Methods | Pseudo‐randomised controlled trial, duration 3 months | |
| Participants | 100 participants with chronic bronchitis or emphysema, or both, chest X‐ray used to confirm presence of emphysema | |
| Interventions | Intervention: "a course of breathing exercises according to accepted principles". Participants attended weekly for 6 weeks for supervision by a physiotherapist and were encouraged to practice at home Control: not stated |
|
| Outcomes | MRC symptom questionnaire, PEFR, FEV1 and FVC, recorded at baseline, 6 weeks and 3 months | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐randomised according to date of birth "One hundred out‐patients who satisfied the above criteria were randomly divided into two groups according to their date of birth; 51 (even dates) to the control and 49 (odd dates) to the treated group" |
| Allocation concealment (selection bias) | High risk | Pseudo‐randomised according to date of birth, allocation not concealed. "One hundred out‐patients who satisfied the above criteria were randomly divided into two groups according to their date of birth; 51 (even dates) to the control and 49 (odd dates) to the treated group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely that participants and personnel were blinded owing to nature of intervention. May have affected symptom ratings |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported. May have affected symptom ratings |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23 drop‐outs, data not provided |
| Selective reporting (reporting bias) | Unclear risk | Baseline data not presented |
| Other bias | Low risk | None evident |
Sun 2003.
| Methods | Randomised controlled trial, 6‐month intervention period | |
| Participants | 99 participants (57 males) with COPD class II or III (FEV1 < 80% predicted); mean age 69 years; condition stable for 1 month | |
| Interventions | Intervention: PLB: 3 times per day (morning, midday and evening). 10 minute per session. Breathing frequency ‐ 7 to 10 breaths per minute Respiratory muscle gymnastics: 5 parts ‐ various forms of breathing with trunk rotation or bending, as well as breathing with leg exercise. Twice a day (morning and evening), 10 minute each session Control ‐ no details provided |
|
| Outcomes | Quality of Life questionnaire for COPD; resting FEV1, FEV1/FVC in the morning; blood gases measured in supine position: PaO2, PaCO2. Measured at baseline and 6 months | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From a random number table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. Unlikely that participants and personnel were blinded owing to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. May have affected quality of life measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | Low risk | None evident |
van Gestel 2011.
| Methods | Randomised controlled trial, 4‐week intervention period | |
| Participants | 40 participants with COPD, mean age 66 years, mean FEV1 46% predicted | |
| Interventions | Intervention: 4‐week pulmonary rehabilitation programme (as per control group) with addition of controlled breathing using respiratory biofeedback. 10 sessions of RBF, 30 minutes each session. Practiced for 10 minutes at rest, and during the 20 minutes of endurance training on a cycle ergometer. Instructions for daily home practice. Patients trained to modify 4 respiratory characteristics: rapid shallow breathing, breath to breath irregularity in rate and depth, predominant thoracic breathing. Patients encouraged to deep breathe with outwards motion of abdominal wall, while reducing upper rib cage motion; prolonged expiration using PLB. Breathing pattern was monitored using respiration sensors at umbilical and abdominal level; RBF training provides simple acoustic tones and visual graphic signals to inform patient of their breathing pattern Control: conventional 4‐week pulmonary rehabilitation programme ‐ 3 times a week for 3 to 4 weeks, 10 sessions in total, 1.5‐hour sessions, dynamic strength training 3 x 10 repetitions starting at 70% 1RM, cycle ergometer 20 minutes starting at 30% peak work load, stepping exs and arm cranking |
|
| Outcomes | FEV1, 6MWT, CRQ, cardiac autonomic function. Measured at baseline and 4 weeks | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel unlikely to be blinded due to nature of intervention. Not clear whether intervention and control participants trained together or separately. May have affected outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessors blinded for respiratory function tests and 6MWT, but not for quality‐of‐life outcome. May have affected quality of life outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 drop‐outs, no ITT analysis |
| Selective reporting (reporting bias) | Low risk | All expected data were reported |
| Other bias | Low risk | None evident |
Wu 2006.
| Methods | Randomised controlled trial, 3‐month intervention period. | |
| Participants | 30 inpatients with mean age 70 years, condition stabilised after an exacerbation | |
| Interventions | Intervention: PLB training in hospital for 2 weeks, 10 minutes each session, 3 times per day. Continue exercises at home for 3 months Control: routine medical treatment | |
| Outcomes | FEV1, FVC, FEV1/FVC, Quality of Life questionnaire for COPD, Activities of Daily Living assessment. Measured at baseline and 3 months | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely that participants and personnel were blinded, given nature of intervention. Lack of placebo or attention control may have affected quality of life outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated. May have affected quality of life outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs not reported |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | Unclear risk | All males. Adherence to intervention unclear |
Yamaguti 2012.
| Methods | Randomised controlled trial with 4‐week intervention period | |
| Participants | 30 participants with mean FEV1 42% predicted | |
| Interventions | Intervention: diaphragmatic breathing training programme, supervised 3 times a week for 4 weeks | |
| Outcomes | Ratio of rib cage to abdominal movement (primary outcome), diaphragmatic mobility; 6MWT, modified MRC dyspnoea scale; St Georges Respiratory Questionnaire. Measured at baseline and 4 weeks | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; blinding of participants and personnel unlikely owing to nature of intervention. May have affected quality‐of‐life outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The technicians who collected data for all outcome measures were blinded to the patients' group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants in the control group lost to follow‐up. ITT analysis performed with last observation carried forward |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Low risk | ‐ |
Yan 1996.
| Methods | Randomised controlled trial with treatment period of between 1 and 20 months. Follow‐up and measurements at 1 month (n = 23); 2 months (n = 51); 3 months (n = 24); 6 months (n = 28); 12 months (n = 18); > 12 months (n = 18) | |
| Participants | 324 participants with a clinical diagnosis of chronic bronchitis and PEFR < 75% predicted | |
| Interventions | Intervention: PLB, diaphragmatic breathing in sitting, standing or walking. PLB and diaphragmatic breathing with trunk movement 2 times a day, 30 minute each session, or 4 times a day, 15 minutes each session. Exercise taught while in hospital. After discharge from hospital, followed up every 1 to 2 weeks by the same physician Control: oral placebo "healthy lung capsule" 2 times a day, 1 capsule each time |
|
| Outcomes | MIP, MEP, Pdi, Pdimax. Time point of measurement from 1 to 20 months | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded ‐ placebo capsule twice a day |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. Unlikely to have affected outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data for drop‐outs not reported |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | High risk | Outcomes measured at different time points; not clear whether participants in the control group had outcomes measured at the same time as those in the intervention group |
Zhang 2008.
| Methods | Randomised controlled trial with 8‐week intervention period | |
| Participants | 60 participants (51 male) in GOLD stage III or IV, no acute exacerbation in last 4 weeks | |
| Interventions | Intervention 1: quick inspiration (0.8 to 1 second) and slow expiration (3 to 4 seconds). Reported to be "in relation to respiratory pathophysiology": quick inspiration to total lung capacity, hold, slow expiration ‐ 3 times per day, 15 minutes each session, for 8 weeks Intervention 2: PLB ‐ 3 times per day, 15 minutes each session, for 8 weeks Control: no breathing training |
|
| Outcomes | 6MWT, MRC Dyspnoea scale, Activity of Daily living, Quality of Life score, MIP, MEP. Measured at baseline and 8 weeks | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; blinding of participants and personnel unlikely owing to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear. States "The first author was responsible for design and intervention, the second author was responsible for assessment". Did not mention how blinding was done, but stated that all researchers "received proper, stringent, training and experimental, randomised control design was employed" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis not reported. Reports 3 dropped out from the quick inspiration group, 5 dropped out from the PLB and 5 dropped out from the negative control group |
| Selective reporting (reporting bias) | Unclear risk | Unclear. No between‐group comparisons |
| Other bias | Low risk | None evident |
6MWT: six‐minute walk test; CESD: Center for Epidemiological Studies Depression; COPD: chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Questionnaire; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; IVC: inspiratory vital capacity; MEP: maximal expiratory pressure; MIP: maximal inspiratory pressure; MMSE: Mini‐Mental Status Examination; MRC: Medical Research Council; Pdi: transdiaphragmatic pressure; Pdimax: maximal transdiaphragmatic pressure; PEF: peak expiratory flow; PEFR: peak expiratory flow rate; PEmax: maximal expiratory muscle pressure; PLB: pursed lip breathing; ITT: intention to treat; RBF: respiratory biofeedback training; S‐FPI: Functional Performance Inventory short form; SSAI: Spielberger State Anxiety Inventory; TCQ: Tai Chi Qigong; VAS: visual analogue scale; VEmax: minute ventilation at peak exercise; VF: ventilation feedback.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Booker 1984 | Intervention < 50% breathing exercises |
| Cai 2003 | Intervention < 50% breathing exercises |
| Campbell 1955 | Not a randomised controlled trial |
| Chen 2005 | Participants not clinically stable |
| Di Marzo 2002 | Control group also performed breathing exercises |
| Esteve 1996 | Control group also performed breathing exercises |
| Faager 2008 | Cross‐over trial |
| Falk 1981 | Letter to editor about randomised controlled trial of inspiratory muscle training |
| Farquhar 2009 | Intervention < 50% breathing exercises |
| Filshie 1994 | Intervention not breathing exercises |
| Garrod 2003 | Cross‐over trial, same study as Garrod 2005 |
| Garrod 2005 | Cross‐over trial |
| Garuti 1998 | Control group also performed breathing exercises |
| Gosselink 1995 | Not a randomised controlled trial |
| Ito 1999 | Cross‐over trial |
| Izumizaki 2008 | Cross‐over trial |
| Jones 2003 | Cross‐over trial |
| Kulpati 1982 | Breathing exercises < 50% of intervention |
| Kurabayashi 1998 | Control group also performed breathing exercises |
| Kurabayashi 2000 | Control group also performed breathing exercises |
| Liu 2002 | Not a randomised controlled trial |
| Lustig 1972 | Breathing exercises < 50% of intervention |
| Marcq 1975 | Not a randomised controlled trial |
| McNeill 1955 | Not a randomised controlled trial |
| Minoguchi 2002 | Cross‐over trial |
| Mularski 2009 | Breathing exercises < 50% of intervention |
| Padkao 2010 | Intervention not breathing exercises |
| Pearce 2006 | Breathing exercises < 50% of intervention |
| Qiying 1996 | No relevant outcomes |
| Reybrouck 1987 | Control group also performed breathing exercises |
| Sassi‐Dambron 1995 | Breathing exercises < 50% of intervention |
| Sergysels 1979 | Control group also performed breathing exercises |
| Sutcu Cicek 2004 | Participants not clinically stable |
| Tandon 1978 | Control group also performed breathing exercises |
| Tiep 1986 | Cross‐over trial |
| Yamanaka 2009 | Not a randomised controlled trial |
| Zakerimoghadam 2006 | Participants not in a stable clinical state. Same study as Zakerimoghadam 2011 |
| Zakerimoghadam 2011 | Participants not in a stable clinical state |
Differences between protocol and review
Dr Alice Jones has been added as an author for the review.
We had planned to conduct subgroup analyses to examine the effects of breathing exercises performed during exercise versus rest, and in severe disease versus mild to moderate disease; however, insufficient data were available. Insufficient data were available to construct funnel plots and to conduct sensitivity analyses to determine the effects of allocation concealment, assessor blinding and use of intention‐to‐treat analysis on results.
Contributions of authors
Anne Holland: initiation and writing of protocol and manuscript, data extraction and analysis.
Catherine Hill: protocol development, data extraction and analysis, manuscript review.
Alice Jones: data extraction, manuscript review.
Christine McDonald: protocol development and review, manuscript review.
Sources of support
Internal sources
La Trobe University, Australia.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Chan 2011 {published data only}
- Chan AW, Lee A, Suen LK, Tam WW. Effectiveness of a Tai chi Qigong program in promoting health‐related quality of life and perceived social support in chronic obstructive pulmonary disease clients. Quality of Life Research 2010;19(5):653‐64. [DOI] [PubMed] [Google Scholar]
- Chan AW, Lee A, Suen LK, Tam WW. Tai chi Qigong improves lung functions and activity tolerance in COPD clients: a single blind, randomized controlled trial. Complementary Therapies in Medicine 2011;19(1):3‐11. [DOI] [PubMed] [Google Scholar]
Chauhan 1992 {published data only}
- Chauhan AJ, McLindon JP, Dillon P, Sawyer JP, Gray L, Leahy BC. Regular balloon inflation for patients with chronic bronchitis: a randomised controlled trial. British Medical Journal 1992;304:1668‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2008 {published data only (unpublished sought but not used)}
- Collins EG, Fehr L, Bammert C, O'Connell S, Laghi F, Hanson K, et al. Effect of ventilation‐feedback training on endurance and perceived breathlessness during constant work‐rate leg‐cycle exercise in patients with COPD. Journal of Rehabilitation Research & Development 2003;40:35‐44. [DOI] [PubMed] [Google Scholar]
- Collins EG, Langbein WE, Fehr L, Bammert C, O'Connell S, Hagarty E, et al. Can ventilation‐feedback training augment exercise tolerance in patients with COPD?. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego California. 2005; Vol. A26, Poster 511.
- Collins EG, Langbein WE, Fehr L, Bammert C, O'Connell S, Hanson K, et al. Can ventilation‐feedback training decrease exertional dyspnoea and improve exercise tolerance in patients with COPD? [Abstract]. American Thoracic Society 99th International Conference. 2003; Vol. B023, Poster 910.
- Collins EG, Langbein WE, Fehr L, O'Connell S, Jelinek C, Hagarty E, et al. Can ventilation‐feedback training augment exercise tolerance in patients with chronic obstructive pulmonary disease?. American Journal of Respiratory & Critical Care Medicine 2008;177:844‐52. [DOI] [PubMed] [Google Scholar]
Donesky‐Cuenco 2009 {published data only}
- Donesky‐Cuenco D, Nguyen HQ, Paul S, Carrieri‐Kohlman V. Yoga therapy decreases dyspnea‐related distress and improves functional performance in people with chronic obstructive pulmonary disease: a pilot study. Journal of Alternative and Complementary Medicine 2009;15:225‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katiyar 2006 {published and unpublished data}
- Katiyar SK, Bihari S. Role of pranayama in the rehabilitation of COPD patients ‐ a randomized controlled study. Indian Journal of Allergy and Applied Immunology 2006;20:98‐104. [Google Scholar]
- Katiyar SK, Singh L, Bihari S. Role of yogic exercises in rehabilitation of patients with chronic obstructive pulmonary disease. Indian Journal of Allergy Asthma and Immunology 2005;19:123. [Google Scholar]
Lausin 2009 {published data only (unpublished sought but not used)}
- Lausin G, Gouilly P. Study of the effects of controlled abdominodiaphragmatic ventilation in patients with level I and II COPD [Étude des effets de la ventilation dirigée abdomino‐diaphragmatique (Vdad) chez des patients BPCO de stade I et II]. Kinésithérapie, la Revue 2009;87:29‐38. [Google Scholar]
Li 2002 {published data only}
- Li YL. Nutritional supplementation and respiratory gym in patients with chronic obstructive pulmonary disease. Zhongguo Linchuang Kangfu 2002;6:1260‐2. [Google Scholar]
Nield 2007 {published data only (unpublished sought but not used)}
- Nield M, Soo Hoo G, Roper J, Santiago S. Pursed‐lips breathing decreases dyspnea and improves health‐related quality of life in 4‐group randomized, controlled study. Chest 2004;126:805S‐a. [Google Scholar]
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed‐lips breathing: a breathing pattern retraining strategy for dyspnea reduction. Journal of Cardiopulmonary Rehabilitation and Prevention 2007;27:237‐44. [DOI] [PubMed] [Google Scholar]
Noseda 1987 {published data only}
- Noseda A, Carpiaux JP, Vandeput W, Prigogine T, Schmerber J. Resistive inspiratory muscle training and exercise performance in COPD patients. A comparative study with conventional breathing retraining. Bulletin European de Physiopathologie Respiratoire 1987;23:457‐63. [PubMed] [Google Scholar]
Saunders 1965 {published data only}
- Saunders KB, White JE. Controlled trial of breathing exercises. British Medical Journal 1965;2:680‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2003 {published data only}
- Sun JX, Yin MX, Shao H, Li ZS, Li SW. Effect of respiratory muscle gymnastics on lung function and quality of life in the old patients with chronic obstructive pulmonary disease. Zhonghua Linchuang Kangfu Zazhi 2003;7:3698‐9. [Google Scholar]
van Gestel 2011 {published data only}
- Gestel AJ, Kohler M, Steier J, Teschler S, Russi EW, Teschler H. The effects of controlled breathing during pulmonary rehabilitation in patients with COPD. Respiration 2011;82:Abstracts pp 67‐107. [DOI] [PubMed] [Google Scholar]
- Gestel AJR, Kohler M, Steier J, Teschler S, Russi EW, Teschler H. The effect of controlled breathing during pulmonary rehabilitation in patients with COPD. Respiration 2012;83:115‐24. [DOI] [PubMed] [Google Scholar]
Wu 2006 {published data only}
- Wu X, Hou L, Bai W. Effects of breathing training on quality of life and activities of daily living in elderly patients with stable severe chronic obstructive pulmonary disease. Zhongguo Kangfu Yixue Zazhi 2006;21:307‐10. [Google Scholar]
Yamaguti 2012 {published and unpublished data}
- Yamaguti W, Claudino R, Neto A, Moriya H, Gomes A, Chammas M, et al. Diaphragmatic breathing training in COPD patients: a randomized, controlled and blinded trial. European Respiratory Society Annual Congress. 2010:P4901.
- Yamaguti WP, Claudino RC, Neto AP, Chammas MC, Gomes AC, Salge JM, et al. Diaphragmatic breathing training program improves abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomised controlled trial. Archives of Physical Medicine and Rehabilitation 2012;93:571‐77. [DOI] [PubMed] [Google Scholar]
Yan 1996 {published data only}
- Yan Q, Sun Y. Quantitative research for improving respiratory muscle contraction by breathing exercise. Chinese Medical Journal 1996;109:771‐5. [PubMed] [Google Scholar]
- Yan Q, Sun Y, Lin J. A quantitative study on the effect of breathing exercises in improving respiratory muscle contraction. Chung‐Hua Nei Ko Tsa Chih Chinese Journal of Internal Medicine 1996;35:235‐8. [PubMed] [Google Scholar]
Zhang 2008 {published data only}
- Zhang ZQ, Chen RC, Yang QK, Li P, Wang CZ, Zhang ZH. A randomized controlled trial study of pulmonary rehabilitation with respiratory physiology as the guide on prognosis in patients with chronic obstructive pulmonary disease. Chinese Critical Care Medicine 2008;20:607‐10. [PubMed] [Google Scholar]
- Zhang ZQ, Chen RC, Yang QK, Li P, Wang CZ, Zhang ZH. Effects of respiratory training in relation to respiratory pathophysiology on respiratory muscle function and exercise tolerance in chronic obstructive pulmonary disease patients. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12:3966‐71. [Google Scholar]
References to studies excluded from this review
Booker 1984 {published data only}
- Booker HA. Exercise training and breathing control in patients with chronic airflow limitation. Physiotherapy 1984;70(7):258‐60. [PubMed] [Google Scholar]
Cai 2003 {published data only}
- Cai H. Rehabilitation effect of combination of respiration exercise, Jinshuibao capsule and external application in stable stage of chronic obstructive pulmonary diseases. Zhonghua Linchuang Kangfu Zazhi 2003;7:877. [Google Scholar]
Campbell 1955 {published data only}
- Campbell EJ, Friend J. Action of breathing exercises in pulmonary emphysema. Lancet 1955;268(6859):325‐9. [DOI] [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Chen YL, Chen LM. Effects of respiratory training plus nutritional support on quality of life and pulmonary function in patients with chronic obstructive pulmonary disease at acute exacerbation. Zhongguo Linchuang Kangfu 2005;9(43):17‐9. [Google Scholar]
Di Marzo 2002 {published data only}
- Marzo A, Mancuso A, Bevignani G. Breathing reeducation with a pressure threshold device in advanced COPD patients. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A506. [Google Scholar]
Esteve 1996 {published data only}
- Esteve F, Blanc‐Gras N, Gallego J, Benchetrit G. The effects of breathing pattern training on ventilatory function in patients with COPD. Biofeedback & Self Regulation 1996;21(4):311‐21. [DOI] [PubMed] [Google Scholar]
Faager 2008 {published data only}
- Faager G, Stahle A, Larsen FF. Influence of spontaneous pursed lips breathing on walking endurance and oxygen saturation in patients with moderate to severe chronic obstructive pulmonary disease. Clinical Rehabilitation 2008;22(8):675‐83. [DOI] [PubMed] [Google Scholar]
Falk 1981 {published data only}
- Falk P, Eriksen AM, Kolliker K, Andersen JB. Exercise and the breathless bronchitic. Lancet 1981;1(8210):54‐5. [DOI] [PubMed] [Google Scholar]
Farquhar 2009 {published data only}
- Farquhar MC, Higginson IJ, Fagan P, Booth S. The feasibility of a single‐blinded fast‐track pragmatic randomised controlled trial of a complex intervention for breathlessness in advanced disease. BMC Palliative Care 2009;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Filshie 1994 {published data only}
- Filshie J, Davis CL, Molen B. Palliation of breathlessness in patients with COAD and anxiety without provoking sedation or respiratory depression. Progress in Palliative Care 1994;2(1):9. [Google Scholar]
Garrod 2003 {published data only}
- Garrod R, Cook J, Dallimore K, Davies V, Quade K. Pursed lips breathing in naive COPD subjects, effects on exercise tolerance and breathlessness: a randomised controlled trial. Thorax 2003;58(Suppl 3):iii77. [Google Scholar]
Garrod 2005 {published data only}
- Garrod R, Dallimore K, Cook J, Davies V, Quade K. An evaluation of the acute impact of pursed lips breathing on walking distance in nonspontaneous pursed lips breathing chronic obstructive pulmonary disease patients. Chronic Respiratory Disease 2005;2(2):67‐72. [DOI] [PubMed] [Google Scholar]
Garuti 1998 {published data only}
- Garuti GC, Rossi G, Totaro L, Lorenzi C, Nobile MT, Marchioni CF. Physiological evaluation of selected maneouvres of breathing in emphysema. European Respiratory Journal Supplement 1998;12 Suppl 28:170S. [Google Scholar]
Gosselink 1995 {published data only}
- Gosselink R, Wagenaar RC, Rijswijk H, Sargeant AJ, Decramer MLA. Diaphragmatic breathing reduces efficiency of breathing in patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1995;151(4):1136‐42. [DOI] [PubMed] [Google Scholar]
Ito 1999 {published data only}
- Ito M, Kakizaki F, Tsuzura Y, Yamada M. Immediate effect of respiratory muscle stretch gymnastics and diaphragmatic breathing on respiratory pattern. Internal Medicine 1999;38(2):126‐32. [DOI] [PubMed] [Google Scholar]
Izumizaki 2008 {published data only}
- Izumizaki M, Satake M, Takahashi H, Sugawara K, Shioya T, Homma I. Effects of inspiratory muscle thixotropy on the 6‐min walk distance in COPD. Respiratory Medicine 2008;102(7):970‐7. [DOI] [PubMed] [Google Scholar]
Jones 2003 {published data only}
- Jones AYM, Dean E, Chow CCS. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Physical Therapy 2003;83(5):424‐31. [PubMed] [Google Scholar]
Kulpati 1982 {published data only}
- Kulpati DD, Kamath RK, Chauhan MR. The influence of physical conditioning by yoga asanas and breathing exercises in patients of chronic obstructive pulmonary disease. Journal of the Association of Physicians in India 1982;30:865‐8. [PubMed] [Google Scholar]
Kurabayashi 1998 {published data only}
- Kurabayashi H, Machida I, Handa H, Akiba T, Kubota K. Comparison of three protocols for breathing exercises during immersion in 38 degrees C water for chronic obstructive pulmonary disease. American Journal of Physical Medicine & Rehabilitation 1998;77(2):145‐8. [PubMed] [Google Scholar]
Kurabayashi 2000 {published data only}
- Kurabayashi H, Machida I, Tamura K, Iwai F, Tamura J, Kubota K. Breathing out into water during subtotal immersion: a therapy for chronic pulmonary emphysema. American Journal of Physical Medicine & Rehabilitation 2000;79(2):150‐3. [DOI] [PubMed] [Google Scholar]
Liu 2002 {published data only}
- Liu YF. Effects of the comprehensive pulmonary rehabilitation programme on the quality of life of the patients with COPD in recovery period. Zhongguo Linchuang Kangfu 2002;6(21):3170‐1. [Google Scholar]
Lustig 1972 {published data only}
- Lustig FM, Haas A, Castillo R. Clinical and rehabilitation regime in patients with chronic obstructive pulmonary diseases. Archives of Physical Medicine and Rehabilitation 1972;53(7):315‐22. [PubMed] [Google Scholar]
Marcq 1975 {published data only}
- Marcq M, Frans A, Stanescu D, et al. Practical problems in the rehabilitation of coal‐miners with chronic bronchitis at the first stage of respiratory insufficiency. Rehabilitation (London) 1975;92:23‐33. [Google Scholar]
McNeill 1955 {published data only}
- McNeill RS, McKenzie JM. An assessment of the value of breathing exercises in chronic bronchitis and asthma. Thorax 1955;10:250‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Minoguchi 2002 {published data only}
- Minoguchi H, Shibuya M, Miyagawa T, Kokubu F, Yamada M, Tanaka H, et al. Cross‐over comparison between respiratory muscle stretch gymnastics and inspiratory muscle training. Internal Medicine 2002;41(10):805‐12. [DOI] [PubMed] [Google Scholar]
Mularski 2009 {published data only}
- Mularski RA, Munjas BA, Lorenz KA, Sun S, Robertson SJ, Schmelzer W, et al. Randomized controlled trial of mindfulness‐based therapy for dyspnea in chronic obstructive lung disease. Journal of alternative and complementary medicine (New York, NY) 2009;15(10):1083‐90. [DOI] [PubMed] [Google Scholar]
Padkao 2010 {published data only}
- Padkao T, Boonsawat W, Jones CU. Conical‐PEP is safe, reduces lung hyperinflation and contributes to improved exercise endurance in patients with COPD: a randomised cross‐over trial. Journal of physiotherapy 2010;56(1):33‐9. [DOI] [PubMed] [Google Scholar]
Pearce 2006 {published data only}
- Pearce L, MacLead V, Baker A, Grant A, Dotesio‐Eyes N, Sydor M, et al. Randomised controlled trial of nurse led breathlessness intervention to improve the management of breathlessness in patients with chronic obstructive pulmonary disease at a district general hospital. Thorax 2006;61(Suppl 2):ii84. [Google Scholar]
Qiying 1996 {published data only}
- Qiying Q, Yinxiang S. Quantitative research for improving respiratory muscle contraction by breathing exercise. Chinese Medical Journal 1996;109(10):771‐5. [PubMed] [Google Scholar]
Reybrouck 1987 {published data only}
- Reybrouck T, Wertelaers A, Bertrand P, Demedts M. Myofeedback training of the respiratory muscles in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation 1987;7(1):20‐2. [Google Scholar]
Sassi‐Dambron 1995 {published data only}
- Sassi‐Dambron DE, Eakin EG, Ries AL, Kaplan RM. Treatment of dyspnea in COPD. A controlled clinical trial of dyspnea management strategies. Chest 1995;107(3):724‐9. [DOI] [PubMed] [Google Scholar]
Sergysels 1979 {published data only}
- Sergysels R, De CA, Degre S, Denolin H. Functional evaluation of a physical rehabilitation program including breathing exercises and bicycle training in chronic obstructive lung disease. Respiration 1979;38(2):105‐11. [DOI] [PubMed] [Google Scholar]
Sutcu Cicek 2004 {published data only}
- Sutcu Cicek H, Akbayrak N. The effects of breathing exercises on pulmonary functions tests and arterial blood gas for the patients with chronic obstructive pulmonary disease (COPD). Gulhane Medical Journal 2004;46(1):1‐9. [Google Scholar]
Tandon 1978 {published data only}
- Tandon MK. Adjunct treatment with yoga in chronic severe airways obstruction. Thorax 1978;33:514‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiep 1986 {published data only}
- Tiep BL, Burns M, Kao D, Madison R, Herrera J. Pursed lips breathing training using ear oximetry. Chest 1986;90(2):218‐21. [DOI] [PubMed] [Google Scholar]
Yamanaka 2009 {published data only}
- Yamanaka Y, Ishikawa A, Miyasaka T, Totsu Y, Urabe Y, Inui K. [The effect of unsupervised home exercise program for patients with chronic obstructive pulmonary disease]. Nippon Ronen Igakkai Zasshi ‐ Japanese Journal of Geriatrics 2009;46(2):154‐9. [DOI] [PubMed] [Google Scholar]
Zakerimoghadam 2006 {published data only}
- Zakerimoghadam M, Shaban M, Kazemnejad A, Tavasoli K. The effect of breathing exercises on fatigue level of COPD patients. HAYAT: The Journal of Tehran Faculty of Nursing & Midwifery 2006;12(3):17‐25. [Google Scholar]
Zakerimoghadam 2011 {published data only}
- Zakerimoghadam M, Tavasoli K, Nejad AK, Khoshkesht S. The effect of breathing exercises on the fatigue levels of patients with chronic obstructive pulmonary disease. Acta Medica Indonesiana 2011;43:29‐33. [PubMed] [Google Scholar]
Additional references
Benzo 2010
- Benzo RP, Chang CC, Farrell MH, Kaplan R, Ries A, Martinez FJ, et al. Physical activity, health status and risk of hospitalization in patients with severe chronic obstructive pulmonary disease. Respiration 2010;80:10‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berlowitz 2011
- Berlowitz DJ, Spong J, O'Donoghue FJ, Pierce RJ, Brown DJ, Campbell DA, et al. Transcutaneous measurement of carbon dioxide tension during extended monitoring: evaluation of accuracy and stability, and an algorithm for correcting calibration drift. Respiratory Care 2011;56:442‐8. [DOI] [PubMed] [Google Scholar]
Bianchi 2007
Breslin 1992
Cahalin 2002
- Cahalin LP, Braga M, Matsuo Y, Hernandez ED. Efficacy of diaphragmatic breathing in persons with chronic obstructive pulmonary disease: a review of the literature. Journal of Cardiopulmonary Rehabilitation 2002;22:7‐21. [DOI] [PubMed] [Google Scholar]
Collins 2001
- Collins EG, Langbein WE, Fehr L, Maloney C. Breathing pattern retraining and exercise in persons with chronic obstructive pulmonary disease. AACN Clinical Issues 2001;12:202‐9. [DOI] [PubMed] [Google Scholar]
Dechman 2004
- Dechman G, Wilson CR. Evidence underlying breathing retraining in people with stable chronic obstructive pulmonary disease. Physical Therapy 2004;84:1189‐97. [PubMed] [Google Scholar]
Facchiano 2011
- Facchiano L, Hoffman Snyder C, Nunez DE. A literature review on breathing retraining as a self‐management strategy operationalized through Rosswurm and Larrabee's evidence‐based practice model. Journal of the American Academy of Nurse Practitioners 2011;23:421‐6. [DOI] [PubMed] [Google Scholar]
Gigliotti 2003
- Gigliotti F, Romagnoli I, Scano G. Breathing retraining and exercise conditioning in patients with chronic obstructive pulmonary disease (COPD): a physiological approach. Respiratory Medicine 2003;97:197‐204. [DOI] [PubMed] [Google Scholar]
Gilmartin 1984
- Gilmartin JJ, Gibson GJ. Abnormalities of chest wall motion in patients with chronic airflow obstruction. Thorax 1984; Vol. 39, issue 4:264‐71. [0040‐6376: (Print)] [DOI] [PMC free article] [PubMed]
Gosselink 2003
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hiratsuka 1993
- Hiratsuka T, Kida K. Quality of life measurements using a linear analogue scale for elderly patients with chronic lung disease. Internal Medicine 1993;32:832‐6. [DOI] [PubMed] [Google Scholar]
Holland 2010
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six‐minute walk distance in patients with chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation 2010;91:221‐5. [DOI] [PubMed] [Google Scholar]
Jones 1992
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self‐complete measure for chronic airflow limitation ‐ the St George's Respiratory Questionnaire. American Review of Respiratory Disease 1992;145:1321‐27. [DOI] [PubMed] [Google Scholar]
Lacasse 2006
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003793.pub2] [DOI] [PubMed] [Google Scholar]
Levine 1988
Martinez 1990
Mueller 1970
Parshall 2012
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. on behalf of the ATS Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. American Journal of Respiratory and Critical Care Medicine 2012;185:435‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2008
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6‐minute walk distance in patients with COPD. European Respiratory Journal 2008;32:637‐43. [DOI] [PubMed] [Google Scholar]
Rabe 2007
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 2007;176:532‐55. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.


