Abstract
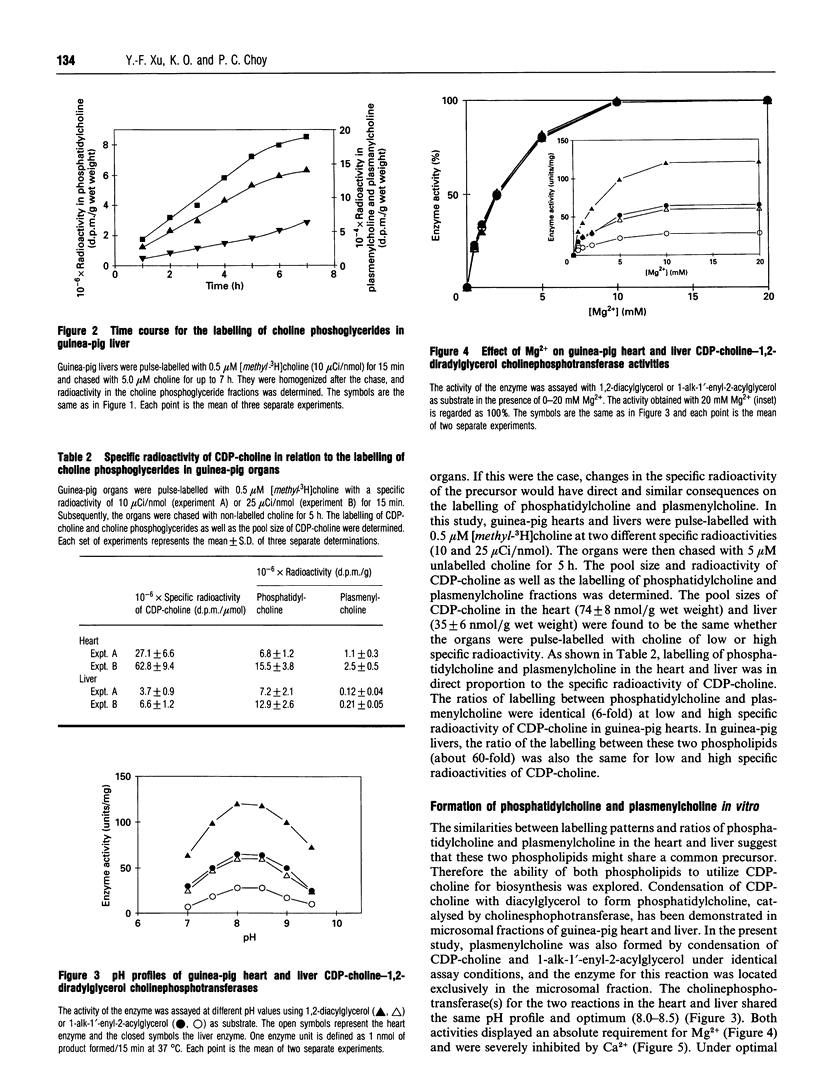
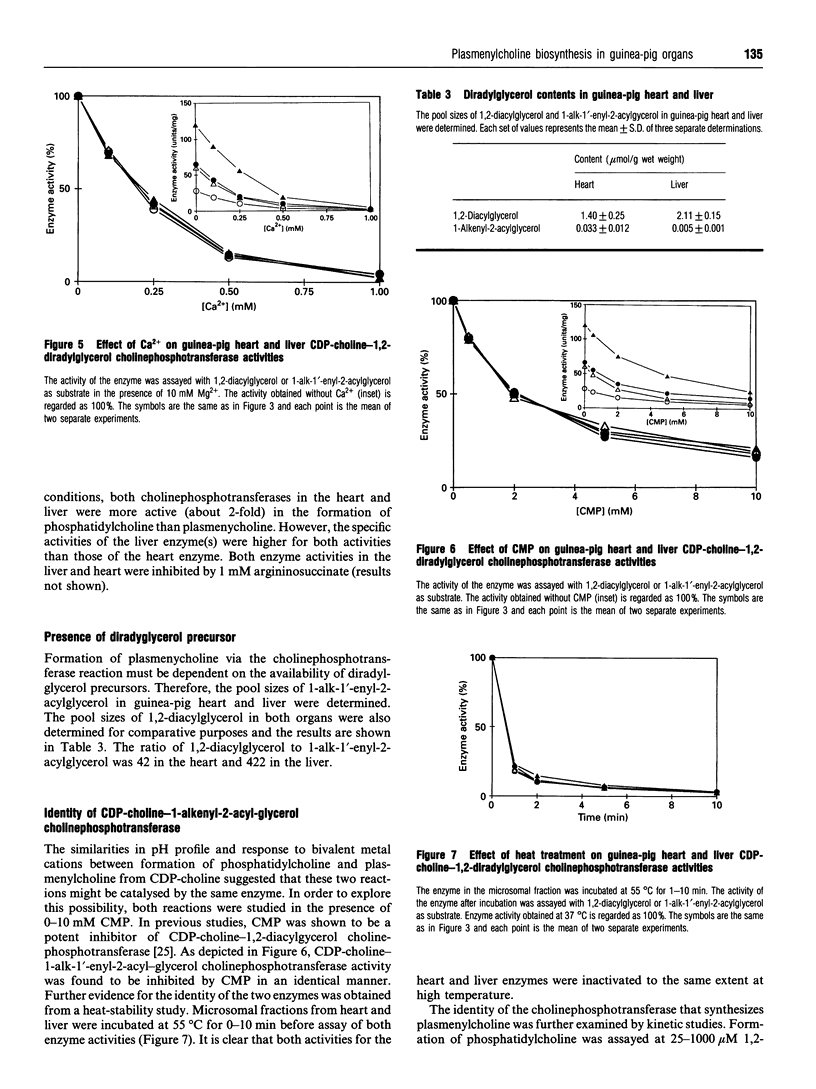
Plasmenylcholine is present in significant proportion (32% of choline phosphoglycerides) in the guinea-pig heart but exists as a minor component (3% of choline phosphoglycerides) in the guinea-pig liver. In this study, the biosynthesis of plasmenylcholine in these two organs was examined. The organs were perfused with labelled choline for 15 min and chased with unlabelled choline for up to 7 h. The labelling of phosphatidylcholine was 6-fold higher than that of plasmenylcholine in the heart and about 60-fold higher in the liver. However, the same labelling ratio was maintained throughout the chase period in both organs. Alterations in the specific radioactivity of CDP-choline caused corresponding changes in the labelling of phosphatidylcholine and plasmenylcholine. Our results suggest that in guinea-pig heart and liver, CDP-choline is the immediate precursor of biosynthesis of phosphatidylcholine and plasmenylcholine. The biochemical cause for the difference in their rates of formation between the two organs was explored. The enzyme activities for the formation of both choline phosphoglycerides were determined. The two reactions share the same characteristics, and 1,2-diacylglycerol and 1-alk-1'-enyl-2-acylglcerol were found to be mutually inhibitory in a competitive fashion. The pool sizes of 1,2-diacylglycerol and 1-alk-1'-enyl-2-acylglycerol were determined, and their ratios were found to be 42 in the heart and 422 in the liver. We conclude that cholinephosphotransferase catalyses the formation of both phosphatidylcholine and plasmenylcholine in the guinea-pig tissues and the rate of plasmenylcholine biosynthesis is dependent on the availability of 1-alk-1'-enyl-2-acylglycerol. Plasmenylcholine biosynthesis is also subjected to modulation by the 1,2-diacylglycerol content of the tissue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur G., Mock T., Zaborniak C., Choy P. C. The distribution and acyl composition of plasmalogens in guinea pig heart. Lipids. 1985 Oct;20(10):693–698. doi: 10.1007/BF02534389. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Choy P. C. Control of phosphatidylcholine biosynthesis in myopathic hamster hearts. J Biol Chem. 1982 Sep 25;257(18):10928–10933. [PubMed] [Google Scholar]
- Cornell R. B. Cholinephosphotransferase from mammalian sources. Methods Enzymol. 1992;209:267–272. doi: 10.1016/0076-6879(92)09033-y. [DOI] [PubMed] [Google Scholar]
- Diagne A., Fauvel J., Record M., Chap H., Douste-Blazy L. Studies on ether phospholipids. II. Comparative composition of various tissues from human, rat and guinea pig. Biochim Biophys Acta. 1984 Apr 18;793(2):221–231. doi: 10.1016/0005-2760(84)90324-2. [DOI] [PubMed] [Google Scholar]
- Ford D. A., Gross R. W. Identification of endogenous 1-O-alk-1'-enyl-2-acyl-sn-glycerol in myocardium and its effective utilization by choline phosphotransferase. J Biol Chem. 1988 Feb 25;263(6):2644–2650. [PubMed] [Google Scholar]
- Ford D. A., Hazen S. L., Saffitz J. E., Gross R. W. The rapid and reversible activation of a calcium-independent plasmalogen-selective phospholipase A2 during myocardial ischemia. J Clin Invest. 1991 Jul;88(1):331–335. doi: 10.1172/JCI115296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. A., Rosenbloom K. B., Gross R. W. The primary determinant of rabbit myocardial ethanolamine phosphotransferase substrate selectivity is the covalent nature of the sn-1 aliphatic group of diradyl glycerol acceptors. J Biol Chem. 1992 Jun 5;267(16):11222–11228. [PubMed] [Google Scholar]
- Frenkel R. A., Johnston J. M. Metabolic conversion of platelet-activating factor into ethanolamine plasmalogen in an amnion-derived cell line. J Biol Chem. 1992 Sep 25;267(27):19186–19191. [PubMed] [Google Scholar]
- GOTTFRIED E. L., RAPPORT M. M. The biochemistry of plasmalogens. I. Isolation and characterization of phosphatidal choline, a pure native plasmalogen. J Biol Chem. 1962 Feb;237:329–333. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McMaster C. R., Lu C. Q., Choy P. C. The existence of a soluble plasmalogenase in guinea pig tissues. Lipids. 1992 Dec;27(12):945–949. doi: 10.1007/BF02535569. [DOI] [PubMed] [Google Scholar]
- Morikawa S., Taniguchi S., Fujii K., Mori H., Kumada K., Fujiwara M., Fujiwara M. Preferential synthesis of diacyl and alkenylacyl ethanolamine and choline glycerophospholipids in rabbit platelet membranes. J Biol Chem. 1987 Jan 25;262(3):1213–1217. [PubMed] [Google Scholar]
- O K. M., Choy P. C. Effects of fasting on phosphatidylcholine biosynthesis in hamster liver: regulation of cholinephosphotransferase activity by endogenous argininosuccinate. Biochem J. 1993 Feb 1;289(Pt 3):727–733. doi: 10.1042/bj2890727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O K. M., Choy P. C. Solubilization and partial purification of cholinephosphotransferase in hamster tissues. Lipids. 1990 Feb;25(2):122–124. doi: 10.1007/BF02562217. [DOI] [PubMed] [Google Scholar]
- Paltauf F. Biosynthesis of plasmalogens from alkyl- and alkyl-acyl-glycerophosphoryl ethanolamine in the rat brain. FEBS Lett. 1971 Sep 15;17(1):118–120. doi: 10.1016/0014-5793(71)80578-1. [DOI] [PubMed] [Google Scholar]
- Rüstow B., Kunze D. Further evidence for the existence of different diacylglycerol pools of the phosphatidylcholine synthesis in microsomes. Biochim Biophys Acta. 1987 Oct 17;921(3):552–558. doi: 10.1016/0005-2760(87)90083-x. [DOI] [PubMed] [Google Scholar]
- Strum J. C., Emilsson A., Wykle R. L., Daniel L. W. Conversion of 1-O-alkyl-2-acyl-sn-glycero-3-phosphocholine to 1-O-alk-1'-enyl-2-acyl-sn-glycero-3-phosphoethanolamine. A novel pathway for the metabolism of ether-linked phosphoglycerides. J Biol Chem. 1992 Jan 25;267(3):1576–1583. [PubMed] [Google Scholar]
- Tardi P. G., Man R. Y., Choy P. C. The effect of methyl-lidocaine on the biosynthesis of phospholipids de novo in the isolated hamster heart. Biochem J. 1992 Jul 1;285(Pt 1):161–166. doi: 10.1042/bj2850161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wientzek M., Man R. Y., Choy P. C. Choline glycerophospholipid biosynthesis in the guinea pig heart. Biochem Cell Biol. 1987 Oct;65(10):860–868. doi: 10.1139/o87-112. [DOI] [PubMed] [Google Scholar]
- Wolf R. A., Gross R. W. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J Biol Chem. 1985 Jun 25;260(12):7295–7303. [PubMed] [Google Scholar]
- Wykle R. L., Blank M. L., Malone B., Snyder F. Evidence for a mixed function oxidase in the biosynthesis of ethanolamine plasmalogens from 1-alkyl-2-acyl-sn-glycero-3-phosphorylethanolamine. J Biol Chem. 1972 Sep 10;247(17):5442–5447. [PubMed] [Google Scholar]
- Xu Z. L., Byers D. M., Palmer F. B., Spence M. W., Cook H. W. Serine utilization as a precursor of phosphatidylserine and alkenyl-(plasmenyl)-, alkyl-, and acylethanolamine phosphoglycerides in cultured glioma cells. J Biol Chem. 1991 Feb 5;266(4):2143–2150. [PubMed] [Google Scholar]
- Zelinski T. A., Savard J. D., Man R. Y., Choy P. C. Phosphatidylcholine biosynthesis in isolated hamster heart. J Biol Chem. 1980 Dec 10;255(23):11423–11428. [PubMed] [Google Scholar]
- Zoeller R. A., Morand O. H., Raetz C. R. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J Biol Chem. 1988 Aug 15;263(23):11590–11596. [PubMed] [Google Scholar]


