Abstract
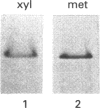
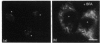
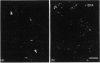
The lethal chicken mutation nanomelia leads to severe skeletal defects because of a deficiency of aggrecan, which is the largest aggregating chondroitin sulphate proteoglycan of cartilage. In previous work, we have demonstrated that nanomelic chondrocytes produce a truncated aggrecan precursor that fails to be secreted, and is apparently arrested in the endoplasmic reticulum (ER). In this study, we investigated the biosynthesis and extent of processing of the abnormal aggrecan precursor. The truncated precursor was translated directly in cell-free reactions, indicating that it does not arise post-translationally. Further studies addressed the processing capabilities of the defective precursor. We found that the mutant precursor was modified by N-linked, mannose-rich oligosaccharides and by the addition of xylose, but was not further processed; this is consistent with the conclusion that it moves no further along the secretory pathway than the ER. Using brefeldin A we demonstrated that the defective precursor can function as a substrate for Golgi-mediated glycosaminoglycan chains, but does not do so in the nanomelic chondrocyte because it fails to be translocated to the appropriate membrane compartment. These studies illustrate how combined cell biological/biochemical and molecular investigations may contribute to our understanding of the biological consequences and molecular basis of genetic diseases, particularly those involving errors in large, highly modified molecules such as proteoglycans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara J. F., Cheng S. H., Smith A. E. Intracellular protein trafficking defects in human disease. Trends Cell Biol. 1992 May;2(5):145–149. doi: 10.1016/0962-8924(92)90101-r. [DOI] [PubMed] [Google Scholar]
- Balch W. E. Biochemistry of interorganelle transport. A new frontier in enzymology emerges from versatile in vitro model systems. J Biol Chem. 1989 Oct 15;264(29):16965–16968. [PubMed] [Google Scholar]
- Beckers C. J., Keller D. S., Balch W. E. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell. 1987 Aug 14;50(4):523–534. doi: 10.1016/0092-8674(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Lippincott-Schwartz J. Degradation of proteins within the endoplasmic reticulum. Curr Opin Cell Biol. 1991 Aug;3(4):592–600. doi: 10.1016/0955-0674(91)90028-w. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Wallis G. A., Willing M. C. Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet. 1991 Jul;28(7):433–442. doi: 10.1136/jmg.28.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. C., Schwartz N. B. Kinetics of intracellular processing of chondroitin sulfate proteoglycan core protein and other matrix components. J Cell Biol. 1988 Jun;106(6):2191–2202. doi: 10.1083/jcb.106.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran L., Tanzer M. L. Molecular cloning of chicken aggrecan. Structural analyses. Biochem J. 1992 Dec 15;288(Pt 3):903–910. doi: 10.1042/bj2880903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessler S. D., Byers P. H. Defective folding and stable association with protein disulfide isomerase/prolyl hydroxylase of type I procollagen with a deletion in the pro alpha 2(I) chain that preserves the Gly-X-Y repeat pattern. J Biol Chem. 1992 Apr 15;267(11):7751–7757. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Doege K., Sasaki M., Horigan E., Hassell J. R., Yamada Y. Complete primary structure of the rat cartilage proteoglycan core protein deduced from cDNA clones. J Biol Chem. 1987 Dec 25;262(36):17757–17767. [PubMed] [Google Scholar]
- Fülöp C., Walcz E., Valyon M., Glant T. T. Expression of alternatively spliced epidermal growth factor-like domains in aggrecans of different species. Evidence for a novel module. J Biol Chem. 1993 Aug 15;268(23):17377–17383. [PubMed] [Google Scholar]
- Halberg D. F., Proulx G., Doege K., Yamada Y., Drickamer K. A segment of the cartilage proteoglycan core protein has lectin-like activity. J Biol Chem. 1988 Jul 5;263(19):9486–9490. [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Hayes C. E., Goldstein I. J. An alpha-D-galactosyl-binding lectin from Bandeiraea simplicifolia seeds. Isolation by affinity chromatography and characterization. J Biol Chem. 1974 Mar 25;249(6):1904–1914. [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Kearns A. E., Vertel B. M., Schwartz N. B. Topography of glycosylation and UDP-xylose production. J Biol Chem. 1993 May 25;268(15):11097–11104. [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- LANDAUER W. NANOMELIA, A LETHAL NUTATION OF THE FOWL. J Hered. 1965 May-Jun;56:131–138. doi: 10.1093/oxfordjournals.jhered.a107392. [DOI] [PubMed] [Google Scholar]
- Lau M. M., Neufeld E. F. A frameshift mutation in a patient with Tay-Sachs disease causes premature termination and defective intracellular transport of the alpha-subunit of beta-hexosaminidase. J Biol Chem. 1989 Dec 15;264(35):21376–21380. [PubMed] [Google Scholar]
- Li H., Schwartz N. B., Vertel B. M. cDNA cloning of chick cartilage chondroitin sulfate (aggrecan) core protein and identification of a stop codon in the aggrecan gene associated with the chondrodystrophy, nanomelia. J Biol Chem. 1993 Nov 5;268(31):23504–23511. [PubMed] [Google Scholar]
- O'Donnell C. M., Kaczman-Daniel K., Goetinck P. F., Vertel B. M. Nanomelic chondrocytes synthesize a glycoprotein related to chondroitin sulfate proteoglycan core protein. J Biol Chem. 1988 Nov 25;263(33):17749–17754. [PubMed] [Google Scholar]
- Pacifici M., Soltesz R., Thal G., Shanley D. J., Boettiger D., Holtzer H. Immunological characterization of the major chick cartilage proteoglycan and its intracellular localization in cultured chondroblasts: a comparison with Type II procollagen. J Cell Biol. 1983 Dec;97(6):1724–1736. doi: 10.1083/jcb.97.6.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Mörgelin M., Wiedemann H., Beardmore-Gray M., Dunham D., Hardingham T., Heinegård D., Timpl R., Engel J. Extended and globular protein domains in cartilage proteoglycans. Biochem J. 1987 Aug 1;245(3):763–772. doi: 10.1042/bj2450763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pennypacker J. P., Goetinck P. F. Biochemical and ultrastructural studies of collagen and proteochondroitin sulfate in normal and nanomelic cartilage. Dev Biol. 1976 May;50(1):35–47. doi: 10.1016/0012-1606(76)90065-8. [DOI] [PubMed] [Google Scholar]
- Prockop D. J. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J Biol Chem. 1990 Sep 15;265(26):15349–15352. [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Sai S., Tanaka T., Kosher R. A., Tanzer M. L. Cloning and sequence analysis of a partial cDNA for chicken cartilage proteoglycan core protein. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5081–5085. doi: 10.1073/pnas.83.14.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger J., Maroteaux P. The lethal osteochondrodysplasias. Adv Hum Genet. 1990;19:1-103, 331-2. doi: 10.1007/978-1-4757-9065-8_1. [DOI] [PubMed] [Google Scholar]
- Stanescu V., Stanescu R., Maroteaux P. Pathogenic mechanisms in osteochondrodysplasias. J Bone Joint Surg Am. 1984 Jul;66(6):817–836. doi: 10.2106/00004623-198466060-00002. [DOI] [PubMed] [Google Scholar]
- Stirpe N. S., Argraves W. S., Goetinck P. F. Chondrocytes from the cartilage proteoglycan-deficient mutant, nanomelia, synthesize greatly reduced levels of the proteoglycan core protein transcript. Dev Biol. 1987 Nov;124(1):77–81. doi: 10.1016/0012-1606(87)90461-1. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Vertel B. M., Dorfman A. Translation and characterization of messenger RNAs in differentiating chicken cartilage. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4847–4851. doi: 10.1073/pnas.76.10.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertel B. M., Barkman L. L., Morrell J. J. Intracellular features of type II procollagen and chondroitin sulfate proteoglycan synthesis in chondrocytes. J Cell Biochem. 1985;27(3):215–229. doi: 10.1002/jcb.240270304. [DOI] [PubMed] [Google Scholar]
- Vertel B. M., Hitti Y. Biosynthetic precursors of cartilage chondroitin sulfate proteoglycan. Coll Relat Res. 1987 Apr;7(1):57–75. doi: 10.1016/s0174-173x(87)80021-3. [DOI] [PubMed] [Google Scholar]
- Vertel B. M., Morrell J. J., Barkman L. L. Immunofluorescence studies on cartilage matrix synthesis. The synthesis of link protein, chondroitin sulfate proteoglycan monomer and type II collagen. Exp Cell Res. 1985 Jun;158(2):423–432. doi: 10.1016/0014-4827(85)90466-5. [DOI] [PubMed] [Google Scholar]
- Vertel B. M., Velasco A., LaFrance S., Walters L., Kaczman-Daniel K. Precursors of chondroitin sulfate proteoglycan are segregated within a subcompartment of the chondrocyte endoplasmic reticulum. J Cell Biol. 1989 Oct;109(4 Pt 1):1827–1836. doi: 10.1083/jcb.109.4.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertel B. M., Walters L. M., Flay N., Kearns A. E., Schwartz N. B. Xylosylation is an endoplasmic reticulum to Golgi event. J Biol Chem. 1993 May 25;268(15):11105–11112. [PubMed] [Google Scholar]
- Vertel B. M., Walters L. M., Grier B., Maine N., Goetinck P. F. Nanomelic chondrocytes synthesize, but fail to translocate, a truncated aggrecan precursor. J Cell Sci. 1993 Mar;104(Pt 3):939–948. doi: 10.1242/jcs.104.3.939. [DOI] [PubMed] [Google Scholar]
- Wiedemann H., Paulsson M., Timpl R., Engel J., Heinegård D. Domain structure of cartilage proteoglycans revealed by rotary shadowing of intact and fragmented molecules. Biochem J. 1984 Nov 15;224(1):331–333. doi: 10.1042/bj2240331. [DOI] [PMC free article] [PubMed] [Google Scholar]