Abstract
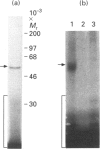
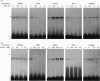
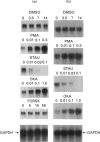
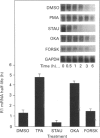
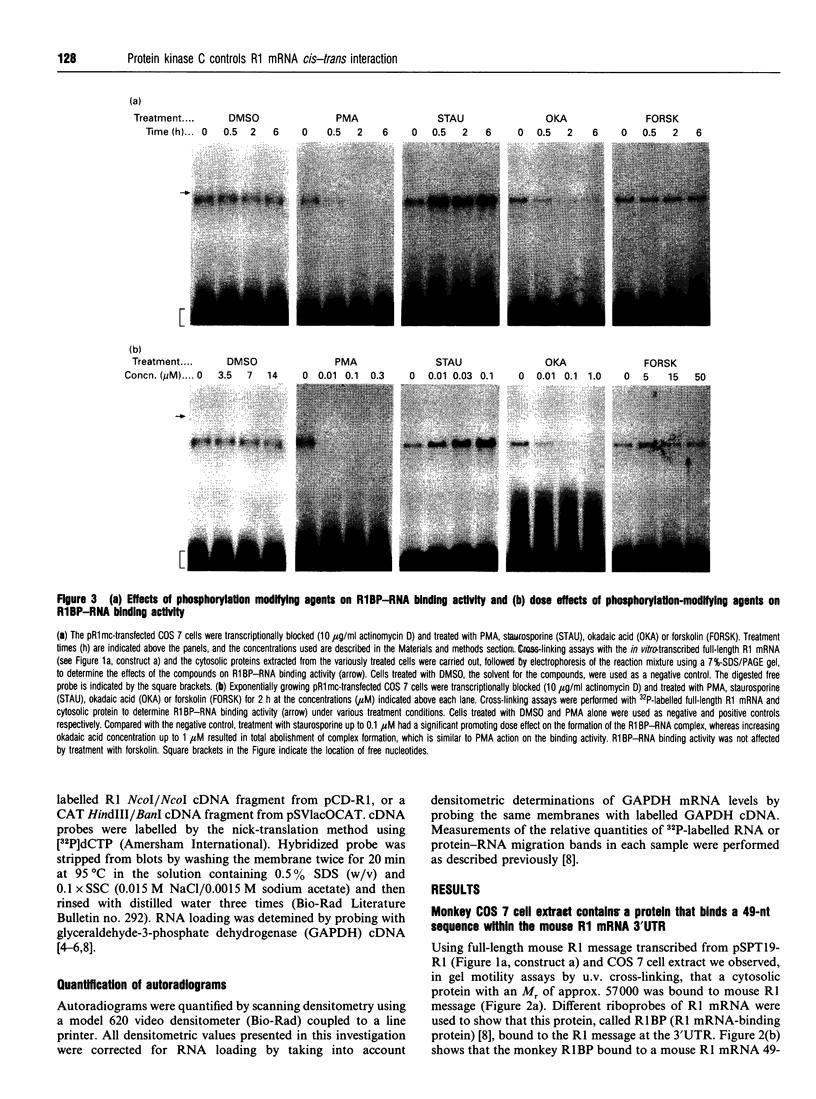
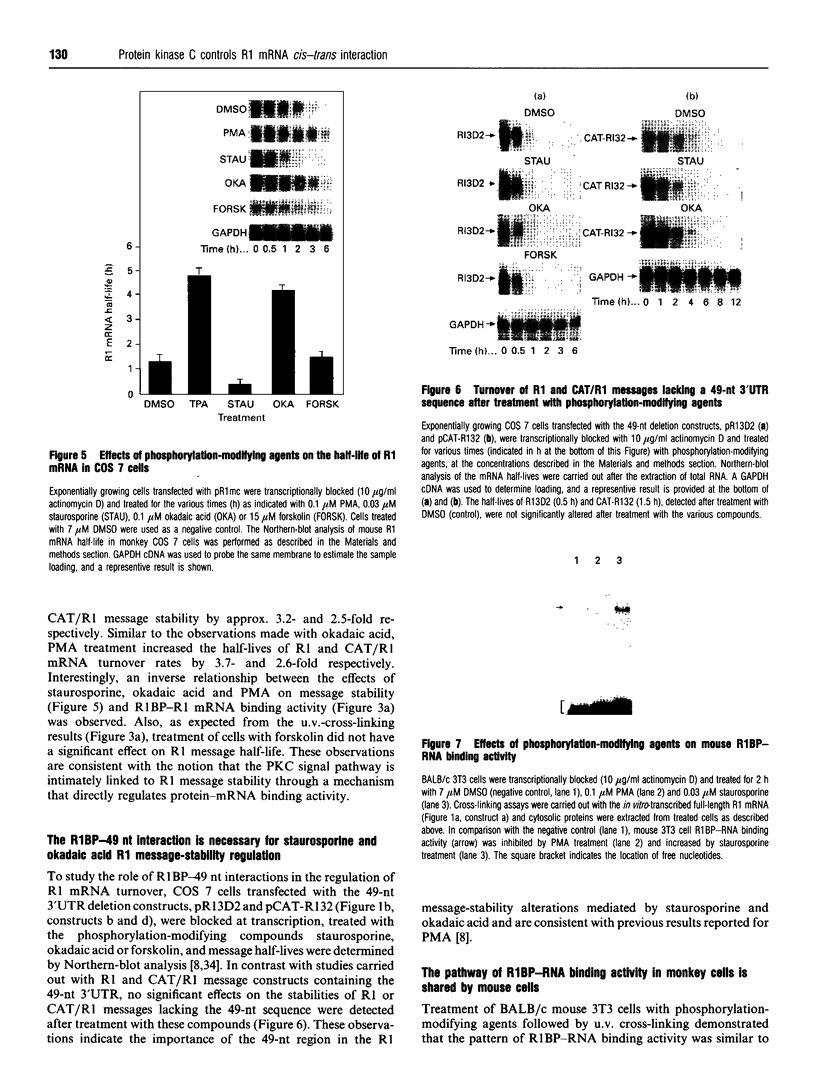
Ribonucleotide reductase catalyses the reaction that eventually provides the four deoxyribonucleotides required for the synthesis and repair of DNA. U.v.-cross-linking and band-shift experiments have identified in COS 7 monkey cells an approx. 57 kDa ribonucleotide reductase R1 mRNA-binding protein called R1BP, which binds specifically to a 49-nt region of the R1 mRNA 3'-untranslated region (3'UTR). The R1BP-RNA binding activity was down-regulated by the tumour promoters phorbol 12-myristate 13-acetate (PMA; 'TPA') and okadaic acid, and up-regulated by the protein kinase C inhibitor staurosporine, in a dose-dependent fashion. Furthermore, staurosporine treatment decreased the stability of R1 and CAT (chloramphenicol acetyltransferase)/R1 hybrid mRNAs, whereas PMA and okadaic acid increased the stability of these messages, in a dose-dependent manner. In contrast, treatment of cells with forskolin, a protein kinase A inhibitor, did not alter either R1BP-RNA binding or R1 mRNA-stability characteristics. Transfectants containing R1 or CAT/R1 cDNA constructs with a deletion of the 49-nt 3'UTR sequence failed to respond in message-stability studies to the effects of PMA, staurosporine or okadaic acid. These observations indicate that a protein kinase C signal pathway regulates ribonucleotide reductase R1 gene expression post-transcriptionally, through a mechanism involving a specific cis-trans interaction at a 49-nt region within the R1 mRNA 3'UTR.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara F. M., Chen F. Y., Wright J. A. A novel transforming growth factor-beta 1 responsive cytoplasmic trans-acting factor binds selectively to the 3'-untranslated region of mammalian ribonucleotide reductase R2 mRNA: role in message stability. Nucleic Acids Res. 1993 Oct 11;21(20):4803–4809. doi: 10.1093/nar/21.20.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blosmanis R., Wright J. A., Goldenberg G. J. Sensitivity to melphalan as a function of transport activity and proliferative rate in BALB/c 3T3 fibroblasts. Cancer Res. 1987 Mar 1;47(5):1273–1277. [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3' untranslated region of lymphokine mRNA. Mol Cell Biol. 1991 Jun;11(6):3288–3295. doi: 10.1128/mcb.11.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. An A + U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991 May;11(5):2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Figge J., Hansen U., Wright C., Jeang K. T., Khoury G., Livingston D. M., Roberts T. M. lac repressor can regulate expression from a hybrid SV40 early promoter containing a lac operator in animal cells. Cell. 1987 Jun 5;49(5):603–612. doi: 10.1016/0092-8674(87)90536-8. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. Y., Amara F. M., Wright J. A. Mammalian ribonucleotide reductase R1 mRNA stability under normal and phorbol ester stimulating conditions: involvement of a cis-trans interaction at the 3' untranslated region. EMBO J. 1993 Oct;12(10):3977–3986. doi: 10.1002/j.1460-2075.1993.tb06075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. K., Holtzer S., Chacko S. A., Lin Z. X., Hoffman R. K., Holtzer H. Phorbol esters selectively and reversibly inhibit a subset of myofibrillar genes responsible for the ongoing differentiation program of chick skeletal myotubes. Mol Cell Biol. 1991 Sep;11(9):4473–4482. doi: 10.1128/mcb.11.9.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamonici O. R., Trepel J. B., Vidal C. A., Neckers L. M. Phorbol ester induces c-sis gene transcription in stem cell line K-562. Mol Cell Biol. 1986 May;6(5):1847–1850. doi: 10.1128/mcb.6.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio V., Huber B. E., Jacobs S. Partial down-regulation of protein kinase C reverses the growth inhibitory effect of phorbol esters on HepG2 cells. J Cell Physiol. 1990 Nov;145(2):381–389. doi: 10.1002/jcp.1041450225. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Wright J. A., Jarolim L., Yanagihara K., Bassin R. H., Greenberg A. H. Transformation by oncogenes encoding protein kinases induces the metastatic phenotype. Science. 1987 Oct 9;238(4824):202–205. doi: 10.1126/science.3659911. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Goode N., Hughes K., Woodgett J. R., Parker P. J. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992 Aug 25;267(24):16878–16882. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hurta R. A., Samuel S. K., Greenberg A. H., Wright J. A. Early induction of ribonucleotide reductase gene expression by transforming growth factor beta 1 in malignant H-ras transformed cell lines. J Biol Chem. 1991 Dec 15;266(35):24097–24100. [PubMed] [Google Scholar]
- Hurta R. A., Wright J. A. Alterations in the activity and regulation of mammalian ribonucleotide reductase by chlorambucil, a DNA damaging agent. J Biol Chem. 1992 Apr 5;267(10):7066–7071. [PubMed] [Google Scholar]
- Hurta R. A., Wright J. A. Alterations in the cyclic AMP signal transduction pathway regulating ribonucleotide reductase gene expression in malignant H-ras transformed cell lines. J Cell Physiol. 1994 Jan;158(1):187–197. doi: 10.1002/jcp.1041580123. [DOI] [PubMed] [Google Scholar]
- Hurta R. A., Wright J. A. Regulation of mammalian ribonucleotide reductase by the tumor promoters and protein phosphatase inhibitors okadaic acid and calyculin A. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):1081–1087. doi: 10.1139/o92-153. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Isakov N., McMahon P., Altman A. Selective post-transcriptional down-regulation of protein kinase C isoenzymes in leukemic T cells chronically treated with phorbol ester. J Biol Chem. 1990 Feb 5;265(4):2091–2097. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Malter J. S., Hong Y. A redox switch and phosphorylation are involved in the post-translational up-regulation of the adenosine-uridine binding factor by phorbol ester and ionophore. J Biol Chem. 1991 Feb 15;266(5):3167–3171. [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- McClarty G. A., Chan A. K., Choy B. K., Wright J. A. Increased ferritin gene expression is associated with increased ribonucleotide reductase gene expression and the establishment of hydroxyurea resistance in mammalian cells. J Biol Chem. 1990 May 5;265(13):7539–7547. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Kour G., Marais R. M., Mitchell F., Pears C., Schaap D., Stabel S., Webster C. Protein kinase C--a family affair. Mol Cell Endocrinol. 1989 Aug;65(1-2):1–11. doi: 10.1016/0303-7207(89)90159-7. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Suganuma M., Fujiki H., Suguri H., Yoshizawa S., Hirota M., Nakayasu M., Ojika M., Wakamatsu K., Yamada K., Sugimura T. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Berg P. Isolation and characterization of expressible cDNA clones encoding the M1 and M2 subunits of mouse ribonucleotide reductase. Mol Cell Biol. 1986 Oct;6(10):3433–3442. doi: 10.1128/mcb.6.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991 Aug 25;266(24):15771–15781. [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- Wright J. A., Chan A. K., Choy B. K., Hurta R. A., McClarty G. A., Tagger A. Y. Regulation and drug resistance mechanisms of mammalian ribonucleotide reductase, and the significance to DNA synthesis. Biochem Cell Biol. 1990 Dec;68(12):1364–1371. doi: 10.1139/o90-199. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Turley E. A., Greenberg A. H. Transforming growth factor beta and fibroblast growth factor as promoters of tumor progression to malignancy. Crit Rev Oncog. 1993;4(5):473–492. [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]