Abstract
In vitro refolding of pig mitochondrial malate dehydrogenase is investigated in the presence of Escherichia coli chaperonins cpn60 (groEL) and cpn10 (groES). When the enzyme is initially denatured with 3 M guanidinium chloride, chaperonin-assisted refolding is 100% efficient. C.d. spectroscopy reveals that malate dehydrogenase is almost unfolded in 3 M guanidinium chloride, suggesting that a state with little or no residual secondary structure is the optimal 'substrate' for chaperonin-assisted refolding. Malate dehydrogenase denatured to more highly structured states proves to refold less efficiently with chaperonin assistance. The enzyme is shown not to aggregate under the refolding conditions, so that losses in refolding efficiency result from irreversible misfolding. Evidence is advanced to suggest that the chaperonins are unable to rescue irreversibly misfolded malate dehydrogenase. A novel use is made of 100 K Centricon concentrators to study the binding of [14C]acetyl-labelled malate dehydrogenase to groEL by an ultrafiltration binding assay. Analysis of the data by Scatchard plot shows that acetyl-malate dehydrogenase, which has previously been extensively unfolded with guanidinium chloride, binds to groEL at a specific binding site(s). At saturation, one acetyl-malate dehydrogenase homodimer (two polypeptides) is shown to bind to each groEL homooligomer with a binding constant of approx. 10 nM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochkareva E. S., Lissin N. M., Flynn G. C., Rothman J. E., Girshovich A. S. Positive cooperativity in the functioning of molecular chaperone GroEL. J Biol Chem. 1992 Apr 5;267(10):6796–6800. [PubMed] [Google Scholar]
- Braig K., Simon M., Furuya F., Hainfeld J. F., Horwich A. L. A polypeptide bound by the chaperonin groEL is localized within a central cavity. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3978–3982. doi: 10.1073/pnas.90.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J., Schmidt M., Fuchs M., Jaenicke R., Rudolph R., Schmid F. X., Kiefhaber T. GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry. 1991 Feb 12;30(6):1586–1591. doi: 10.1021/bi00220a020. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Rohrbach M. S., Harrison J. H. Selective chemical modification of malate dehydrogenase. N-ethylmaleimide modification of active center sulfhydryl residues. J Biol Chem. 1971 Sep 10;246(17):5491–5497. [PubMed] [Google Scholar]
- Hayer-Hartl M. K., Hartl F. U. A comment on: 'The aromatic amino acid content of the bacterial chaperone protein groEL (cpn60): evidence for the presence of a single tryptophan', by N.C. Price, S.M. Kelly, S. Wood and A. auf der Mauer (1991) FEBS Lett. 292, 9-12. FEBS Lett. 1993 Mar 29;320(1):83–85. doi: 10.1016/0014-5793(93)81663-k. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Huang T. M., Chang G. G. Characterization of the tetramer-dimer-monomer equilibrium of the enzymatically active subunits of pigeon liver malic enzyme. Biochemistry. 1992 Dec 22;31(50):12658–12664. doi: 10.1021/bi00165a016. [DOI] [PubMed] [Google Scholar]
- Jackson G. S., Staniforth R. A., Halsall D. J., Atkinson T., Holbrook J. J., Clarke A. R., Burston S. G. Binding and hydrolysis of nucleotides in the chaperonin catalytic cycle: implications for the mechanism of assisted protein folding. Biochemistry. 1993 Mar 16;32(10):2554–2563. doi: 10.1021/bi00061a013. [DOI] [PubMed] [Google Scholar]
- Kupke D. W., Dorrier T. E. Protein concentration measurements: the dry weight. Methods Enzymol. 1978;48:155–162. doi: 10.1016/s0076-6879(78)48008-5. [DOI] [PubMed] [Google Scholar]
- Landry S. J., Jordan R., McMacken R., Gierasch L. M. Different conformations for the same polypeptide bound to chaperones DnaK and GroEL. Nature. 1992 Jan 30;355(6359):455–457. doi: 10.1038/355455a0. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
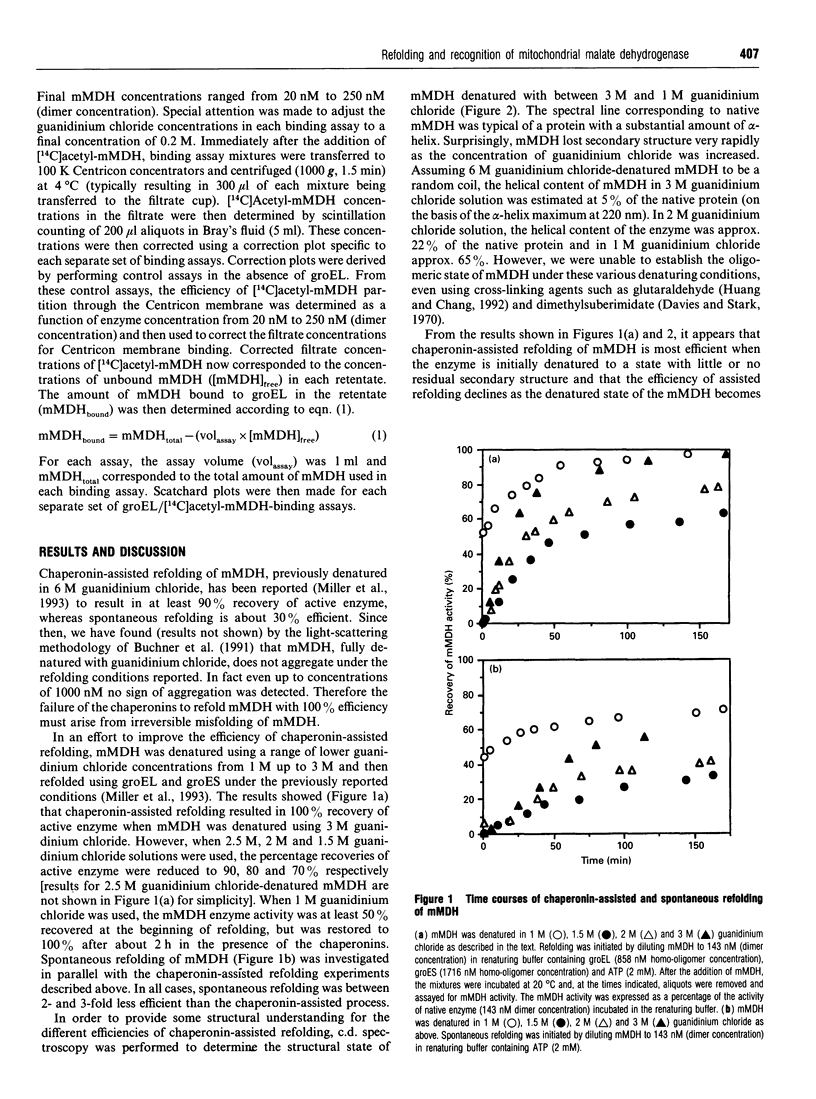
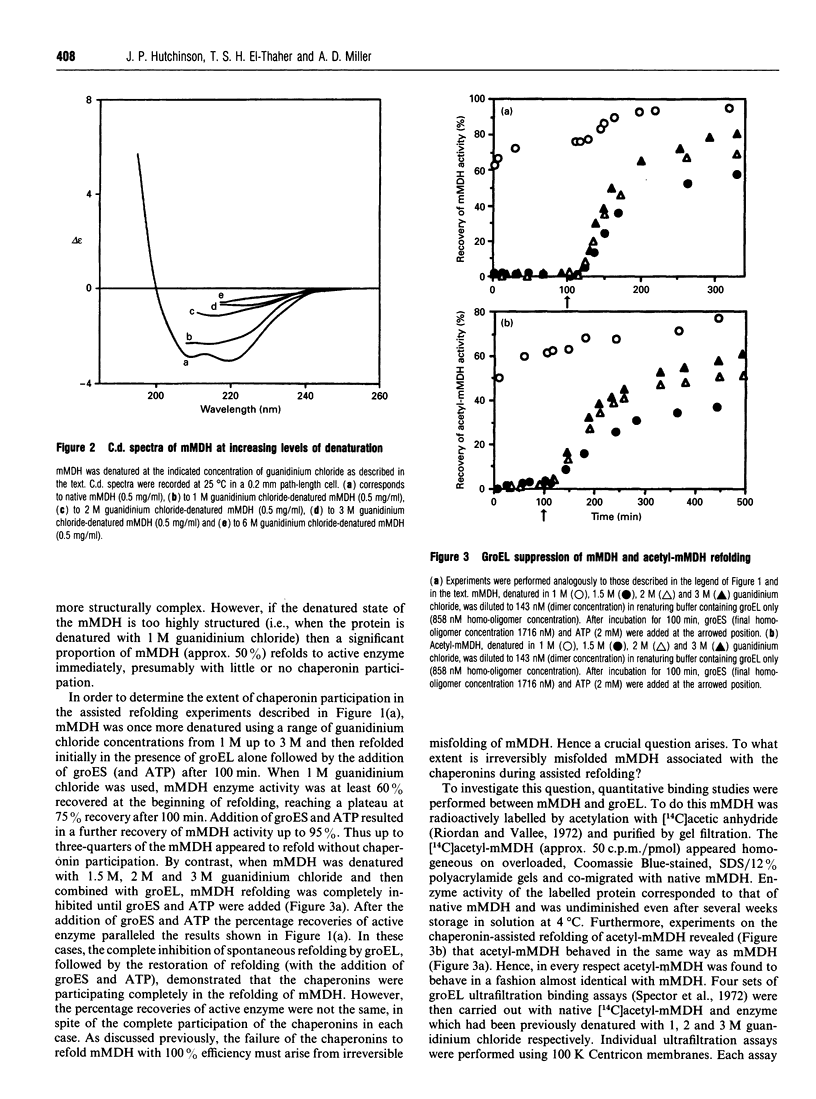
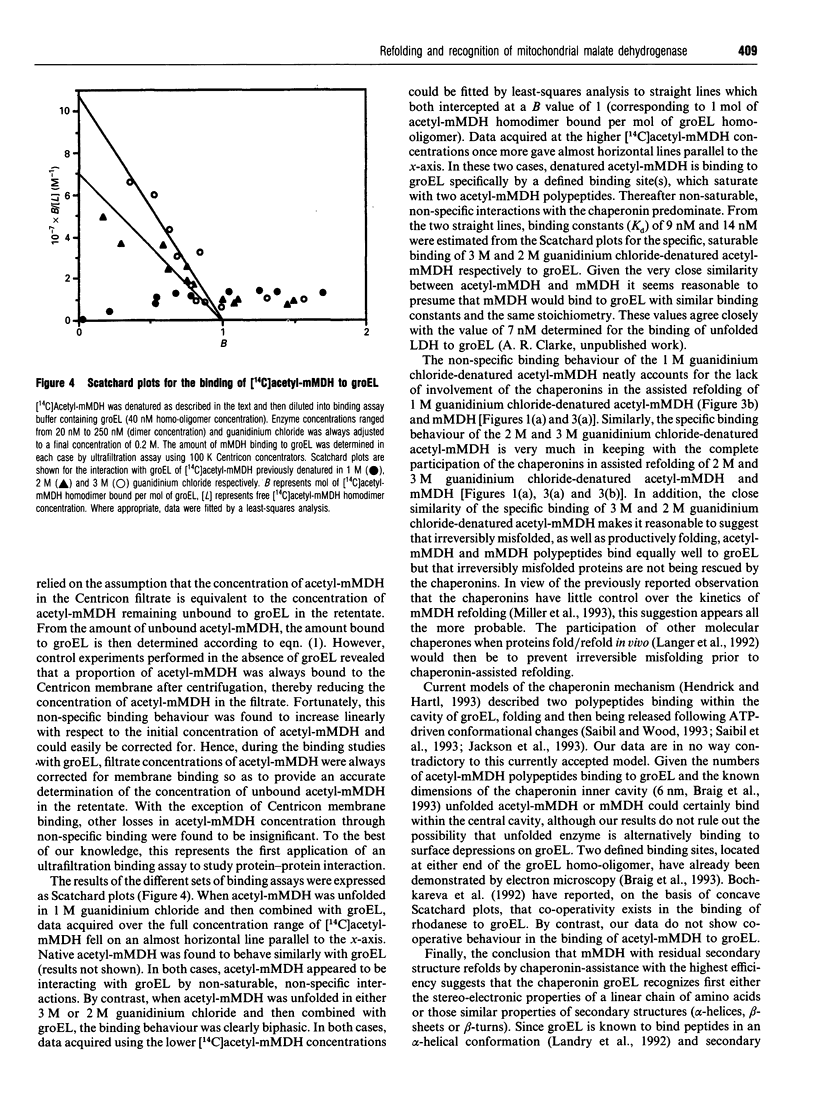
- Miller A. D., Maghlaoui K., Albanese G., Kleinjan D. A., Smith C. Escherichia coli chaperonins cpn60 (groEL) and cpn10 (groES) do not catalyse the refolding of mitochondrial malate dehydrogenase. Biochem J. 1993 Apr 1;291(Pt 1):139–144. doi: 10.1042/bj2910139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibil H. R., Zheng D., Roseman A. M., Hunter A. S., Watson G. M., Chen S., Auf Der Mauer A., O'Hara B. P., Wood S. P., Mann N. H. ATP induces large quaternary rearrangements in a cage-like chaperonin structure. Curr Biol. 1993 May 1;3(5):265–273. doi: 10.1016/0960-9822(93)90176-o. [DOI] [PubMed] [Google Scholar]
- Spector R., Korkin D. T., Lorenzo A. V. A rapid method for the determination of salicylate binding by the use of ultrafilters. J Pharm Pharmacol. 1972 Oct;24(10):786–789. doi: 10.1111/j.2042-7158.1972.tb08883.x. [DOI] [PubMed] [Google Scholar]
- THORNE C. J., KAPLAN N. O. Physicochemical properties of pig and horse heart mitochondrial malate dehydrogenase. J Biol Chem. 1963 May;238:1861–1868. [PubMed] [Google Scholar]
- Todd M. J., Viitanen P. V., Lorimer G. H. Hydrolysis of adenosine 5'-triphosphate by Escherichia coli GroEL: effects of GroES and potassium ion. Biochemistry. 1993 Aug 24;32(33):8560–8567. doi: 10.1021/bi00084a024. [DOI] [PubMed] [Google Scholar]
- Viitanen P. V., Lubben T. H., Reed J., Goloubinoff P., O'Keefe D. P., Lorimer G. H. Chaperonin-facilitated refolding of ribulosebisphosphate carboxylase and ATP hydrolysis by chaperonin 60 (groEL) are K+ dependent. Biochemistry. 1990 Jun 19;29(24):5665–5671. doi: 10.1021/bi00476a003. [DOI] [PubMed] [Google Scholar]
- Wright P. E., Dyson H. J., Lerner R. A. Conformation of peptide fragments of proteins in aqueous solution: implications for initiation of protein folding. Biochemistry. 1988 Sep 20;27(19):7167–7175. doi: 10.1021/bi00419a001. [DOI] [PubMed] [Google Scholar]
- Zahn R., Plückthun A. GroE prevents the accumulation of early folding intermediates of pre-beta-lactamase without changing the folding pathway. Biochemistry. 1992 Mar 31;31(12):3249–3255. doi: 10.1021/bi00127a029. [DOI] [PubMed] [Google Scholar]


