Abstract
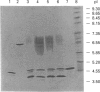
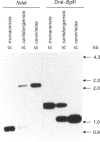
Acyl-CoA-binding protein (ACBP) is a 10 kDa protein characterized in vertebrates. We have isolated two ACBP homologues from the yeast Saccharomyces carlsbergensis, named yeast ACBP types 1 and 2. Both proteins contain 86 amino acid residues and are identical except for four conservative substitutions. In comparison with human ACBP, yeast ACBPs exhibit 48% (type 1) and 49% (type 2) conservation of amino acid residues. The amino acid sequence of S. carlsbergensis ACBP type 1 was found to be identical with the one ACBP present in Saccharomyces cerevisiae. A recombinant form of this protein was expressed in Escherichia coli and S. cerevisiae, purified, and its acyl-CoA-binding properties were characterized by isoelectric focusing and microcalorimetric analyses. The yeast ACBP was found to bind acyl-CoA esters with high affinity (Kd 0.55 x 10(-10) M). Overexpression of yeast ACBP in S. cerevisiae resulted in a significant expansion of the intracellular acyl-CoA pool. Finally, Southern-blotting analysis of the two genes encoding ACBP types 1 and 2 in S. carlsbergensis strongly indicated that this species is a hybrid between S. cerevisiae and Saccharomyces monacensis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besman M. J., Yanagibashi K., Lee T. D., Kawamura M., Hall P. F., Shively J. E. Identification of des-(Gly-Ile)-endozepine as an effector of corticotropin-dependent adrenal steroidogenesis: stimulation of cholesterol delivery is mediated by the peripheral benzodiazepine receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4897–4901. doi: 10.1073/pnas.86.13.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfman M., Morales M. N., Orellana A. Diacylglycerol activation of protein kinase C is modulated by long-chain acyl-CoA. Biochem Biophys Res Commun. 1988 May 16;152(3):987–992. doi: 10.1016/s0006-291x(88)80381-4. [DOI] [PubMed] [Google Scholar]
- Casey G. P., Pedersen M. B. DNA sequence polymorphisms in the genus Saccharomyces. V. Cloning and characterization of a LEU2 gene from S. carlsbergensis. Carlsberg Res Commun. 1988;53(3):209–219. doi: 10.1007/BF02904408. [DOI] [PubMed] [Google Scholar]
- Casey P. G proteins. Visual differences. Nature. 1992 Oct 22;359(6397):671–672. doi: 10.1038/359671a0. [DOI] [PubMed] [Google Scholar]
- Chen Z. W., Agerberth B., Gell K., Andersson M., Mutt V., Ostenson C. G., Efendić S., Barros-Söderling J., Persson B., Jörnvall H. Isolation and characterization of porcine diazepam-binding inhibitor, a polypeptide not only of cerebral occurrence but also common in intestinal tissues and with effects on regulation of insulin release. Eur J Biochem. 1988 Jun 1;174(2):239–245. doi: 10.1111/j.1432-1033.1988.tb14088.x. [DOI] [PubMed] [Google Scholar]
- Deschenes R. J., Resh M. D., Broach J. R. Acylation and prenylation of proteins. Curr Opin Cell Biol. 1990 Dec;2(6):1108–1113. doi: 10.1016/0955-0674(90)90164-a. [DOI] [PubMed] [Google Scholar]
- DiRusso C. C., Heimert T. L., Metzger A. K. Characterization of FadR, a global transcriptional regulator of fatty acid metabolism in Escherichia coli. Interaction with the fadB promoter is prevented by long chain fatty acyl coenzyme A. J Biol Chem. 1992 Apr 25;267(12):8685–8691. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Forchetti C. M., Corda M. G., Konkel D., Bennett C. D., Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hach M., Pedersen S. N., Börchers T., Højrup P., Knudsen J. Determination by photoaffinity labelling of the hydrophobic part of the binding site for acyl-CoA esters on acyl-CoA-binding protein from bovine liver. Biochem J. 1990 Oct 1;271(1):231–236. doi: 10.1042/bj2710231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., Cronan J. E., Jr A new mechanism of transcriptional regulation: release of an activator triggered by small molecule binding. Cell. 1992 Aug 21;70(4):671–679. doi: 10.1016/0092-8674(92)90435-f. [DOI] [PubMed] [Google Scholar]
- Knudsen J., Nielsen M. Diazepam-binding inhibitor: a neuropeptide and/or an acyl-CoA ester binding protein? Biochem J. 1990 Feb 1;265(3):927–929. doi: 10.1042/bj2650927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokame K., Fukada Y., Yoshizawa T., Takao T., Shimonishi Y. Lipid modification at the N terminus of photoreceptor G-protein alpha-subunit. Nature. 1992 Oct 22;359(6397):749–752. doi: 10.1038/359749a0. [DOI] [PubMed] [Google Scholar]
- Kragelund B. B., Andersen K. V., Madsen J. C., Knudsen J., Poulsen F. M. Three-dimensional structure of the complex between acyl-coenzyme A binding protein and palmitoyl-coenzyme A. J Mol Biol. 1993 Apr 20;230(4):1260–1277. doi: 10.1006/jmbi.1993.1240. [DOI] [PubMed] [Google Scholar]
- Li Q. L., Yamamoto N., Inoue A., Morisawa S. Fatty acyl-CoAs are potent inhibitors of the nuclear thyroid hormone receptor in vitro. J Biochem. 1990 May;107(5):699–702. doi: 10.1093/oxfordjournals.jbchem.a123111. [DOI] [PubMed] [Google Scholar]
- Li Q., Yamamoto N., Morisawa S., Inoue A. Fatty acyl-CoA binding activity of the nuclear thyroid hormone receptor. J Cell Biochem. 1993 Apr;51(4):458–464. doi: 10.1002/jcb.2400510411. [DOI] [PubMed] [Google Scholar]
- Mandrup S., Andreasen P. H., Knudsen J., Kristiansen K. Genome organization and expression of the rat ACBP gene family. Mol Cell Biochem. 1993 Jun 9;123(1-2):55–61. doi: 10.1007/BF01076475. [DOI] [PubMed] [Google Scholar]
- Mandrup S., Hummel R., Ravn S., Jensen G., Andreasen P. H., Gregersen N., Knudsen J., Kristiansen K. Acyl-CoA-binding protein/diazepam-binding inhibitor gene and pseudogenes. A typical housekeeping gene family. J Mol Biol. 1992 Dec 5;228(3):1011–1022. doi: 10.1016/0022-2836(92)90888-q. [DOI] [PubMed] [Google Scholar]
- Mandrup S., Højrup P., Kristiansen K., Knudsen J. Gene synthesis, expression in Escherichia coli, purification and characterization of the recombinant bovine acyl-CoA-binding protein. Biochem J. 1991 Jun 15;276(Pt 3):817–823. doi: 10.1042/bj2760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup S., Jepsen R., Skøtt H., Rosendal J., Højrup P., Kristiansen K., Knudsen J. Effect of heterologous expression of acyl-CoA-binding protein on acyl-CoA level and composition in yeast. Biochem J. 1993 Mar 1;290(Pt 2):369–374. doi: 10.1042/bj2900369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen I. B., Schulenberg H., Hansen H. O., Spener F., Knudsen J. A novel acyl-CoA-binding protein from bovine liver. Effect on fatty acid synthesis. Biochem J. 1987 Jan 1;241(1):189–192. doi: 10.1042/bj2410189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J. T., Börchers T., Knudsen J. Comparison of the binding affinities of acyl-CoA-binding protein and fatty-acid-binding protein for long-chain acyl-CoA esters. Biochem J. 1990 Feb 1;265(3):849–855. doi: 10.1042/bj2650849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J. T., Faergeman N. J., Kristiansen K., Knudsen J. Acyl-CoA-binding protein (ACBP) can mediate intermembrane acyl-CoA transport and donate acyl-CoA for beta-oxidation and glycerolipid synthesis. Biochem J. 1994 Apr 1;299(Pt 1):165–170. doi: 10.1042/bj2990165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J. T., Rosendal J., Knudsen J. Interaction of acyl-CoA binding protein (ACBP) on processes for which acyl-CoA is a substrate, product or inhibitor. Biochem J. 1993 Jun 15;292(Pt 3):907–913. doi: 10.1042/bj2920907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocque W. J., McWherter C. A., Wood D. C., Gordon J. I. A comparative analysis of the kinetic mechanism and peptide substrate specificity of human and Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase. J Biol Chem. 1993 May 15;268(14):9964–9971. [PubMed] [Google Scholar]
- Rose T. M., Schultz E. R., Todaro G. J. Molecular cloning of the gene for the yeast homolog (ACB) of diazepam binding inhibitor/endozepine/acyl-CoA-binding protein. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11287–11291. doi: 10.1073/pnas.89.23.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal J., Ertbjerg P., Knudsen J. Characterization of ligand binding to acyl-CoA-binding protein. Biochem J. 1993 Mar 1;290(Pt 2):321–326. doi: 10.1042/bj2900321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S. Fatty acylation of proteins. Annu Rev Cell Biol. 1988;4:611–647. doi: 10.1146/annurev.cb.04.110188.003143. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Gentry L. E., Marquardt H., Todaro G. J. Isolation and characterization of a putative endogenous benzodiazepineoid (endozepine) from bovine and human brain. J Biol Chem. 1986 Sep 15;261(26):11968–11973. [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Yanagibashi K., Ohno Y., Kawamura M., Hall P. F. The regulation of intracellular transport of cholesterol in bovine adrenal cells: purification of a novel protein. Endocrinology. 1988 Oct;123(4):2075–2082. doi: 10.1210/endo-123-4-2075. [DOI] [PubMed] [Google Scholar]