Abstract
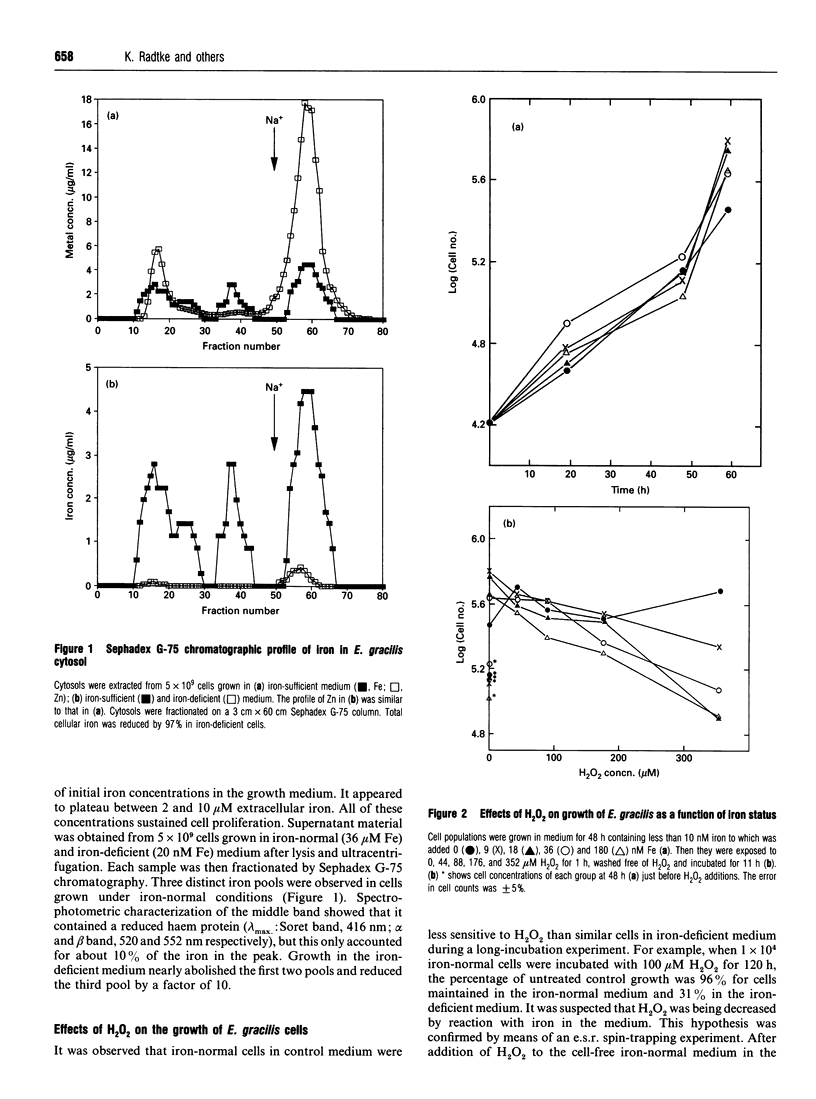
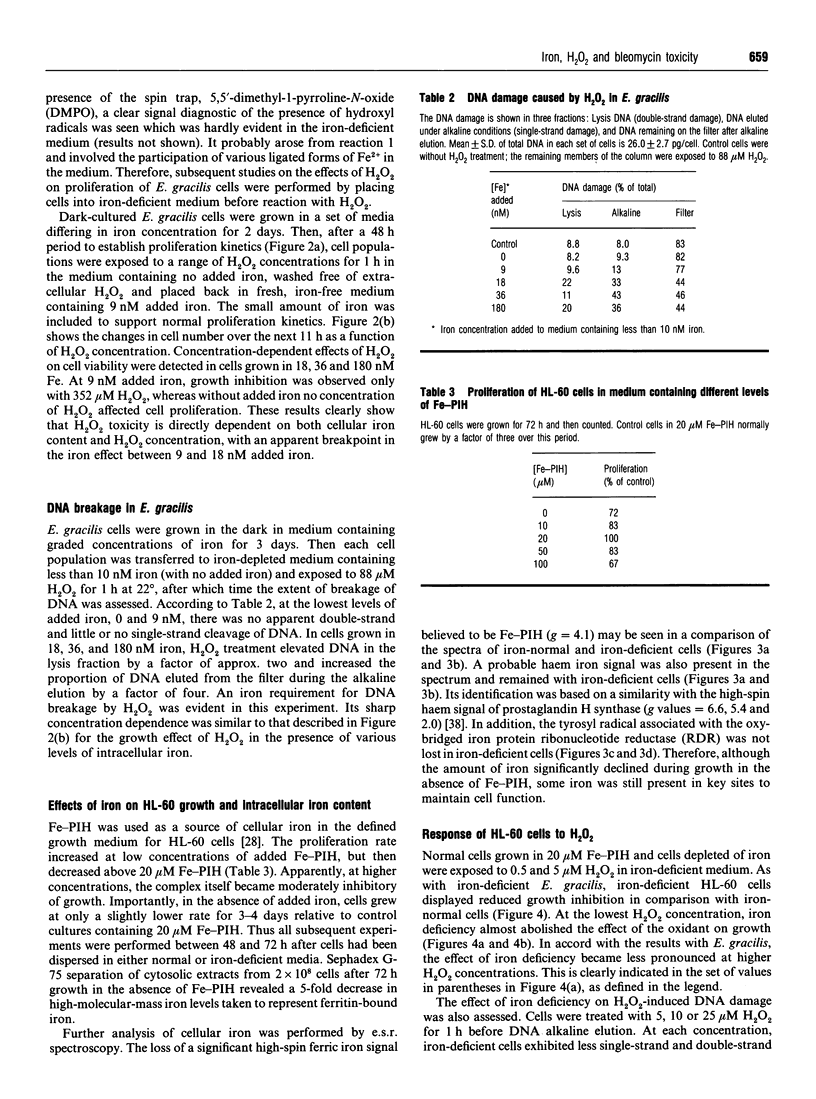
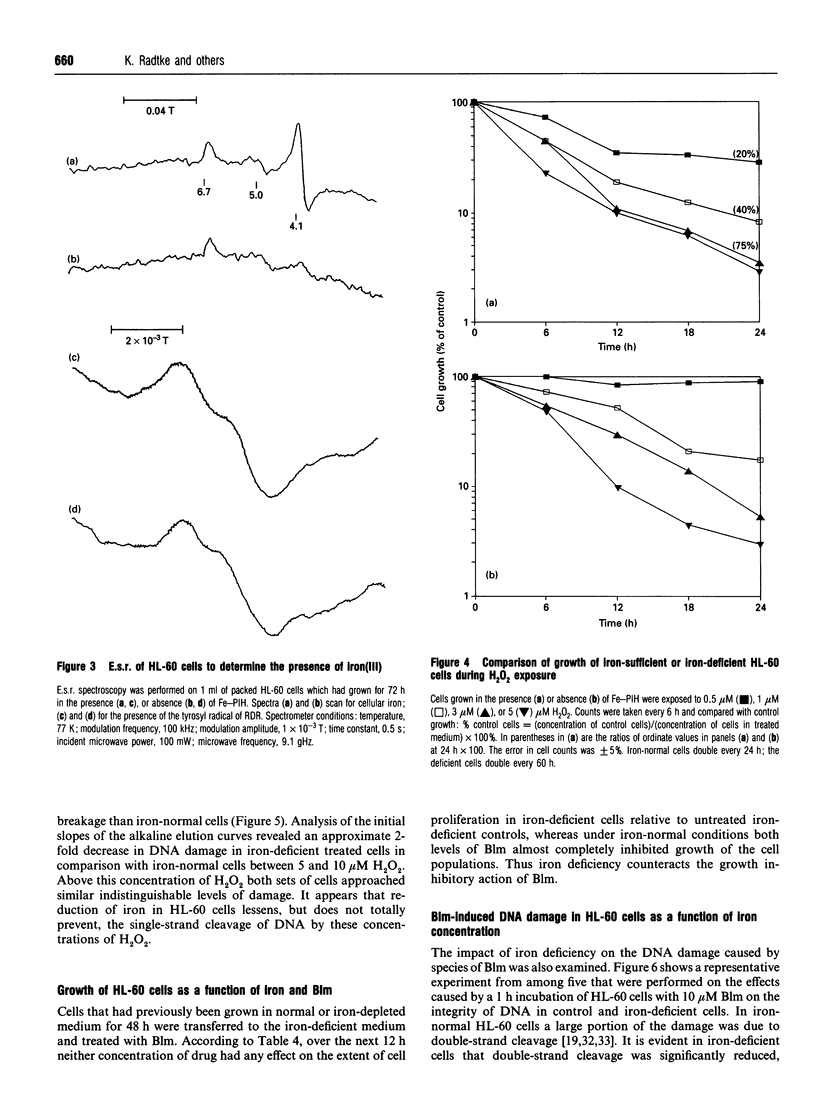
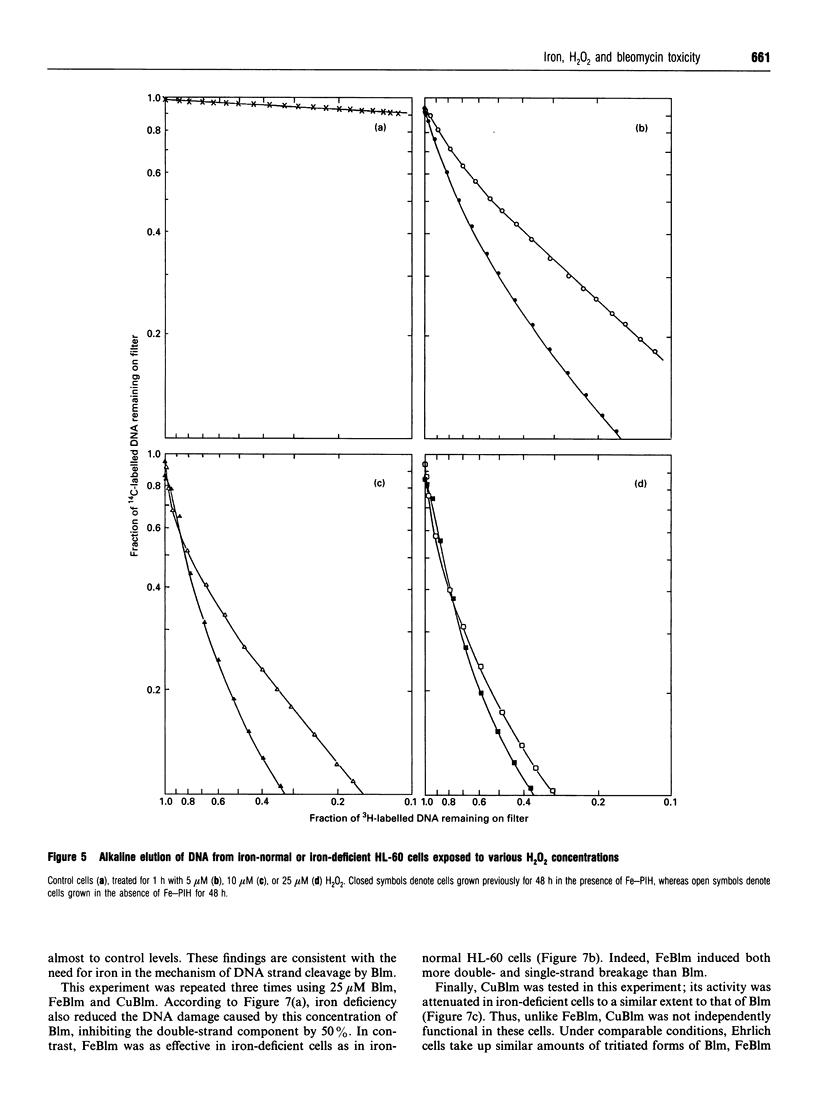
Studies with Euglena gracilis and HL-60 cells have assessed the need for intracellular iron in the mechanisms of inhibition of cell growth and DNA damage by H2O2 and bleomycin. Cell culture media were directly depleted of iron in order to deprive cells of nutrient iron. Major pools of cellular iron were reduced in both cell types. Nevertheless, iron bound in e.s.r.-observable haem protein and ribonucleotide diphosphate reductase in HL-60 cells was not decreased. In both control cell populations, there was a concentration-dependent reduction in proliferation and cell survival caused by H2O2. In comparison, the proliferation rates of both iron-deficient cell types were significantly less sensitive to H2O2. H2O2 caused concentration-dependent single-strand breakage in DNA in control HL-60 and Euglena gracilis cells. Iron deficiency reduced the amount of strand breaks in HL-60 cells at each concentration of H2O2 used. Single-strand breakage caused by H2O2 in Euglena gracilis was a direct function of the concentration of iron in which the cells had been grown. Growth inhibition and both single- and double-strand DNA damage caused by bleomycin were substantially reduced or eliminated in iron-deficient cells. Copper bleomycin behaved like metal-free bleomycin when assayed for the capacity to cause DNA damage in iron-normal and iron-deficient HL-60 cells. In contrast, iron bleomycin was equally active under the two conditions in these cells.
Full text
PDF


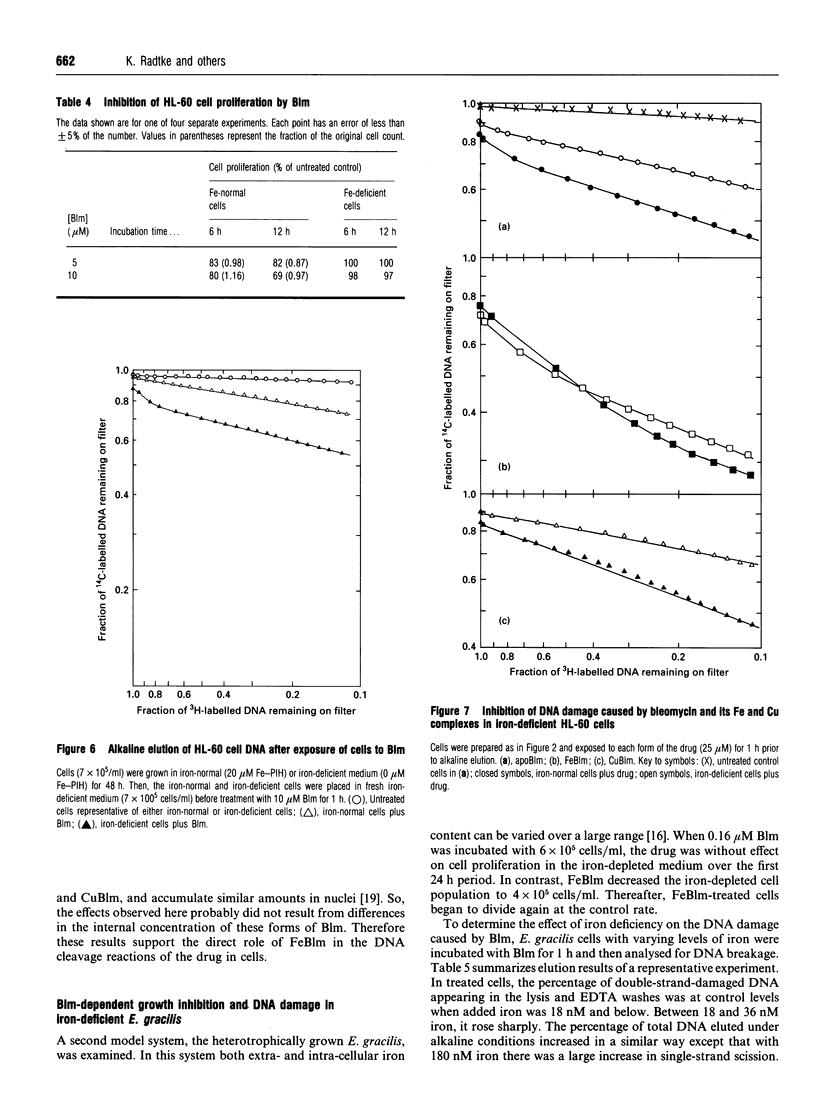
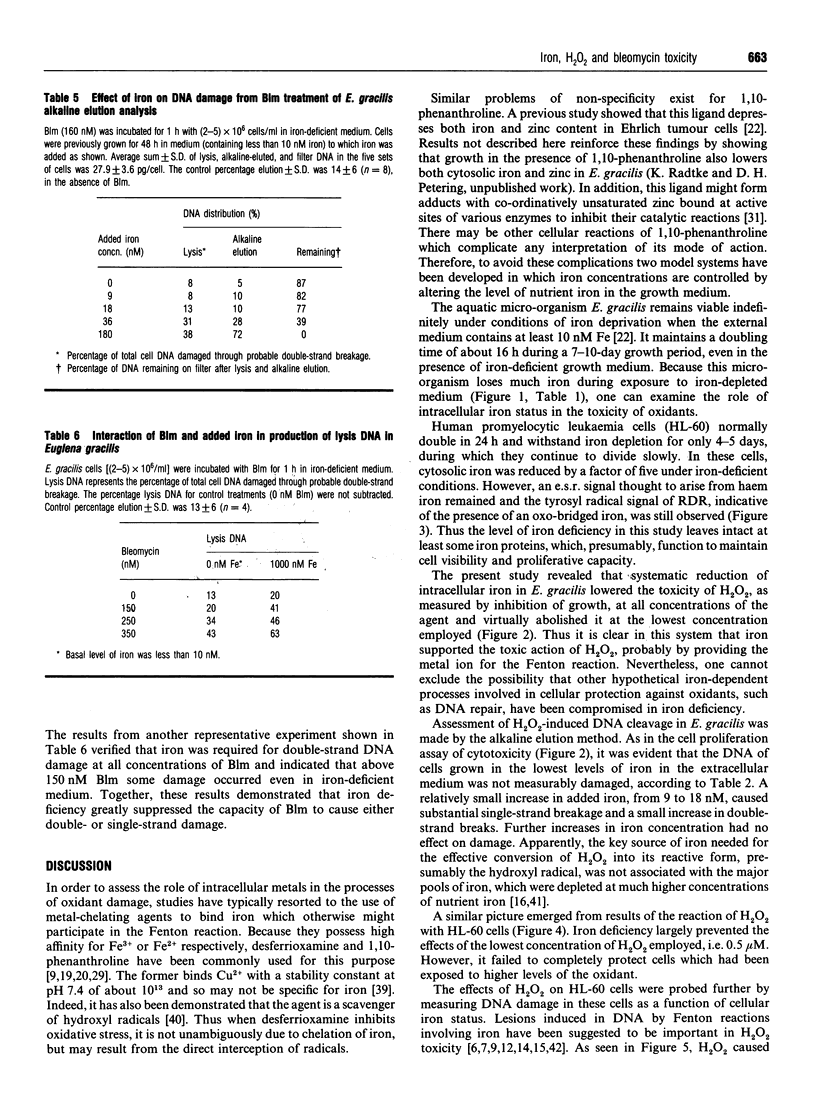

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthaswamy H. N., Eisenstark A. Repair of hydrogen peroxide-induced single-strand breaks in Escherichia coli deoxyribonucleic acid. J Bacteriol. 1977 Apr;130(1):187–191. doi: 10.1128/jb.130.1.187-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antholine W. E., Solaiman D., Saryan L. A., Petering D. H. Studies on the chemical reactivity of copper bleomycin. J Inorg Biochem. 1982 Oct;17(2):75–94. doi: 10.1016/s0162-0134(00)80077-x. [DOI] [PubMed] [Google Scholar]
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Kanabus-Kaminska M. The production of DNA strand breaks in human leukocytes by superoxide anion may involve a metabolic process. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6820–6824. doi: 10.1073/pnas.82.20.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O., Erickson L. C. Comparison of the effects of hydrogen peroxide and x-ray irradiation on toxicity, mutation, and DNA damage/repair in mammalian cells (V-79). Biochim Biophys Acta. 1981 Jun 26;654(1):135–141. doi: 10.1016/0005-2787(81)90146-5. [DOI] [PubMed] [Google Scholar]
- Burger R. M., Peisach J., Horwitz S. B. Activated bleomycin. A transient complex of drug, iron, and oxygen that degrades DNA. J Biol Chem. 1981 Nov 25;256(22):11636–11644. [PubMed] [Google Scholar]
- Byrnes R. W., Mohan M., Antholine W. E., Xu R. X., Petering D. H. Oxidative stress induced by a copper-thiosemicarbazone complex. Biochemistry. 1990 Jul 31;29(30):7046–7053. doi: 10.1021/bi00482a014. [DOI] [PubMed] [Google Scholar]
- Byrnes R. W., Petering D. H. DNA double-strand breakage by bleomycin in Ehrlich ascites tumor cells as measured by nondenaturing filter elution. Radiat Res. 1994 Feb;137(2):162–170. [PubMed] [Google Scholar]
- Byrnes R. W., Petering D. H. Inhibition of bleomycin-induced cellular DNA strand scission by 1,10-phenanthroline. Biochem Pharmacol. 1991 Apr 15;41(8):1241–1248. doi: 10.1016/0006-2952(91)90664-q. [DOI] [PubMed] [Google Scholar]
- Byrnes R. W., Petering D. H. Repair of bleomycin-induced DNA double-strand breakage in Ehrlich ascites tumor cells. Radiat Res. 1993 Jun;134(3):343–348. [PubMed] [Google Scholar]
- Byrnes R. W., Templin J., Sem D., Lyman S., Petering D. H. Intracellular DNA strand scission and growth inhibition of Ehrlich ascites tumor cells by bleomycins. Cancer Res. 1990 Sep 1;50(17):5275–5286. [PubMed] [Google Scholar]
- Chitambar C. R., Zivkovic Z. Inhibition of hemoglobin production by transferrin-gallium. Blood. 1987 Jan;69(1):144–149. [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- DiGuiseppi J., Fridovich I. The toxicology of molecular oxygen. Crit Rev Toxicol. 1984;12(4):315–342. doi: 10.3109/10408448409044213. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld G. M., Shipley J. B., Heimbrook D. C., Sugiyama H., Long E. C., van Boom J. H., van der Marel G. A., Oppenheimer N. J., Hecht S. M. Copper-dependent cleavage of DNA by bleomycin. Biochemistry. 1987 Feb 10;26(3):931–942. doi: 10.1021/bi00377a038. [DOI] [PubMed] [Google Scholar]
- Girotti A. W. Mechanisms of lipid peroxidation. J Free Radic Biol Med. 1985;1(2):87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Use of desferrioxamine as a 'probe' for iron-dependent formation of hydroxyl radicals. Evidence for a direct reaction between desferal and the superoxide radical. Biochem Pharmacol. 1985 Jan 15;34(2):229–233. doi: 10.1016/0006-2952(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. E., Meneghini R. Action of hydrogen peroxide on human fibroblast in culture. Photochem Photobiol. 1979 Jul;30(1):151–155. doi: 10.1111/j.1751-1097.1979.tb07128.x. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Iqbal Z. M., Kohn K. W., Ewig R. A., Fornace A. J., Jr Single-strand scission and repair of DNA in mammalian cells by bleomycin. Cancer Res. 1976 Oct;36(10):3834–3838. [PubMed] [Google Scholar]
- Karthein R., Nastainczyk W., Ruf H. H. EPR study of ferric native prostaglandin H synthase and its ferrous NO derivative. Eur J Biochem. 1987 Jul 1;166(1):173–180. doi: 10.1111/j.1432-1033.1987.tb13499.x. [DOI] [PubMed] [Google Scholar]
- Krishnamurti C., Saryan L. A., Petering D. H. Effects of ethylenediaminetetraacetic acid and 1,10-phenanthroline on cell proliferation and DNA synthesis of Ehrlich ascites cells. Cancer Res. 1980 Nov;40(11):4092–4099. [PubMed] [Google Scholar]
- Lewis J. G., Adams D. O. Induction of 5,6-ring-saturated thymine bases in NIH-3T3 cells by phorbol ester-stimulated macrophages: role of reactive oxygen intermediates. Cancer Res. 1985 Mar;45(3):1270–1275. [PubMed] [Google Scholar]
- Lyman S., Taylor P., Lornitzo F., Wier A., Stone D., Antholine W. E., Petering D. H. Activity of bleomycin in iron- and copper-deficient cells. Biochem Pharmacol. 1989 Dec 1;38(23):4273–4282. doi: 10.1016/0006-2952(89)90526-1. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Hoffmann M. E., Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D., vanAnkeren S. C., Meyn R. E. Applicability of the alkaline elution procedure as modified for the measurement of DNA damage and its repair in nonradioactively labeled cells. Anal Biochem. 1987 Jan;160(1):149–159. doi: 10.1016/0003-2697(87)90625-7. [DOI] [PubMed] [Google Scholar]
- Price C. A., Vallee B. L. Euglena gracilis, A Test Organism for Study of Zinc. Plant Physiol. 1962 May;37(3):428–433. doi: 10.1104/pp.37.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prise K. M., Davies S., Michael B. D. Cell killing and DNA damage in Chinese hamster V79 cells treated with hydrogen peroxide. Int J Radiat Biol. 1989 Apr;55(4):583–592. doi: 10.1080/09553008914550631. [DOI] [PubMed] [Google Scholar]
- Rao E. A., Saryan L. A., Antholine W. E., Petering D. H. Cytotoxic and antitumor properties of bleomycin and several of its metal complexes. J Med Chem. 1980 Dec;23(12):1310–1318. doi: 10.1021/jm00186a006. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Rubin R., Farber J. L. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984 Feb 1;228(2):450–459. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Sahlin M., Petersson L., Gräslund A., Ehrenberg A., Sjöberg B. M., Thelander L. Magnetic interaction between the tyrosyl free radical and the antiferromagnetically coupled iron center in ribonucleotide reductase. Biochemistry. 1987 Aug 25;26(17):5541–5548. doi: 10.1021/bi00391a049. [DOI] [PubMed] [Google Scholar]
- Sausville E. A., Peisach J., Horwitz S. B. Effect of chelating agents and metal ions on the degradation of DNA by bleomycin. Biochemistry. 1978 Jul 11;17(14):2740–2746. doi: 10.1021/bi00607a007. [DOI] [PubMed] [Google Scholar]
- Sausville E. A., Stein R. W., Peisach J., Horwitz S. B. Properties and products of the degradation of DNA by bleomycin and iron(II). Biochemistry. 1978 Jul 11;17(14):2746–2754. doi: 10.1021/bi00607a008. [DOI] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Hinshaw D. B., Spragg R. G., Sklar L. A., Cochrane C. G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Kuwahara J., Sugiura Y. Copper-bleomycin has no significant DNA cleavage activity. Biochemistry. 1985 Aug 27;24(18):4719–4721. doi: 10.1021/bi00339a001. [DOI] [PubMed] [Google Scholar]


