Abstract
Objective
Determine the efficacy, effectiveness, and safety of fluoroscopically- or ultrasound-guided caudal epidural steroid injections (ESIs) with or without catheter placement for the treatment of chronic low back (CLBP), radicular pain, and/or chronic post-surgical back pain (CPSBP).
Design
Systematic review.
Population
Adults ≥18 years with CLBP, radicular pain, or CPSBP ≥3 months.
Intervention
Fluoroscopically- or ultrasound-guided caudal ESI with or without a catheter including epidural neuroplasty.
Comparison
Sham, placebo procedure, active standard care treatment, or none.
Outcomes
The primary outcome was the proportion of individuals with reduction of pain by ≥ 50%. Secondary outcomes included functional improvement, analgesic use, subsequent spinal surgery, healthcare utilization, and mean improvement in pain. Reported adverse events were also cataloged.
Methods
Four reviewers independently assessed publications before January 2, 2022 in PubMed, Ovid MEDLINE, and Scopus. Quality of evidence was evaluated using the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) framework.
Results
Of 364 records screened, 23 publications met inclusion criteria. The success rates for the primary outcome could only be extrapolated from one study. Another study used a composite improvement scale that included pain and functional outcomes. The reported success rates in these two studies ranged from 40 to 58% at three months, 25%–67% at six months, and 58%–61% at one year. Data on secondary outcomes were limited; however, rates of functional improvement as measured by mean improvement in Oswestry Disability Index (ODI) ranged from 2% to 55%.
Conclusion
There is moderate-quality evidence that caudal ESIs using an in-dwelling catheter for two days is an effective treatment for pain and dysfunction associated with disc herniation with radicular pain and for CPSBP at three, six, and 12 months. There is low-quality evidence supporting the effectiveness of other caudal ESI techniques for pain and dysfunction associated with central lumbar spinal stenosis with neurogenic claudication, discogenic CLBP, and CLBP without disc herniation or radiculitis.
Keywords: Low back pain, Radiculopathy, Chronic post-surgical back pain, Lumbar spinal stenosis, Steroids, Corticosteroids, Analgesics, Catheter, Indwelling, Caudal
1. Introduction
Epidural steroid injections (ESIs) are commonly utilized to treat radicular pain syndromes throughout the spine. They have been used to treat lumbar radiculopathy since 1953 [1]. Additionally, they have been used in the treatment of spinal stenosis with neurogenic claudication or other neural involvement and axial chronic low back pain (CLBP), including chronic post-surgical back pain (CPSBP) [[2], [3], [4], [5]]. Various methods allow for access of the epidural space. Depending on the spinal segmental origin of the pain, epidural access routes include interlaminar, transforaminal, and caudal approaches, with the last method used almost solely for lumbosacral pain [2,[6], [7], [8]]. The present systematic review focuses on the caudal route of epidural access and steroid administration with or without other adjunctive medications.
The caudal approach for ESI is often chosen due to perceived safety features associated with this route of administration. In addition, the point of entry to the caudal epidural space is almost always left intact following spine surgeries, which is often not the case with interlaminar access following posterior spinal decompression. Surgical hardware may also obscure transforaminal access in some individuals. The point of entry to the caudal epidural space is located below the vertebrae that are commonly decompressed and fused surgically, thereby reducing the incidence of dural puncture during epidural injection [6,9]. However, since this point of entry is distant from affected lumbar nerve roots and discs, interventionalists rely on higher volumes of injectate [6,7] or catheter use to ensure medication delivery to the targeted level(s) [10].
The caudal epidural approach also allows the safe use of particulate steroid, as this region of access contains venous plexus but no radiculomedullary arteries, similar to access of the interlaminar space, and in contrast to transforaminal access [11]. While the published evidence is mixed, use of particulate steroid for the treatment of radicular pain syndromes has been associated with a more durable treatment response in some studies [[12], [13], [14]]. As such, some physicians perceive an advantage of the caudal epidural approach with deposition of particulate steroid compared to transforaminal ESI with non-particulate steroid when an interlaminar route of access is not possible due to scarring of or obstruction of the dorsal epidural space in post-surgical patients.
In some cases, a catheter-based access method is used to deposit a low volume of epidural injectate with a relatively high concentration of steroid at the target level where lumbosacral pathology is present (compared to the high-volume injection needed to reach various lumbar levels using a caudal approach without a catheter) [15]. Typically, a “soft” epidural catheter is used with this method. In other cases, the catheter-based access method is paired with a technique initially described as a lysis of adhesions (LOA), adhesiolysis, or percutaneous epidural neuroplasty [16]. In this technique, the caudal epidural space is accessed via a percutaneous approach, and a stiff catheter is advanced to the targeted level(s) of disc herniation or scar tissue encroaching upon the affected nerve root(s) [16]. Typically, local anesthetic, steroid, and a substance used to “lyse” the adhesions, such as a 10% hypertonic saline or hyaluronidase, is injected through the catheter [17]. In addition to the injectate that provides chemical or enzymatic LOA, a balloon or other device may be used to provide mechanical LOA [18]. The catheter may be left in place for several days to allow repeated administration of medications (without steroid) prior to removal [16,17], though many studies also describe single or multiple injections in the same day followed by immediate removal of the catheter [[19], [20], [21], [22], [23], [24]]. In this systematic review, all procedures in which steroids were injected via a caudal approach were included as our primary inclusion criterion to include single shot ESIs, ESIs with a catheter, and LOA procedures. For clarity, procedures involving use of a catheter where the intention was to chemically or mechanically “lyse” adhesions (LOA) or resolve a filling defect are hereafter termed “neuroplasty”.
A large volume of literature on caudal ESI and epidural neuroplasty has been published by a single common primary author [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]], including several systematic reviews [[37], [38], [39]]. Regarding caudal ESI, evidence in one review was rated as “good” for disc herniation or radiculitis and “fair” for axial or discogenic pain without disc herniation, radiculitis, or facet joint pain; spinal stenosis; and CPSBP. Lumbar percutaneous adhesiolysis (neuroplasty) was rated at a “fair” level of evidence for CLBP and lower extremity pain secondary to post-surgery syndrome and spinal stenosis [38]. However, there is significant concern for bias regarding the data and conclusions in these articles, including the reporting of primarily continuous data, poor description of intention-to-treat analysis, the authors' methods of addressing missing data, absent sensitivity analyses, and significant references to the authors’ own work. Other reviewers noted similar concerns, stating “there is weak positive evidence that LOA is more effective for CPSBP and spinal stenosis, and that LOA is more effective than sham adhesiolysis and conservative management for lumbosacral radiculopathy” [40].
Until more conclusive studies are published, this systematic review was constructed to evaluate the quality of the existing evidence regarding the efficacy, effectiveness, and safety of caudal access and steroid administration in treating CLBP, radicular pain, and CPSBP. This systematic review was performed with the knowledge that studies would likely be found that utilized steroids in the caudal space but relied upon other injectates, catheter placement, etc. as the primary intervention being studied. For the purpose of this review, prior to evaluating the published literature, the authors elected to analyze any study that included steroid as an injectate in the caudal space, regardless of whether the steroid was the intended primary treatment of the original researchers.
1.1. Objectives and rationale
The objective of this systematic review was to identify and evaluate the quality of studies regarding the effectiveness and adverse events associated with procedures that introduce steroids into the caudal space.
2. Methods
2.1. Protocol and registration
This IRB-exempt study was registered on PROSPERO (ID: CRD42020192039, June 2020).
2.2. Eligibility criteria
2.2.1. Population
Adults 18 years or older with CLBP, radicular pain, or CPSBP (often also referred to as “failed back surgery syndrome”) with symptoms for greater than 3 months. This criterion was changed from “symptoms for greater than 6 months” after the review was registered on PROSPERO during the literature search period based upon consensus from the research team. The intent was to capture the majority of the published literature, for which inclusion criteria more frequently required duration of symptoms of 3 months. Studies that did not report duration of pain or that reported duration of pain less than 3 months were omitted from the analysis.
2.2.2. Intervention
Fluoroscopically- or ultrasound-guided caudal ESI with or without a catheter. For clarity, procedures involving use of catheter where the intention was to mechanically or chemically “lyse” adhesions (LOA) or resolve a filling defect are hereafter termed “neuroplasty”.
2.2.3. Comparison
Sham, placebo procedure, active standard care treatment, or none.
2.2.4. Outcome
The primary outcome of interest was the proportion of individuals with ≥50% pain reduction as measured by a validated scale such as the visual analog scale (VAS) or numeric rating scale (NRS). Secondary outcomes included the proportion of individuals with ≥50% functional improvement compared to baseline as measured by the Oswestry Disability Index (ODI), mean changes in ODI and NRS/VAS scores, analgesic medication use, patient satisfaction, subsequent lumbosacral spinal surgery, and utilization of healthcare related to the index symptoms of interest. No restrictions were placed on the number of procedures performed. No restrictions were imposed regarding a minimum duration of outcome assessment. Outcomes were analyzed at two timeframes: between three and six months post-procedure and greater than six months post-procedure.
2.2.5. Studies
The present study included randomized controlled trials (RCTs), non-randomized comparative studies, and single group observational studies. Case reports, expert opinion, and non-English language manuscripts were excluded. No publication date restrictions were applied. For the purpose of this review, the authors analyzed any study that included steroid as an injectate in the caudal space, regardless of whether the steroid was the intended primary treatment of the original researchers.
2.3. Information sources and search
Clinical outcome studies on the effectiveness of caudal ESI for the treatment of CLBP or radicular pain were obtained by searching PubMed, Ovid MEDLINE, and Scopus, using the search terms “radiculopathy,” “failed back,” “chronic low back pain,” “disc degeneration,” “caudal,” “catheter,” and “adhesiolysis.” The searches were performed on February 1, 2022. Literature was also identified from the cited references of retrieved publications.
2.4. Study selection
Three authors (AN, BB, TV) independently assessed a subset of abstracts according to screening criteria. Two reviewers (BB, TV) assessed each abstract for eligibility. Discrepancies were resolved by a third reviewer (AN) not originally assigned to review those specific studies to reach a final decision regarding study inclusion. Subsequently, the full publications were independently reviewed by at least two authors (BB, TV) and assessed for inclusion. Discrepancies were again resolved by a third reviewer (AN).
Studies were included if they met the following criteria: 1) presentation of clinically relevant data on efficacy or effectiveness of caudal ESI for the treatment of CLBP or radicular pain and 2) presentation of valid information based on appropriate procedural technique, study methodology, and data analyses according to principles of EBM [41] or the ability to perform appropriate data analysis from raw published data.
2.5. Data items and collection
The following information was extracted from each study: 1) bibliographic details including year of publication and authors; 2) study design; 3) participant selection criteria; 4) technical description of the procedure; and 5) any relevant author disclosures or study funding. Additionally, outcome measures were recorded for pain reduction, functional improvement, patient satisfaction, analgesic medication use, lumbosacral spine surgery, healthcare resource utilization, and adverse events/complications.
2.6. Risk of bias and methodologic assessment
All reviewers have successfully achieved accreditation in the Spine Intervention Society's (SIS) Accreditation in Evidence-based Medicine Training Program. The program consists of two courses – Accreditation Course in Assessing Studies of Treatment (EBM I) and Accreditation Course in Assessing Studies of Diagnostic Tests and Strategies (EBM II). Each of these courses consist of 3–5 h of didactic presentations and require participation in a 2-h, small group discussion session with SIS EBM faculty. In order to attain accreditation, participants must pass all quizzes (six for EBM I and three for EBM 2), participate in the 2-h discussion sessions, and demonstrate competency on exams demonstrating understanding of statistical concepts and calculations and the ability to rigorously critique studies.
Reviewers evaluated all studies for various methodologic qualities known to impact study quality [41]. These considerations included a number of factors including; 1) selection of a patient sample representative of a realistic clinical population; 2) use of validated outcome measures; 3) <20% loss to follow-up (censoring); 4) use of controls for co-interventions; 5) authors’ conflicts of interest; and 6) the validity of diagnostic criteria and assessment tools.
The body of evidence was evaluated using the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) system of appraisal to determine the quality of the evidence of the effectiveness of caudal ESI [42]. In the GRADE system, reviewers analyze the body of evidence pertaining to an intervention in domains including imprecision, risk of bias, inconsistency, indirectness, and publication bias. GRADE gives an initial quality rating based upon the existing evidence and allows for upgrading or downgrading of the overall evidence quality rating (e.g., upgrading for large magnitude of effect, or downgrading for risk of bias). Disagreements regarding GRADE evaluation were resolved by consensus decision among the reviewers.
2.7. Summary measures and synthesis of results
The primary outcome of interest was the proportion of “responders”, defined by ≥ 50% pain reduction from baseline at three months. Secondary outcomes included the proportion of individuals with ≥50% functional improvement compared to baseline as measured by the Oswestry Disability Index (ODI), mean changes in ODI and NRS/VAS scores, analgesic medication use, subsequent lumbosacral spinal surgery, and utilization of healthcare related to the index symptoms of interest. The GRADE system was applied to assess the quality of evidence related to the effectiveness of caudal ESI [43]. A meta-analysis was not performed due to significant heterogeneity of the study designs, populations studied, and primary and secondary outcomes.
3. Results
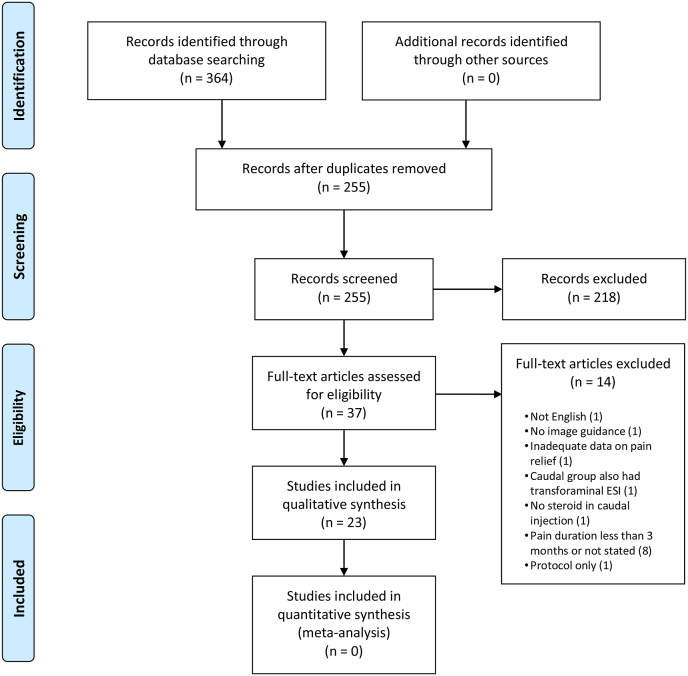
A total of 364 records were identified from the initial search. After abstract screening for relevant publications and removal of duplicates, 37 full text articles were assessed for eligibility and 23 were ultimately included (Fig. 1). Studies that met the inclusion criteria consisted of two explanatory trials, 20 pragmatic trials, and one retrospective comparison of two pragmatic RCTs. Results were organized by study design and characteristics of individual studies and are summarized in Table 1. Categorical outcomes are reported in Table 2. Continuous outcomes for improvement in pain are reported in Table 3, and continuous outcomes for secondary outcomes are reported in Table 4. Adverse events are listed in Table 5. Because none of the included studies reported data on subsequent lumbosacral spinal surgery or utilization of healthcare related to the index symptoms of interest, these secondary outcomes are not represented in the tables.
Fig. 1.
Prisma 2009 flow diagram.
Table 1.
Study characteristics.
| Study | Study Design | Diagnosis/Question Addressed | Injectate/Catheter Duration | Follow-Up Interval |
|---|---|---|---|---|
| Akbas 2018 [46] | Pragmatic RCT | CPSBP To compare 3 adhesiolysis∗ approaches (caudal, S1 foraminal, L5-S1 transforaminal) |
Caudal: •catheter (procedure only) •1500U hyaluronidase •10 mL 1% lidocaine •80 mg methylprednisolone |
1 month 3 months 6 months |
| Chun-jing 2012 [47] | Pragmatic RCT | CPSBP To compare adhesiolysis vs caudal ESI |
Adhesiolysis: •catheter (procedure only) •50–80 mL saline •10 mg dexamethasone Caudal ESI only: •no catheter •10 mg dexamethasone |
7 days 1 month 6 months |
| Gerdesmeyer 2013 [44] | Explanatory RCT | Radicular pain with disc herniation or CPSBP To compare neurolysis∗ vs placebo |
Neurolysis: •catheter (remained 2 days) •10 mL 0.25% bupivacaine •10 mL saline and 150U/mL hyaluronidase + each subsequent day (2 days): •10 mL saline •40 mg triamcinolone •2 mL 0.25% bupivacaine Placebo: •catheter in subcutaneous tissue •10 mL saline each day |
3 months 6 months 12 months |
| Gerdesmeyer 2021 [45] | Explanatory RCT | Radicular pain with disc herniation or CPSBP To compare neurolysis vs placebo |
Neurolysis: •catheter (remained 3 days) •10 mL 0.25% bupivacaine •10 mL saline and 150U/mL hyaluronidase + Post-neurolysis: •10 mL saline •40 mg triamcinolone •2 mL 0.25% bupivacaine + each subsequent day (2 days): •10 mL 0.25% bupivacaine •10 mL saline •2 mL 0.25% bupivacaine Placebo: •catheter in subcutaneous tissue •10 mL saline each day |
12 months 120 months |
| Karm 2018 [18] | Pragmatic RCT | Central lumbar spinal stenosis with neurogenic claudication To compare inflatable catheter (ZiNeu) to balloon-less catheter (Racz) adhesiolysis |
ZiNeu: •catheter (remained 2 days) •4 mL 1% lidocaine •1500 IU hyaluronidase •5 mg dexamethasone and 1% lidocaine •4 mL 10% hypertonic saline After 2 days: •5 mg dexamethasone •2 mL 1% lidocaine •4 mL 10% hypertonic saline Racz: •catheter (remained 2 days) •1500 IU hyaluronidase •5 mg dexamethasone and 1% lidocaine •4 mL 10% hypertonic saline After 2 days: •5 mg dexamethasone •2 mL 1% lidocaine •4 mL 10% hypertonic saline |
1 month 3 months 6 months |
| Manchikanti 2008 [25] | Pragmatic RCT | Radicular pain and disc herniation To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine •2 mL 0.9% sodium chloride solution Group 2: •9 mL 0.5% lidocaine •1 mL 6 mg betamethasone or 40 mg methylprednisolone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months |
| Manchikanti 2008 [26] | Pragmatic RCT | CLBP and radicular pain CPSBP To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine •2 mL 0.9% sodium chloride solution Group 2: •9 mL 0.5% lidocaine •1 mL 6 mg betamethasone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months |
| Manchikanti 2008 [27] | Pragmatic RCT | CLBP and radicular pain Stenosis To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine •2 mL 0.9% sodium chloride solution Group 2: •9 mL 0.5% lidocaine •1 mL 6 mg betamethasone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months |
| Manchikanti 2008 [28] | Pragmatic RCT | CLBP, discogenic pain without disc herniation or radiculitis To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine •2 mL 0.9% sodium chloride solution Group 2: •9 mL 0.5% lidocaine •1 mL 6 mg betamethasone or 40 mg methylprednisolone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months |
| Manchikanti 2009 [24] | Pragmatic RCT | CLBP and radicular pain CPSBP To evaluate caudal epidural steroid versus epidural adhesiolysis |
Caudal ESI: •catheter (procedure only) •5 mL 2% lidocaine •6 mL saline •1 mL 6 mg betamethasone •1 mL normal saline Adhesiolysis: •Racz catheter (procedure only, adhesiolysis performed) •5 mL 2% lidocaine •6 mL 10% sodium chloride •1 mL 6 mg betamethasone •1 mL normal saline |
3 months 6 months 12 months |
| Manchikanti 2009 [20] | Pragmatic RCT | CLBP and radicular pain Stenosis To evaluate caudal epidural steroid versus epidural adhesiolysis |
Caudal ESI: •catheter (procedure only) •5 mL 2% lidocaine •6 mL saline •1 mL 6 mg betamethasone •1 mL normal saline Adhesiolysis: •Racz catheter (procedure only, adhesiolysis performed) •5 mL 2% lidocaine •6 mL 10% sodium chloride •1 mL 6 mg betamethasone •1 mL normal saline |
3 months 6 months 12 months |
| Manchikanti 2010 [29] | Pragmatic RCT | CLBP and radicular pain CPSBP To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine Group 2: •9 mL 0.5% lidocaine •6 mg betamethasone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months |
| Manchikanti 2011 [30] | Pragmatic RCT | Radiculitis, disc herniation To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine •2 mL 0.9% sodium chloride solution Group 2: •9 mL 0.5% lidocaine •1 mL 6 mg betamethasone or 40 mg methylprednisolone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months |
| Manchikanti 2011 [31] | Pragmatic RCT | CLBP without herniation or radiculitis To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine •2 mL 0.9% sodium chloride solution Group 2: •9 mL 0.5% lidocaine •1 mL 6 mg betamethasone or 40 mg methylprednisolone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months |
| Manchikanti 2012 [32] | Pragmatic RCT | CLBP and radicular pain Stenosis To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine •2 mL 0.9% sodium chloride solution Group 2: •9 mL 0.5% lidocaine •1 mL 6 mg betamethasone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months 18 months 24 months |
| Manchikanti 2012 [33] | Pragmatic RCT | CLBP and radicular pain CPSBP To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine •2 mL 0.9% sodium chloride solution Group 2: •9 mL 0.5% lidocaine •1 mL 6 mg betamethasone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months 18 months 24 months |
| Manchikanti 2012 [23] | Pragmatic RCT | CLBP and radicular pain CPSBP To compare caudal epidural steroid injections with epidural adhesiolysis |
Caudal ESI: •catheter (procedure only) •5 mL 2% lidocaine •6 mL 0.9% saline •1 mL 6 mg betamethasone •1 mL sodium chloride solution •6 mL 0.9% saline Neuroplasty: •Racz catheter (procedure only, adhesiolysis performed) •5 mL 2% lidocaine •6 mL 10% sodium chloride •1 mL 6 mg betamethasone •1 mL sodium chloride solution |
3 months 6 months 12 months 18 months 24 months |
| Manchikanti 2012 [34] | Pragmatic RCT | CLBP and radicular pain Stenosis To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine •2 mL 0.9% sodium chloride solution Group 2: •9 mL 0.5% lidocaine •1 mL 6 mg betamethasone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months |
| Manchikanti 2012 [35] | Pragmatic RCT | Radiculitis, disc herniation To compare caudal epidural injections with and without steroids |
Group 1: •10 mL 0.5% lidocaine •2 mL 0.9% sodium chloride solution Group 2: •9 mL 0.5% lidocaine •1 mL 6 mg betamethasone or 40 mg methylprednisolone •2 mL 0.9% sodium chloride solution |
3 months 6 months 12 months 18 months 24 months |
| Manchikanti 2013 [19] | Pragmatic RCT | CLBP and radicular pain Stenosis To evaluate percutaneous adhesiolysis |
•Racz catheter (procedure only, adhesiolysis performed in all) •5 mL 2% lidocaine •6 mL 10% sodium chloride •1 mL 6 mg betamethasone •1 mL sodium chloride solution |
3 months 6 months 12 months 18 months 24 months |
| Manchikanti 2015 [36] | Retrospective comparison of results of 2 Pragmatic RCTs | CLBP, discogenic pain To compare caudal and lumbar interlaminar approaches of epidural injections with and without steroids |
Caudal, anesthetic only •10 mL 0.5% lidocaine Caudal, steroid •9 mL 0.5% lidocaine •1 mL “steroid” Lumbar interlaminar, anesthetic only •6 mL 0.5% lidocaine Lumbar interlaminar, steroid •5 mL 0.5% lidocaine •1 mL “steroid" |
3 months 6 months 12 months 18 months 24 months |
| Park 2013 [48] | Pragmatic RCT | Disc herniation or stenosis and radicular pain To compare ultrasound vs fluoroscopy guided caudal ESI |
Both groups: •1 mL 1% lidocaine •13 mL 0.5% lidocaine •2 mL 10 mg dexamethasone |
2 weeks 12 weeks |
| Yousef 2010 [49] | Pragmatic RCT | CPSBP To compare caudal ESI with and without hyaluronidase |
Group 1: •10 mL 0.25% bupivacaine •80 mg methylprednisolone •30 mL 3% hypertonic saline Group 2: •10 mL 0.25% bupivacaine •80 mg methylprednisolone •30 mL 3% hypertonic saline •1500 IU hyaluronidase |
6 weeks 3 months 6 months 12 months |
RCT- “randomized controlled trial”; ESI- epidural steroid injection; CPSBP- chronic post-surgical back pain; CLBP- chronic low back pain. ∗The term neuroplasty is used throughout the manuscript to describe procedures involving the use of a catheter where the intention was to chemically or mechanically “lyse” adhesions or resolve a filling defect. In this table the authors' preferred term is used.
Table 2.
Categorical data on pain and functional improvement.
| Study | Determinant of Successful Treatment - Pain | Categorical Pain Improvement at Each Interval | Determinant of Successful Treatment - Function | Categorical Functional Improvement at Each Interval |
|---|---|---|---|---|
| Gerdesmeyer 2013 [44] | ≥50% improvement VAS |
Neurolysis∗ (n = 46) 3 m: 31/46, 67% (95% CI: 54–81%) 6 m: 32/46, 70% (95%CI: 56–83%) 12 m: 29/46, 63% (95%CI: 49–72%) |
≥50% improvement ODI |
Neurolysis (n = 46) 3 m: 26/46, 57% (95%CI: 42–71%) 6 m: 31/46, 67% (95%CI: 54–81%) 12 m: 28/46, 61% (95%CI: 47–75%) |
| Gerdesmeyer 2021 [45] | ≥50% improvement VAS |
Neurolysis (n = 46) 12 m: 29/46, 63% (95%CI: 49–77%) 120 m: 25/46, 54% (95%CI: 40–69%) |
≥50% improvement ODI |
Neurolysis (n = 46) 12 m: 28/46, 61% (95%CI: 47–75%) 120 m: 25/46, 54% (95%CI: 40–69%) |
| Karm 2018 [18] | (1) ≥ 50% (or ≥ 4-point) reduction from baseline leg or lower back NRS-11, and no increase from baseline ODI and MQS, and ≥4 points on the GPES scale, or (2) ≥ 30% (or ≥ 2-point) reduction from baseline NRS with any one of the following criteria: simultaneous ≥30% (or ≥ 10-point) reduction in ODI from baseline, ≥ 6 points on the GPES scale, or ≥ 25% reduction in MQS from baseline |
LOA∗ Racz (n = 20) 1 m: 8/20, 40% (95%CI: 19%–61%) 3 m: 8/20, 40% (95%CI: 19%–61%) 6 m: 5/20, 25% (95%CI: 6%–44%) LOA Zineu (n = 24) 3 m: 14/24, 58% (95%CI: 39%–78%) 6 m: 14/24, 58% (95%CI: 39%–78%) 12 m: 14/24, 58% (95%CI: 39%–78%) |
(1) ≥ 50% (or ≥ 4-point) reduction from baseline leg or lower back NRS-11, and no increase from baseline ODI and MQS, and ≥4 points on the GPES scale, or (2) ≥ 30% (or ≥ 2-point) reduction from baseline NRS with any one of the following criteria: simultaneous ≥30% (or ≥ 10-point) reduction in ODI from baseline, ≥ 6 points on the GPES scale, or ≥ 25% reduction in MQS from baseline ______ ≥30% Improvement in ODI |
LOA Racz (n = 20) 1 m: 8/20, 40% (95%CI: 19%–61%) 3 m: 8/20, 40% (95%CI: 19%–61%) 6 m: 5/20, 25% (95%CI: 6%–44%) LOA ZiNeu (n = 24) 3 m: 14/24, 58% (95%CI: 39%–78%) 6 m: 14/24, 58% (95%CI: 39%–78%) 12 m: 14/24, 58% (95%CI: 39%–78%) _______ LOA Racz (n = 20) 1 m: 6/20, 30% (95%CI: 10%–50%) 3 m: 7/20, 35% (95%CI: 14%–56%) 6 m: 4/20, 20% (95%CI: 2%–38%) LOA ZiNeu (n = 24) 1 m: 13/24, 54% (95%CI: 34%–74%) 3 m: 15/24, 63% (95%CI: 43%–82%) 6 m: 13/24, 54% (95%CI: 34%–74%) |
VAS- Visual Analog Scale; ODI- Oswestry Disability Index; NRS- Numeric Rating Scale; 95%CI: 95% Confidence Interval; MQS- Medication Quantification Scale; GPES- Global Perceived Effect Scale; LOA- Lysis of adhesions; ∗The term neuroplasty is used throughout the manuscript to describe procedures involving the use of a catheter where the intention was to chemically or mechanically “lyse” adhesions or resolve a filling defect. In this table the authors' preferred term is used.
Table 3.
Continuous data - pain relief.
| Study | Follow-Up Interval | Pain Outcome Measure | % Mean Improvement in Remaining Subjects at Each Interval | Diagnosis/Question Addressed |
|---|---|---|---|---|
| Akbas 2018 [46] | 1 month 3 months 6 months |
VAS |
Caudal 69% 59% 53% S1 transforaminal 71% 64% 56% L5-S1 transforaminal 67% 56% 51% |
CPSBP To compare 3 adhesiolysis∗ approaches (caudal, S1 transforaminal, L5-S1 transforaminal) |
| Chun-jing 2012 [47] | 7 days 1 month 6 months |
VAS |
Adhesiolysis 50% 49% 47% Caudal ESI only 22% 15% 12% |
CPSBP To compare adhesiolysis vs caudal ESI |
| Gerdesmeyer 2013 [44] | 3 months 6 months 12 months |
VAS |
Neurolysis∗ 57% 79% 82% |
Radicular pain w/disc herniation or CPSBP To compare neurolysis∗ vs placebo |
| Gerdesmeyer 2021 [45] | 12 months 120 months |
VAS |
Neurolysis 82% 78% |
Radicular pain w/disc herniation or CPSBP To compare neurolysis vs placebo |
| Karm 2018 [18] | 1 month 3 months 6 months |
NRS leg NRS back |
LOA Racz (NRS leg) 34% 25% 13% LOA Racz (NRS back) 34% 30% 22% LOA ZiNeu (NRS leg) 27% 40% 47% LOA ZiNeu (NRS back) 29% 38% 46% |
Central lumbar spinal stenosis w/neurogenic claudication To compare inflatable catheter (ZiNeu) to balloon-less catheter (Racz) LOA |
| Manchikanti 2008 [25] | 3 months 6 months 12 months |
NRS |
Caudal, No Steroid 53% 55% 54% Caudal ESI 57% 56% 56% |
Radicular pain and disc herniation To compare caudal epidural injections with and without steroids |
| Manchikanti 2008 [26] | 3 months 6 months 12 months |
NRS |
Caudal, No Steroid 53% 55% 54% Caudal ESI 57% 56% 56% |
Chronic LBP and radicular pain CPSBP To compare caudal epidural injections with and without steroids |
| Manchikanti 2008 [27] | 3 months 6 months 12 months |
NRS |
Caudal, No Steroid 48% 51% 53% Caudal ESI 44% 45% 45% |
CLBP and radicular pain Stenosis To compare caudal epidural injections with and without steroids |
| Manchikanti 2008 [28] | 3 months 6 months 12 months |
NRS |
Caudal, No Steroid 53% 54% 53% Caudal ESI 53% 52% 51% |
CLBP, discogenic pain without disc herniation or radiculitis To compare caudal epidural injections with and without steroids |
| Manchikanti 2009 [24] | 3 months 6 months 12 months |
NRS |
Caudal ESI 38% 27% 23% Adhesiolysis 58% 54% 51% |
Chronic LBP and radicular pain CPSBP To evaluate caudal epidural steroid versus epidural adhesiolysis |
| Manchikanti 2009 [20] | 3 months 6 months 12 months |
NRS |
Caudal ESI 33% 25% 23% Adhesiolysis 54% 51% 50% |
CLBP and radicular pain Stenosis To evaluate caudal epidural steroid versus epidural adhesiolysis |
| Manchikanti 2010 [29] | 3 months 6 months 12 months |
NRS |
Caudal, No Steroid 47% 44% 43% Caudal ESI 47% 47% 46% |
CLBP and radicular pain CPSBP To compare caudal epidural injections with and without steroids |
| Manchikanti 2011 [30] | 3 months 6 months 12 months |
NRS |
Caudal, No Steroid 49% 52% 49% Caudal ESI 56% 55% 55% |
Radiculitis, disc herniation To compare caudal epidural injections with and without steroids |
| Manchikanti 2011 [31] | 3 months 6 months 12 months |
NRS |
Caudal, No Steroid 48% 49% 46% Caudal ESI 54% 53% 52% |
CLBP without herniation or radiculitis To compare caudal epidural injections with and without steroids |
| Manchikanti 2012 [32] | 3 months 6 months 12 months 18 months 24 months |
NRS |
Caudal, No Steroid 48% 48% 44% 43% 42% Caudal ESI 46% 45% 43% 42% 38% |
CLBP and radicular pain Stenosis To compare caudal epidural injections with and without steroids |
| Manchikanti 2012 [33] | 3 months 6 months 12 months 18 months 24 months |
NRS |
Caudal, No Steroid 46% 45% 42% 41% 44% Caudal ESI 47% 47% 46% 47% 46% |
CLBP and radicular pain CPSBP To compare caudal epidural injections with and without steroids |
| Manchikanti 2012 [23] | 3 months 6 months 12 months 18 months 24 months |
NRS |
Caudal ESI 38% 27% 23% 23% 22% Adhesiolysis 58% 54% 51% 56% 56% |
CLBP and radicular pain CPSBP To compare caudal epidural steroid injections with epidural adhesiolysis |
| Manchikanti 2012 [34] | 3 months 6 months 12 months |
NRS |
Caudal, No Steroid 48% 48% 44% Caudal ESI 46% 45% 43% |
CLBP and radicular pain Stenosis To compare caudal epidural injections with and without steroids |
| Manchikanti 2012 [35] | 3 months 6 months 12 months 18 months 24 months |
NRS |
Caudal, No Steroid 49% 52% 49% 49% 48% Caudal ESI 56% 55% 55% 55% 54% |
Radiculitis, disc herniation To compare caudal epidural injections with and without steroids |
| Manchikanti 2013 [19] | 3 months 6 months 12 months 18 months 24 months |
NRS |
Adhesiolysis 55% 53% 50% 51% 48% |
CLBP and radicular pain Stenosis To evaluate epidural adhesiolysis |
| Manchikanti 2015 [36] | 3 months 6 months 12 months 18 months 24 months |
NRS |
Caudal, No Steroid 51% 51% 49% 48% 48% Caudal ESI 55% 53% 53% 50% 53% |
CLBP, discogenic pain To compare caudal and lumbar interlaminar approaches of epidural injections with and without steroids |
| Park 2013 [48] | 2 weeks 12 weeks |
VAS |
Ultrasound 51% 61% Fluoroscopy 51% 59% |
Disc herniation or stenosis and radicular pain To compare ultrasound vs fluoroscopy guided caudal ESI |
| Yousef 2010 [49] | 6 weeks 3 months 6 months 12 months |
Verbal Scale (0–4, 4 “extremely severe") |
Caudal ESI 54% 49% 28% 15% Caudal ESI + Hyaluronidase 55% 52% 56% 50% |
CPSBP To compare caudal ESI with and without hyaluronidase |
ESI- Epidural Steroid Injection; CLBP- Chronic low back pain; CPSBP- Chronic post-surgical back pain; LOA- Lysis of adhesions; VAS- Visual Analog Scale; NRS- Numeric Rating Scale; ∗The term neuroplasty is used throughout the manuscript to describe procedures involving the use of a catheter where the intention was to chemically or mechanically “lyse” adhesions or resolve a filling defect. In this table the authors' preferred term is used.
Table 4.
Continuous data - functional improvement and analgesic use.
| Study | Follow-Up Interval | Functional Outcome Measure | % Mean Improvement in Remaining Subjects at Each Interval | Analgesic Improvement Outcome Measure | % Mean Improvement in Remaining Subjects at Each Interval |
|---|---|---|---|---|---|
| Akbas 2018 [46] | 1 month 3 months 6 months |
ODI |
Caudal 52% 46% 44% S1 Transforaminal 71% 64% 56% L5-S1 Transforaminal 67% 56% 51% |
||
| Chun-jing 2012 [47] | 7 days 1 month 6 months |
MME reduction |
Adhesiolysis: 50% 50% 51% Caudal ESI only: 33% 21% 20% |
||
| Gerdesmeyer 2013 [44] | 3 months 6 months 12 months |
ODI |
Neurolysis 52% 78% 83% |
||
| Gerdesmeyer 2021 [45] | 12 months 120 months |
ODI |
Neurolysis 83% 79% |
||
| Karm 2018 [18] | 1 month 3 months 6 months |
ODI |
Racz 21% 23% 14% ZiNeu 29% 33% 42% |
||
| Manchikanti 2008 [25] | 3 months 6 months 12 months |
ODI |
Caudal, No Steroid 46% 50% 51% Caudal ESI 52% 53% 56% |
MME reduction |
Caudal, No Steroid 41% 41% 41% Caudal ESI 40% 41% 40% |
| Manchikanti 2008 [26] | 3 months 6 months 12 months |
ODI |
Caudal, No Steroid 45% 44% 45% Caudal ESI 43% 44% 42% |
MME reduction |
Caudal, No Steroid 31% 16% 30% Caudal ESI 32% 33% 34% |
| Manchikanti 2008 [27] | 3 months 6 months 12 months |
ODI |
Caudal, No Steroid 42% 46% 50% CESI 37% 41% 39% |
MME reduction |
Caudal, No Steroid 22% 24% 24% Caudal ESI 36% 38% 38% |
| Manchikanti 2008 [28] | 3 months 6 months 12 months |
ODI |
Caudal, No Steroid 49% 51% 51% Caudal ESI 49% 51% 51% |
MME reduction |
Caudal, No Steroid 25% 25% 25% Caudal ESI 25% 17% 24% |
| Manchikanti 2009 [24] | 3 months 6 months 12 months |
ODI |
Caudal ESI 29% 22% 19% Adhesiolysis 51% 51% 49% |
MME reduction |
Caudal ESI 2% 15% 2% Adhesiolysis 34% 23% 36% |
| Manchikanti 2009 [20] | 3 months 6 months 12 months |
ODI |
Caudal ESI 23% 17% 16% Adhesiolysis 49% 48% 49% |
MME reduction |
Caudal ESI 17% 17% 17% Adhesiolysis 16% 16% 16% |
| Manchikanti 2010 [29] | 3 months 6 months 12 months |
ODI |
Caudal, No Steroid 42% 42% 42% Caudal ESI 42% 44% 43% |
MME reduction |
Caudal, No Steroid 18% 22% 22% Caudal ESI 17% 17% 15% |
| Manchikanti 2011 [30] | 3 months 6 months 12 months |
ODI |
Caudal, No Steroid 43% 47% 47% Caudal ESI 51% 51% 53% |
MME reduction |
Caudal, No Steroid 12% 13% 12% Caudal ESI 33% 31% 31% |
| Manchikanti 2011 [31] | 3 months 6 months 12 months |
ODI |
Caudal, No Steroid 42% 43% 46% Caudal ESI 49% 50% 49% |
MME reduction |
Caudal, No Steroid 17% 9% 9% Caudal ESI 17% 14% 17% |
| Manchikanti 2012 [32] | 3 months 6 months 12 months 18 months 24 months |
ODI |
Caudal, No Steroid 42% 42% 41% 41% 41% Caudal ESI 40% 40% 40% 41% 37% |
MME reduction |
Caudal, No Steroid 27% 25% 21% 22% 22% Caudal ESI 33% 32% 32% 32% 34% |
| Manchikanti 2012 [33] | 3 months 6 months 12 months 18 months 24 months |
ODI |
Caudal, No Steroid 42% 42% 42% 41% 41% Caudal ESI 42% 44% 43% 43% 43% |
||
| Manchikanti 2012 [23] | 3 months 6 months 12 months 18 months 24 months |
ODI |
Caudal ESI 29% 22% 19% 19% 19% Adhesiolysis 51% 51% 49% 53% 55% |
MME reduction |
Caudal ESI 2% 2% 2% 2% 2% Adhesiolysis 23% 24% 21% 23% 23% |
| Manchikanti 2012 [34] | 3 months 6 months 12 months |
ODI |
Caudal, No Steroid 42% 42% 41% Caudal ESI 40% 40% 40% |
MME reduction |
Caudal, No Steroid 28% 25% 21% Caudal ESI 33% 32% 32% |
| Manchikanti 2012 [35] | 3 months 6 months 12 months 18 months 24 months |
ODI |
Caudal, No Steroid 43% 47% 47% 47% 47% Caudal ESI 51% 51% 53% 53% 52% |
MME reduction |
Caudal, No Steroid 37% 36% 37% 37% 37% Caudal ESI 33% 31% 31% 31% 31% |
| Manchikanti 2013 [19] | 3 months 6 months 12 months 18 months 24 months |
ODI |
Adhesiolysis∗ 51% 50% 49% 50% 51% |
MME reduction |
Adhesiolysis 31% 31% 27% 29% 27% |
| Manchikanti 2015 [36] | 3 months 6 months 12 months 18 months 24 months |
ODI |
Caudal, No Steroid 46% 46% 46% 45% 45% Caudal ESI 51% 50% 50% 50% 51% |
||
| Park 2013 [48] | 2 weeks 12 weeks |
ODI |
Ultrasound Caudal ESI 35% 43% Fluoroscopic Caudal ESI 41% 44% |
||
ESI- Epidural Steroid Injection; ODI-Oswestry Disability Index; MME- Morphine Milligram Equivalent; ∗The term neuroplasty is used throughout the manuscript to describe procedures involving the use of a catheter where the intention was to chemically or mechanically “lyse” adhesions or resolve a filling defect. In this table the authors' preferred term is used.
Table 5.
Adverse events.
| Study | Adverse Events |
|---|---|
| Akbas 2018 [46] | No serious adverse events L5-S1 TFESI: 1 headache, 1 post-operative paresthesia S1 TFESI: 1 subdural contrast spread, 1 hypotension, 1 dural puncture Caudal ESI: 1 subdural contrast spread, 1 infection |
| Chun-jing 2012 [47] | No serious adverse events Adhesiolysis∗ failed in 6 cases without any significant change in epidural anterior space angiography or any nerve injury complication |
| Gerdesmeyer 2013 [44] | No serious adverse events 54 patients (34 lysis, 20 placebo) reported intra-procedural pain 5 patients (3 lysis, 2 control) reported swelling without clinical evidence 48 (42 lysis, 6 control) transient neurological deficits following intervention 2 (1 lysis, 1 control) dural punctures with shearing of external catheter coating |
| Gerdesmeyer 2021 [45] | None reported |
| Karm 2018 [18] | No serious adverse events Transient minor events (not tabulated): Pain after adhesiolysis (2–3 days) Paresthesia during adhesiolysis Pain with needle insertion |
| Manchikanti 2008 [25] | No serious adverse events |
| Manchikanti 2008 [26] | No serious adverse events |
| Manchikanti 2008 [27] | No serious adverse events |
| Manchikanti 2008 [28] | No serious adverse events |
| Manchikanti 2009 [24] | No adverse events noted |
| Manchikanti 2009 [20] | 1 subarachnoid placement of the catheter identified in the adhesiolysis group |
| Manchikanti 2010 [29] | No serious adverse events |
| Manchikanti 2011 [30] | No serious adverse events |
| Manchikanti 2011 [31] | No serious adverse events |
| Manchikanti 2012 [32] | No serious adverse events |
| Manchikanti 2012 [33] | No serious adverse events |
| Manchikanti 2012 [23] | No serious adverse events 7 subarachnoid entries in adhesiolysis group 2 patients developed transient postoperative weakness |
| Manchikanti 2012 [34] | No serious adverse events |
| Manchikanti 2012 [35] | No serious adverse events |
| Manchikanti 2013 [19] | No serious adverse events 6 subarachnoid punctures 2 patients developed transient postoperative weakness |
| Manchikanti 2015 [36] | No serious adverse events |
| Park 2013 [48] | No serious adverse events 5 vasovagal (2 ultrasound, 3 fluoro) 3 transient headache (2 ultrasound, 1 fluoro) 9 transient pain (5 ultrasound, 4 fluoro) |
| Yousef 2010 [49] | No serious adverse events 4 minor complications (e.g., rash and itching) |
TFESI- Transforaminal Epidural Steroid Injection; ∗The term neuroplasty is used throughout the manuscript to describe procedures involving the use of a catheter where the intention was to chemically or mechanically “lyse” adhesions or resolve a filling defect. In this table the authors' preferred term is used.
3.1. Explanatory studies
One explanatory study that generated two articles met criteria for inclusion [44,45]. These two articles assess the efficacy of treatment for the same group of subjects; the 2013 manuscript presents three-month, six-month, and one-year follow-up data, and the 2021 manuscript presents one-year and 10-year follow-up data. Subjects with radiculopathy from disc herniation or CPSBP were randomized in a 1:1 ratio to receive either fluoroscopically-guided caudal epidural neuroplasty (n = 46) (defined as a two-day in-dwelling catheter with solution injected as a slow bolus as described in Table 1) or a fluoroscopically-guided subcutaneous catheter placement with daily slow bolus of normal saline only (n = 44). Subjects and assessing physicians were both blinded to group assignment. In a worst-case analysis, greater than 50% improvement in VAS was observed in 67% (95% Confidence Interval [CI]: 54%–81%), 70% (95% CI: 56%–83%), and 63% (95% CI: 49%–72%) of subjects at three, six, and 12 months, respectively. Greater than 50% improvement in ODI was reported by 57% (95% CI: 42%–71%), 67% (95% CI: 54%–81%), and 61% (95% CI: 47%–75%) of subjects at three, six, and 12 months, respectively, which was significantly higher than in the saline group [16% (95% CI: 5%–27%), 9% (95% CI: 1%–18%), and 20% (95% CI: 9%–32%) respectively]. These improvements in VAS and ODI were statistically significant and superior to placebo at all three timepoints (p < 0.05). The mean VAS scores in the neuroplasty group improved from 6.7 ± 1.1 at baseline to 2.9 ± 1.9, 1.4 ± 0.9, and 1.2 ± 1.0 at 3-, 6-, and 12-months post-procedure [44]. Thirty-eight percent and 31% of subjects were lost to follow up by one-year post-intervention in the neuroplasty and placebo groups, respectively.
Gerdesmeyer et al. additionally provided 10-year data from this study cohort [45]. At this time point, greater than 50% improvement in VAS was observed in 54% (95% CI: 40%–69%) of subjects who received the intervention and 34% (95% CI: 28%–48%) of subjects who received placebo. Also at this time point, greater than 50% improvement in ODI was reported by 54% (95% CI: 40%–69%) of subjects who received the intervention and 36% (95% CI: 28%–51%) of subjects who received placebo. In the neuroplasty group, mean VAS scores decreased from a baseline of 6.7 ± 1.1 to 1.2 ± 1.1 at one year and to 1.5 ± 1.4 at 10 years. The differences in VAS and ODI score improvements between the two groups at the 10-year threshold were not statistically significant [45].
3.2. Pragmatic studies
One pragmatic study was identified that provided acceptable data on success rates for pain relief and functional improvement [18]. In this study, an inflatable catheter was compared to a balloon-less catheter introduced by caudal epidural access for subjects with central lumbar spinal stenosis with neurogenic claudication. Because both groups received steroid injections, data from each are reported. This study used a composite categorical score as the criterion for success (see Table 2 for the description of this composite score). In the balloon-less group, 40% (95% CI: 19%–61%), 40% (95% CI: 19%–61%), and 25% (95% CI: 6%–44%) of subjects met the composite categorical definition of treatment success at three, six, and 12 months, respectively. In the balloon group, 58% (95% CI: 39%–78%) met the composite categorical definition of treatment success at all three time points.
None of the other pragmatic studies that met the established inclusion criteria of the present systematic review reported categorical data that could be effectively extrapolated. Fifteen studies reported categorical data and success rates, but these data could not be validly interpreted due to use of the “last data point carried forward” methodology, which represents a misutilization of the intention to treat analysis; allowance for repeat interventions within the study timeframe; unblinding of subjects during the study timeframe; absence of raw data to allow for independent calculations of success rates; and reporting results graphically without numerical representation in the text of the manuscript [19,20,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]].
Improvement in pain using continuous data was reported in 21 pragmatic studies [[18], [19], [20],[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36],[46], [47], [48], [49]]. Various time points were evaluated in these studies. The range of mean improvement in pain scores for three- to six-month post-procedure time points in these studies was 12%–79% (Table 3). While differences exist between how individual studies defined specific diagnoses, and several studies combined diagnoses without separating them for interpretation, there were several identifiable diagnoses that could be grouped for interpretation of continuous data. These included CPSBP, disc herniation with radicular pain, central stenosis with radicular pain, and low back pain without disc herniation or radiculitis. For CPSBP, the range of mean improvement in pain scores at three- to six-months after caudal ESI was 12–79% (Table 3). For disc herniation with radicular pain, the range of mean improvement in pain score at three- to six-month time points after caudal ESI was 57–79% (Table 3). For central stenosis with radicular pain, the range of mean improvement in pain score at three- to six-month time points after caudal ESI was 13–61% (Table 3). For non-radicular LBP, the range of mean improvement in pain scores at three- to six-month time points after caudal ESI was 48–55% (Table 3).
Seventeen studies reported improvement in mean pain score at an endpoint greater than six months post-procedure [19,20,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36],49]. Endpoints included 12 months, 18 months, and 24 months post-procedure. The range of mean improvement in pain score post-procedure in these studies at these endpoints was 45–56% (Table 3). As above, identifiable diagnoses that could be grouped for interpretation of continuous data included CPSBP, disc herniation with radicular pain, central stenosis with radicular pain, and non-radicular LBP. The range of mean improvement in pain scores after six month following caudal ESI was 46–56% for CPSBP, 54–56% for disc herniation with radicular pain, 38–51% for central stenosis with radicular pain, and 51–53% for non-radicular LBP (Table 3).
Nineteen pragmatic studies evaluated functional improvement (Table 4) [[18], [19], [20],[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36],46,48]. All 19 described a mean improvement in ODI. The range of improvement in ODI at three- to six-month time points post-procedure was 14–54%. The range of mean improvement in ODI for the endpoints beyond six months post-procedure was 17–53%.
Fifteen studies assessed change in opioid consumption (see Table 4) [19,20,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32],34,35,47]. All 15 studies described a mean reduction in morphine equivalents (MME). The range of improvement in reduction of MME at three- to six-month time points post-procedure was 14–51%. The range of improvement in reduction of MME for greater than six-month post-procedure endpoints was 15–41%.
3.3. Adverse events
Adverse events data were collected and assessed from each of the included studies. No serious adverse events were identified in any of the studies. Subarachnoid needle or catheter placement was identified in 9% (14/155) [95% CI: 5–14%] of subjects treated with epidural neuroplasty across three studies [19,20,23], while subdural needle placement was noted in 5% (1/20) (95% CI: 0–15%) of subjects treated with a caudal ESI in one study [46]. No other identifiable patterns of adverse events were noted (Table 5).
3.4. GRADE assessment of the evidence
There is only one explanatory randomized controlled trial with valid, interpretable success rates published on the use of caudal ESIs, and data are reported in two separate articles from this trial [44,45]. The second of these studies reports 10-year follow-up data that are unlikely to be related to the original intervention. There is a wide variety of heterogeneity of study design, injectate, and diagnosis inclusions for the remaining pragmatic studies. Gerdesmeyer et al. performed epidural neuroplasty by placing a catheter in the caudal epidural space for two days with a specific injectate (Table 1). With RCT evidence available for this particular form of intervention, the resulting body of evidence is initially assigned a “high” GRADE quality of evidence rating. However, this body of evidence is downgraded due to imprecision of results, which represents a serious risk of bias. This is based upon a small sample of available explanatory data that have not been replicated, leading to large confidence intervals and uncertainty regarding the findings. Therefore, the GRADE level for the quality of evidence for epidural neuroplasty to improve pain and function associated with radiculopathy from disc herniation or CPSBP is moderate at three, six, and 12 months. The quality of evidence for the use of caudal ESI for the treatment of pain and dysfunction associated with radiculopathy in disc herniation or CPSBP using any technique other than the epidural neuroplasty technique described by Gerdesmeyer et al. is low. While there are pragmatic RCTs, which confer an initial GRADE assignment of high quality to the evidence, the quality of the body of evidence is downgraded because the studies are biased by imprecise effect size (small sample sizes and unreliable categorical and continuous data), indirectness (substantial differences in the methodology of caudal ESI technique as well as substantial population differences), and inconsistency in results (large differences in effect between studies).
The GRADE assessment for the use of caudal ESI for pain and dysfunction associated with the following diagnoses at all included time points is low: central lumbar spinal stenosis with neurogenic claudication, discogenic CLBP, and CLBP without disc herniation/radiculitis. While there are pragmatic RCTs, which confer an initial GRADE assignment of high quality to the evidence, the quality of the body of evidence is downgraded because the studies are biased by imprecise effect size, indirectness, and inconsistency.
4. Discussion
The primary focus of the present review was to rigorously and comprehensively analyze the published literature on the efficacy and effectiveness of caudal ESI for the treatment of chronic back and/or lower extremity pain due to a variety of diagnoses. Relevant studies were selected for inclusion using criteria from established guidelines [41,50].
The recommendations for the importance of categorical data analyses have been well-documented in the medical literature. A panel of leading authorities in the field of Pain Medicine published recommendations known as “Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials” (IMMPACT) guidelines. These authors emphasized the importance of reporting categorical data analysis (Anchor-Based Methods) over group (mean) data analysis (Distribution-Based Methods) [50]. While group data may provide a statistical indication that a treatment is effective, it does not provide any information on the proportion of patients in which the treatment is effective, the number of patients for which it is effective, or the magnitude of effectiveness in a particular patient [51].
Trends emerge when assessing the level of evidence in accordance with GRADE. In this review, the authors encountered both explanatory and pragmatic RCTs. There are many studies in which caudal access is obtained and steroids are injected. There is a finite number of diagnoses for which caudal ESIs are generally used. Each of these diagnoses is represented in studies in this systematic review: 1) radiculopathy with disc herniation, 2) CPSBP, 3) central lumbar spinal stenosis with neurogenic claudication, 4) discogenic CLBP, and 5) CLBP without disc herniation/radiculitis. However, the similarities of the studies addressed herein end there.
A variety of techniques and injected medications have been studied for caudal ESIs, more so than interlaminar ESIs or transforaminal ESIs. However, the associated body of literature has not been developed enough to establish a definitive superiority of a specific procedural technique or epidural injectate. This leads to ambiguity when interpreting the available literature. Table 1 describes the 23 different studies that were included in this review, the majority of which include entirely different methods for performing this procedure. For this reason, the authors were unable to perform a meta-analysis. Because of the heterogeneity in the technique for caudal ESI, it is possible that the subtle differences may have contributed to increased or decreased benefit as compared to steroid in isolation. Additionally, 16/23 (70%) of the studies had the same first author, which limits generalizability of findings. Many of the included studies used the “last data point carried forward” method to complete an intention-to-treat analysis, wherein a dropout's score was carried forward throughout the duration of the study, as opposed to accounting for the dropout as a failure. The categorical data within these studies therefore cannot be fairly evaluated, which left only three articles with interpretable categorical data [18,44,45].
Of the three articles with interpretable categorical data, data from the two articles describing the same population support the notion that caudal ESIs may be a viable option for the treatment of radiculopathy with disc herniation, CPSBP, and lumbar spinal stenosis with claudication [44,45]. In Gerdesmeyer et al., the only explanatory RCT with viable categorical data, there was a statistically and clinically meaningful improvement in pain and function at three, six, and 12 months. If a similar study demonstrates comparable results, the reproducibility of findings would likely increase confidence in the results and justify a GRADE quality of evidence rating of “high”. Of note, it is unclear what the role of the included injectates other than steroid may have played in the outcomes in this study. Perhaps most importantly, Gerdesmeyer et al. implemented a two-day in-dwelling catheter neuroplasty technique, which is not commonly used due to the necessity of inpatient hospitalization.
In the one other available article with interpretable categorical data, because the main difference between the two groups in Karm et al. was the use of a catheter with a balloon versus a catheter without a balloon, this study amounts to two separate cohort studies of two distinct types of epidural neuroplasty [18]. Therefore, the findings are less meaningful relative to a prospective trial comparing caudal ESI or epidural neuroplasty to a sham procedure or any type of standard care for the condition of interest.
Given the noted heterogeneity and lack of conclusive results relative to the various diagnoses studied, it is clear that more research is needed to understand the utility of caudal ESI and epidural neuroplasty in the treatment of painful conditions of the lumbosacral spine. This research should include, but is not limited to, appropriate diagnosis/diagnoses for the procedure, optimal timeframe of intervention, precise location(s) of injectate delivery, injectate type(s), catheter use, catheter type, and optimal duration of use of the catheter (if any).
There are several limitations to the present review. It is possible that all relevant data were not captured. Useful data may have been rejected if unavailable in English. Reviewers are also susceptible to confirmation bias and their assessments can be influenced by their previous experience and knowledge of a procedure and its effects. With access to raw data, the possibility exists that several of the studies with published categorical data may have been interpretable and thus added to the body of outcomes for explanatory studies. We did not contact authors to request raw data. Lastly, our review was not exhaustive regarding a review of retrospective case studies, case series, and case studies to assess for other adverse events associated with caudal ESIs and epidural neuroplasty. Rare complications, by definition, are not captured in cohort studies aside from very large registries, which do not exist for these procedures.
5. Conclusion
The published evidence establishes that when the inclusion criteria utilized for this systematic review are applied, caudal epidural neuroplasty performed using an indwelling catheter for two days with a specific epidural injectate described by Gerdesmeyer et al. are effective treatments for lumbosacral radiculopathy with disc herniation and for CPSBP, though the GRADE level of certainty is moderate only for this variant of the procedure. For all other methods of performing caudal ESIs described in the present literature, the level of evidence is low for the treatment of pain and dysfunction associated with central lumbar spinal stenosis with neurogenic claudication, discogenic CLBP, and CLBP without disc herniation/radiculitis, and the available evidence supports a possible benefit. More research is needed to understand the utility of caudal ESI/neuroplasty in the treatment of painful conditions of the lumbosacral spine. This research should include, but is not limited to, appropriate diagnosis/diagnoses for the procedure, optimal timeframe of intervention, location(s) of injectate delivery, injectate type(s), injectate volume, catheter use, catheter type, and optimal duration of use of a catheter (if any).
Funding
No funding was received for this systematic review.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge Dr. Arsenio Avila for his assistance early in the review process with reviewing a subset of abstracts and participating in discussions regarding the scope of the review. We also wish to thank the Spine Intervention Society's Standards Division and Evidence Analysis Committee for their careful review and thoughtful feedback on this manuscript.
References
- 1.Goebert H.W., Jr., Jallo S.J., Gardner W.J., Wasmuth C.E. Painful radiculopathy treated with epidural injections of procaine and hydrocortisone acetate: results in 113 patients. Anesth Analg. 1961;40:130–134. [PubMed] [Google Scholar]
- 2.Bogduk N., editor. Practice guidelines for spinal diagnostic and treatment procedures. second ed. International Spine Intervention Society; San Francisco: 2013. [Google Scholar]
- 3.Smith C.C., McCormick Z.L., Mattie R., MacVicar J., Duszynski B., Stojanovic M.P. The effectiveness of lumbar transforaminal injection of steroid for the treatment of radicular pain: a comprehensive review of the published data. Pain Med. 2020;21(3):472–487. doi: 10.1093/pm/pnz160. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A.K., Vorobeychik Y., Wasserman R., et al. The effectiveness and risks of fluoroscopically guided lumbar interlaminar epidural steroid injections: a systematic review with comprehensive analysis of the published data. Pain Med. 2017;18(2):239–251. doi: 10.1093/pm/pnw131. [DOI] [PubMed] [Google Scholar]
- 5.Clements N., Vydra D., Cushman D.M., et al. Reg Anesth Pain Med; 2019. Trends in steroid agent and diluent choices for epidural steroid injections: a survey of Spine Intervention Society physicians. [published online ahead of print, 2019 May 24] ;rapm-2018-100366. [DOI] [PubMed] [Google Scholar]
- 6.Conn A., Buenaventura R.M., Datta S., Abdi S., Diwan S. Systematic review of caudal epidural injections in the management of chronic low back pain. Pain Physician. 2009;12(1):109–135. [PubMed] [Google Scholar]
- 7.Andreisek G., Jenni M., Klingler D., Wertli M., Elliott M., Ulbrich E.J., Winklhofer S., Steurer J. Access routes and reported decision criteria for lumbar epidural drug injections: a systematic literature review. Skeletal Radiol. 2013;42(12):1683–1692. doi: 10.1007/s00256-013-1713-5. [DOI] [PubMed] [Google Scholar]
- 8.Johnson B.A., Schellhas K.P., Pollei S.R. Epidurography and therapeutic epidural injections: technical considerations and experience with 5334 cases. AJNR Am J Neuroradiol. 1999;20(4):697–705. [PMC free article] [PubMed] [Google Scholar]
- 9.Benyamin R.M., Manchikanti L., Parr A.T., Diwan S., Singh V., Falco F.J., Datta S., Abdi S., Hirsch J.A. The effectiveness of lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain. Pain Physician. 2012;15(4):E363–E404. [PubMed] [Google Scholar]
- 10.Oh C.H., Ji G.Y., Cho P.G., Choi W.S., Shin D.A., Kim K.N., Kang H.A. The catheter tip position and effects of percutaneous epidural neuroplasty in patients with lumbar disc disease during 6-months of follow-up. Pain Physician. 2014;17(5):E599–E608. [PubMed] [Google Scholar]
- 11.Smith C., Sudhakaran S., McCormick Z.L. Spine Intervention Society's Patient Safety Committee. Is there a risk of neurological complications due to vascular infarction associated with particulate steroid use during interlaminar epidural steroid injections? Pain Med. 2021;22(1):212–213. doi: 10.1093/pm/pnaa310. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy D.J., Plastaras C., Casey E., et al. Comparative effectiveness of lumbar transforaminal epidural steroid injections with particulate versus nonparticulate corticosteroids for lumbar radicular pain due to intervertebral disc herniation: a prospective, randomized, double-blind trial. Pain Med. 2014;15(4):548–555. doi: 10.1111/pme.12325. [DOI] [PubMed] [Google Scholar]
- 13.Park C.H., Lee S.H., Kim B.I. Comparison of the effectiveness of lumbar transforaminal epidural injection with particulate and nonparticulate corticosteroids in lumbar radiating pain. Pain Med. 2010;11(11):1654–1658. doi: 10.1111/j.1526-4637.2010.00941.x. [DOI] [PubMed] [Google Scholar]
- 14.Schneider B.J., McCormick Z.L., Smith C.C. Spine Intervention Society's Patient Safety Committee. Particulate or nonparticulate steroids for lumbar transforaminal injections. Pain Med. 2017;18(9):1817–1818. doi: 10.1093/pm/pnx171. [DOI] [PubMed] [Google Scholar]
- 15.Cleary M., Keating C., Poynton A.R. The flow patterns of caudal epidural in upper lumbar spinal pathology. Eur Spine J. 2011;20(5):804–807. doi: 10.1007/s00586-010-1613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racz G.B., Holubec J.T. In: Techniques of neurolysis. Racz G.B., editor. Springer US; Boston, MA: 1989. Lysis of adhesions in the epidural space; pp. 57–72. [Google Scholar]
- 17.Heavner J.E., Racz G.B., Raj P. Percutaneous epidural neuroplasty: prospective evaluation of 0.9% NaCl versus 10% NaCl with or without hyaluronidase. Reg Anesth Pain Med. 1999;24(3):202–207. doi: 10.1016/s1098-7339(99)90128-1. [DOI] [PubMed] [Google Scholar]
- 18.Karm M.H., Choi S.S., Kim D.H., Park J.Y., Lee S., Park J.K., Suh Y.J., Leem J.G., Shin J.W. Percutaneous epidural adhesiolysis using inflatable balloon catheter and balloon-less catheter in central lumbar spinal stenosis with neurogenic claudication: a randomized controlled trial. Pain Physician. 2018;21(6):593–606. [PubMed] [Google Scholar]
- 19.Manchikanti L., Cash K.A., McManus C.D., Pampati V. Assessment of effectiveness of percutaneous adhesiolysis in managing chronic low back pain secondary to lumbar central spinal canal stenosis. Int J Med Sci. 2013;10(1):50–59. doi: 10.7150/ijms.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manchikanti L., Cash K.A., McManus C.D., Pampati V., Singh V., Benyamin R. The preliminary results of a comparative effectiveness evaluation of adhesiolysis and caudal epidural injections in managing chronic low back pain secondary to spinal stenosis: a randomized, equivalence controlled trial. Pain Physician. 2009;12(6):E341–E354. [PubMed] [Google Scholar]
- 21.Manchikanti L., Pampati V., Cash K.A. Protocol for evaluation of the comparative effectiveness of percutaneous adhesiolysis and caudal epidural steroid injections in low back and/or lower extremity pain without post surgery syndrome or spinal stenosis. Pain Physician. 2010;13(2):E91–E110. [PubMed] [Google Scholar]
- 22.Manchikanti L., Rivera J.J., Pampati V., Damron K.S., McManus C.D., Brandon D.E., Wilson S.R. One day lumbar epidural adhesiolysis and hypertonic saline neurolysis in treatment of chronic low back pain: a randomized, double-blind trial. Pain Physician. 2004;7(2):177–186. [PubMed] [Google Scholar]
- 23.Manchikanti L., Singh V., Cash K.A., Pampati V. Assessment of effectiveness of percutaneous adhesiolysis and caudal epidural injections in managing post lumbar surgery syndrome: 2-year follow-up of a randomized, controlled trial. J Pain Res. 2012;5:597–608. doi: 10.2147/JPR.S38999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manchikanti L., Singh V., Cash K.A., Pampati V., Datta S. A comparative effectiveness evaluation of percutaneous adhesiolysis and epidural steroid injections in managing lumbar post surgery syndrome: a randomized, equivalence controlled trial. Pain Physician. 2009;12(6):E355–E368. [PubMed] [Google Scholar]
- 25.Manchikanti L., Singh V., Cash K.A., Pampati V., Damron K.S., Boswell M.V. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 2--Disc herniation and radiculitis. Pain Physician. 2008;11(6):801–815. [PubMed] [Google Scholar]
- 26.Manchikanti L., Singh V., Cash K.A., Pampati V., Datta S. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 3--Post surgery syndrome. Pain Physician. 2008;11(6):817–831. [PubMed] [Google Scholar]
- 27.Manchikanti L., Cash K.A., McManus C.D., Pampati V., Abdi S. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 4--Spinal stenosis. Pain Physician. 2008;11(6):833–848. [PubMed] [Google Scholar]
- 28.Manchikanti L., Cash K.A., McManus C.D., Pampati V., Smith H.S. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 1--Discogenic pain without disc herniation or radiculitis. Pain Physician. 2008;11(6):785–800. [PubMed] [Google Scholar]
- 29.Manchikanti L., Singh V., Cash K.A., Pampati V., Datta S. Management of pain of post lumbar surgery syndrome: one-year results of a randomized, double-blind, active controlled trial of fluoroscopic caudal epidural injections. Pain Physician. 2010;13(6):509–521. [PubMed] [Google Scholar]
- 30.Manchikanti L., Singh V., Cash K.A., Pampati V., Damron K.S., Boswell M.V. A randomized, controlled, double-blind trial of fluoroscopic caudal epidural injections in the treatment of lumbar disc herniation and radiculitis. Spine. 2011;36(23):1897–1905. doi: 10.1097/BRS.0b013e31823294f2. [DOI] [PubMed] [Google Scholar]
- 31.Manchikanti L., Cash K.A., McManus C.D., Pampati V., Smith H.S. One-year results of a randomized, double-blind, active controlled trial of fluoroscopic caudal epidural injections with or without steroids in managing chronic discogenic low back pain without disc herniation or radiculitis. Pain Physician. 2011;14(1):25–36. [PubMed] [Google Scholar]
- 32.Manchikanti L., Cash K.A., McManus C.D., Pampati V., Fellows B. Results of 2-year follow-up of a randomized, double-blind, controlled trial of fluoroscopic caudal epidural injections in central spinal stenosis. Pain Physician. 2012;15(5):371–384. [PubMed] [Google Scholar]
- 33.Manchikanti L., Singh V., Cash K.A., Pampati V., Datta S. Fluoroscopic caudal epidural injections in managing post lumbar surgery syndrome: two-year results of a randomized, double-blind, active-control trial. Int J Med Sci. 2012;9(7):582–591. doi: 10.7150/ijms.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manchikanti L., Cash K.A., McManus C.D., Pampati V., Fellows B. Fluoroscopic caudal epidural injections with or without steroids in managing pain of lumbar spinal stenosis: one-year results of randomized, double-blind, active-controlled trial. J Spinal Disord Tech. 2012;25(4):226–234. doi: 10.1097/BSD.0b013e3182160068. [DOI] [PubMed] [Google Scholar]
- 35.Manchikanti L., Singh V., Cash K.A., Pampati V., Damron K.S., Boswell M.V. Effect of fluoroscopically guided caudal epidural steroid or local anesthetic injections in the treatment of lumbar disc herniation and radiculitis: a randomized, controlled, double blind trial with a two-year follow-up. Pain Physician. 2012;15(4):273–286. [PubMed] [Google Scholar]
- 36.Manchikanti L., Pampati V., Benyamin R.M., Boswell M.V. Analysis of efficacy differences between caudal and lumbar interlaminar epidural injections in chronic lumbar axial discogenic pain: local anesthetic alone vs. local combined with steroids. Int J Med Sci. 2015;12(3):214–222. doi: 10.7150/ijms.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manchikanti L., Knezevic E., Knezevic N.N., Sanapati M.R., Thota S., Abd-Elsayed A., Hirsch J.A. Epidural injections for lumbar radiculopathy or sciatica: a comparative systematic review and meta-analysis of cochrane review. Pain Physician. 2021;24(5) E539-e54. [PubMed] [Google Scholar]
- 38.Manchikanti L., Abdi S., Atluri S., Benyamin R.M., Boswell M.V., Buenaventura R.M., Bryce D.A., Burks P.A., Caraway D.L., Calodney A.K., Cash K.A., Christo P.J., Cohen S.P., Colson J., Conn A., Cordner H., Coubarous S., Datta S., Deer T.R., Diwan S., Falco F.J., Fellows B., Geffert S., Grider J.S., Gupta S., Hameed H., Hameed M., Hansen H., Helm S., 2nd, Janata J.W., Justiz R., Kaye A.D., Lee M., Manchikanti K.N., McManus C.D., Onyewu O., Parr A.T., Patel V.B., Racz G.B., Sehgal N., Sharma M.L., Simopoulos T.T., Singh V., Smith H.S., Snook L.T., Swicegood J.R., Vallejo R., Ward S.P., Wargo B.W., Zhu J., Hirsch J.A. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(2 Suppl):S49–S283. [PubMed] [Google Scholar]
- 39.Parr A.T., Manchikanti L., Hameed H., Conn A., Manchikanti K.N., Benyamin R.M., Diwan S., Singh V., Abdi S. Caudal epidural injections in the management of chronic low back pain: a systematic appraisal of the literature. Pain Physician. 2012;15(3):E159–E198. [PubMed] [Google Scholar]
- 40.Jamison D.E., Hsu E., Cohen S.P. Epidural adhesiolysis: an evidence-based review. J Neurosurg Sci. 2014;58(2):65–76. [PubMed] [Google Scholar]
- 41.Bogduk N., Kennedy D.J., Vorobeychik Y., Engel A. Guidelines for composing and assessing a paper on treatment of pain. Pain Med. 2017;18(11):2096–2104. doi: 10.1093/pm/pnx121. [DOI] [PubMed] [Google Scholar]
- 42.Balshem H., Helfand M., Schunemann H.J., Oxman A.D., Kunz R., Brozek J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerdesmeyer L., Wagenpfeil S., Birkenmaier C., Veihelmann A., Hauschild M., Wagner K., et al. Percutaneous epidural lysis of adhesions in chronic lumbar radicular pain: a randomized, double-blind, placebo-controlled trial. Pain Physician. 2013;16(3):185–196. [PubMed] [Google Scholar]
- 45.Gerdesmeyer L., Noe C., Prehn-Kristensen A., et al. Long-term efficacy of percutaneous epidural neurolysis of adhesions in chronic lumbar radicular pain: 10 year follow-up of a randomized controlled trial. Pain Physician. 2021;24(5):359–367. [PubMed] [Google Scholar]
- 46.Akbas M., Elawamy A.R., Salem H.H., Fouad A.Z., Abbas N.A., Dagistan G. Comparison of 3 approaches to percutaneous epidural adhesiolysis and neuroplasty in post lumbar surgery syndrome. Pain Physician. 2018;21(5):E501–E508. [PubMed] [Google Scholar]
- 47.Chun-jing H., Hao-xiong N., jia-xiang N. The application of percutaneous lysis of epidural adhesions in patients with failed back surgery syndrome. Acta Cir Bras. 2012;27(4):357–362. doi: 10.1590/s0102-86502012000400013. [DOI] [PubMed] [Google Scholar]
- 48.Park Y., Lee J.H., Park K.D., Ahn J.K., Park J., Jee H. Ultrasound-guided vs. fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92(7):575–586. doi: 10.1097/PHM.0b013e318292356b. [DOI] [PubMed] [Google Scholar]
- 49.Yousef A.A., AS E.L.-D., Al-Deeb A.E. The role of adding hyaluronidase to fluoroscopically guided caudal steroid and hypertonic saline injection in patients with failed back surgery syndrome: a prospective, double-blinded, randomized study. Pain Pract. 2010;10(6):548–553. doi: 10.1111/j.1533-2500.2009.00357.x. [DOI] [PubMed] [Google Scholar]
- 50.Dworkin R.H., Turk D.C., Wyrwich K.W., Beaton D., Cleeland C.S., Farrar J.T., et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain: Off J Am Pain Soc. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Smith S.M., Dworkin R.H., Turk D.C., McDermott M.P., Eccleston C., Farrar J.T., Rowbotham M.C., Bhagwagar Z., Burke L.B., Cowan P., Ellenberg S.S., Evans S.R., Freeman R.L., Garrison L.P., Iyengar S., Jadad A., Jensen M.P., Junor R., Kamp C., Katz N.P., Kesslak J.P., Kopecky E.A., Lissin D., Markman J.D., Mease P.J., O'Connor A.B., Patel K.V., Raja S.N., Sampaio C., Schoenfeld D., Singh J., Steigerwald I., Strand V., Tive L.A., Tobias J., Wasan A.D., Wilson H.D. Interpretation of chronic pain clinical trial outcomes: IMMPACT recommended considerations. Pain. 2020 Nov;161(11):2446–2461. doi: 10.1097/j.pain.0000000000001952. PMID: 32520773; PMCID: PMC7572524. [DOI] [PMC free article] [PubMed] [Google Scholar]