Abstract
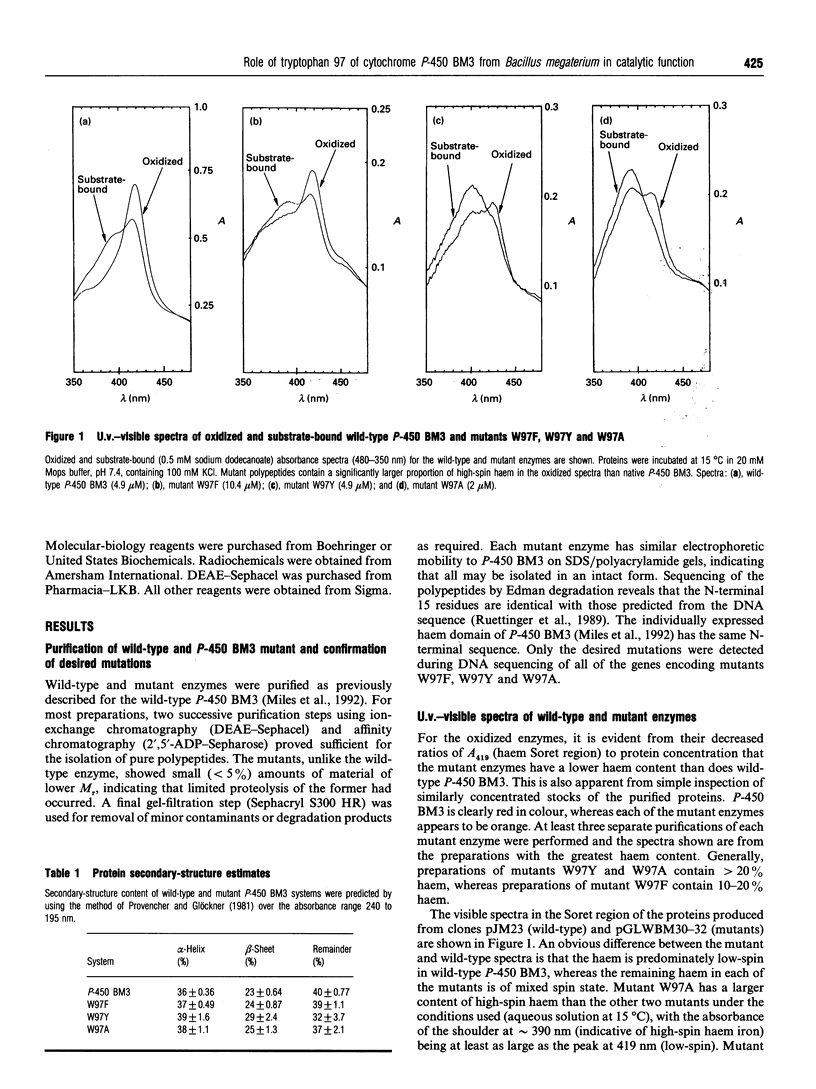
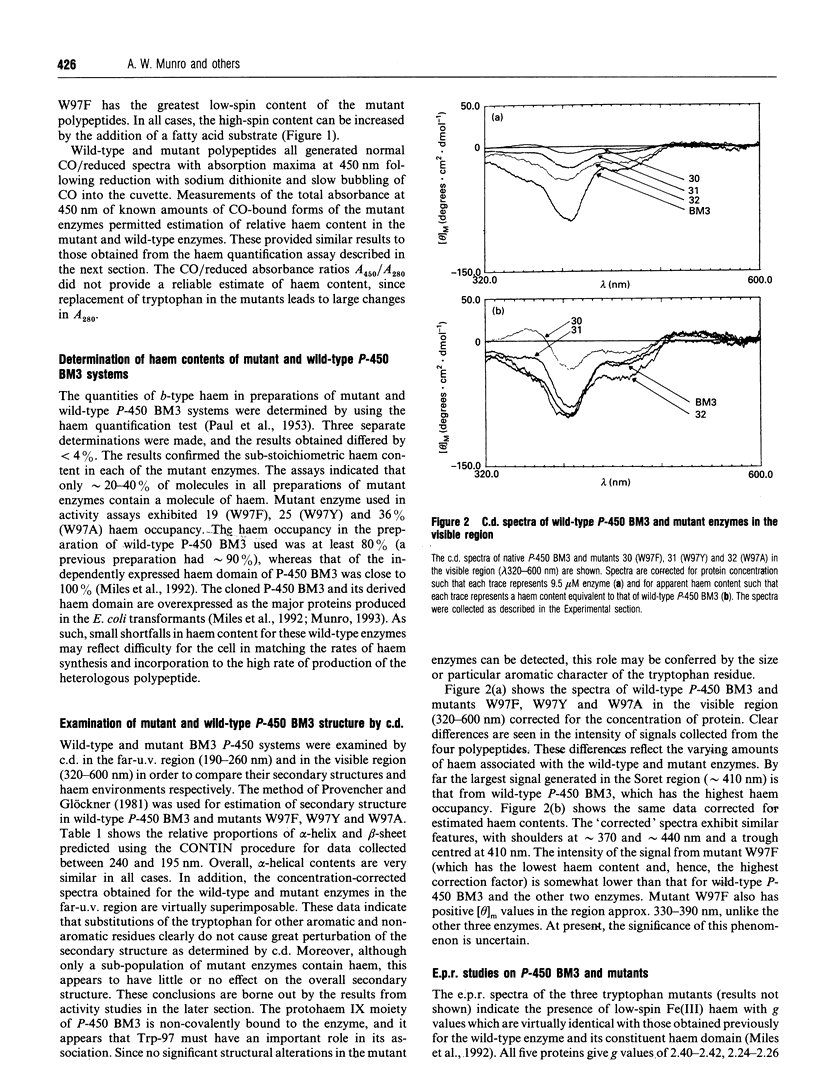
The 'Covalent Switching' hypothesis suggests that a strongly conserved tryptophan residue acts as a mediator of electron-transfer flow between redox partners in cytochrome P-450 systems [Baldwin, Morris and Richards (1991) Proc. R. Soc. London B 245, 43-51]. We have investigated the effect of alteration of the conserved tryptophan (Trp-97) in cytochrome P-450 BM3 (P-450 102) from Bacillus megaterium. Replacement of Trp-97 with Ala, Phe or Tyr results in a decrease in the natural haem content and alters the resting spin state of the remaining haem in the purified mutant enzymes. However, kinetic analyses indicate that the mutant enzymes retain high levels of catalytic activity. C.d. and e.p.r. spectroscopy also reveal little alteration in secondary structure or change in the pattern of haem ligation. These findings cast doubt on the covalent switching mechanism of intermolecular electron flow in the P-450s, but indicate that this residue plays a role in the association of the haem prosthetic group.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. E., Morris G. M., Richards W. G. Electron transport in cytochromes P-450 by covalent switching. Proc Biol Sci. 1991 Jul 22;245(1312):43–51. doi: 10.1098/rspb.1991.0086. [DOI] [PubMed] [Google Scholar]
- Boddupalli S. S., Estabrook R. W., Peterson J. A. Fatty acid monooxygenation by cytochrome P-450BM-3. J Biol Chem. 1990 Mar 15;265(8):4233–4239. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Crespi C. L., Penman B. W., Steimel D. T., Gelboin H. V., Gonzalez F. J. The development of a human cell line stably expressing human CYP3A4: role in the metabolic activation of aflatoxin B1 and comparison to CYP1A2 and CYP2A3. Carcinogenesis. 1991 Feb;12(2):355–359. doi: 10.1093/carcin/12.2.355. [DOI] [PubMed] [Google Scholar]
- Fulco A. J. P450BM-3 and other inducible bacterial P450 cytochromes: biochemistry and regulation. Annu Rev Pharmacol Toxicol. 1991;31:177–203. doi: 10.1146/annurev.pa.31.040191.001141. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Reactions and significance of cytochrome P-450 enzymes. J Biol Chem. 1991 Jun 5;266(16):10019–10022. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Imai Y., Nakamura M. Point mutations at threonine-301 modify substrate specificity of rabbit liver microsomal cytochromes P-450 (laurate (omega-1)-hydroxylase and testosterone 16 alpha-hydroxylase). Biochem Biophys Res Commun. 1989 Feb 15;158(3):717–722. doi: 10.1016/0006-291x(89)92780-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci. 1992 Nov;17(11):463–468. doi: 10.1016/0968-0004(92)90489-v. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Juvonen R., Lindberg R., Negishi M. Alteration of high and low spin equilibrium by a single mutation of amino acid 209 in mouse cytochromes P450. J Biol Chem. 1991 Feb 25;266(6):3380–3382. [PubMed] [Google Scholar]
- Miles J. S., Munro A. W., Rospendowski B. N., Smith W. E., McKnight J., Thomson A. J. Domains of the catalytically self-sufficient cytochrome P-450 BM-3. Genetic construction, overexpression, purification and spectroscopic characterization. Biochem J. 1992 Dec 1;288(Pt 2):503–509. doi: 10.1042/bj2880503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y., Fulco A. J. Omega-1, Omega-2 and Omega-3 hydroxylation of long-chain fatty acids, amides and alcohols by a soluble enzyme system from Bacillus megaterium. Biochim Biophys Acta. 1975 Jun 23;388(3):305–317. doi: 10.1016/0005-2760(75)90089-2. [DOI] [PubMed] [Google Scholar]
- Munro A. W., Malarkey K., Miles J. S. Investigating the function of cytochrome P450 BM-3: a role for the phylogenetically conserved tryptophan residue? Biochem Soc Trans. 1993 Feb;21(1):66S–66S. doi: 10.1042/bst021066s. [DOI] [PubMed] [Google Scholar]
- Munro A. W. Purification schemes for the constituent domains of cytochrome P450 BM3 in E. coli. Biochem Soc Trans. 1993 Aug;21(3):316S–316S. doi: 10.1042/bst021316s. [DOI] [PubMed] [Google Scholar]
- Narhi L. O., Fulco A. J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986 Jun 5;261(16):7160–7169. [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Strobel H. W. On the membrane topology of vertebrate cytochrome P-450 proteins. J Biol Chem. 1988 May 5;263(13):6038–6050. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Okita R. T., Clark J. E., Okita J. R., Masters B. S. Omega- and (omega-1)-hydroxylation of eicosanoids and fatty acids by high-performance liquid chromatography. Methods Enzymol. 1991;206:432–441. doi: 10.1016/0076-6879(91)06112-g. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Finzel B. C., Howard A. J. Crystal structure of substrate-free Pseudomonas putida cytochrome P-450. Biochemistry. 1986 Sep 9;25(18):5314–5322. doi: 10.1021/bi00366a049. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Raag R. Cytochrome P450cam: crystallography, oxygen activation, and electron transfer. FASEB J. 1992 Jan 6;6(2):674–679. doi: 10.1096/fasebj.6.2.1537455. [DOI] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Ravichandran K. G., Boddupalli S. S., Hasermann C. A., Peterson J. A., Deisenhofer J. Crystal structure of hemoprotein domain of P450BM-3, a prototype for microsomal P450's. Science. 1993 Aug 6;261(5122):731–736. doi: 10.1126/science.8342039. [DOI] [PubMed] [Google Scholar]
- Ruettinger R. T., Wen L. P., Fulco A. J. Coding nucleotide, 5' regulatory, and deduced amino acid sequences of P-450BM-3, a single peptide cytochrome P-450:NADPH-P-450 reductase from Bacillus megaterium. J Biol Chem. 1989 Jul 5;264(19):10987–10995. [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligar S. G., Debrunner P. G., Lipscomb J. D., Namtvedt M. J., Gunsalus I. C. A role of the putidaredoxin COOH-terminus in P-450cam (cytochrome m) hydroxylations. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3906–3910. doi: 10.1073/pnas.71.10.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligar S. G., Filipovic D., Stayton P. S. Mutagenesis of cytochromes P450cam and b5. Methods Enzymol. 1991;206:31–49. doi: 10.1016/0076-6879(91)06074-d. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tuck S. F., Peterson J. A., Ortiz de Montellano P. R. Active site topologies of bacterial cytochromes P450101 (P450cam), P450108 (P450terp), and P450102 (P450BM-3). In situ rearrangement of their phenyl-iron complexes. J Biol Chem. 1992 Mar 15;267(8):5614–5620. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- White K. A., Marletta M. A. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992 Jul 28;31(29):6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- Zvelebil M. J., Wolf C. R., Sternberg M. J. A predicted three-dimensional structure of human cytochrome P450: implications for substrate specificity. Protein Eng. 1991 Feb;4(3):271–282. doi: 10.1093/protein/4.3.271. [DOI] [PubMed] [Google Scholar]


