Abstract
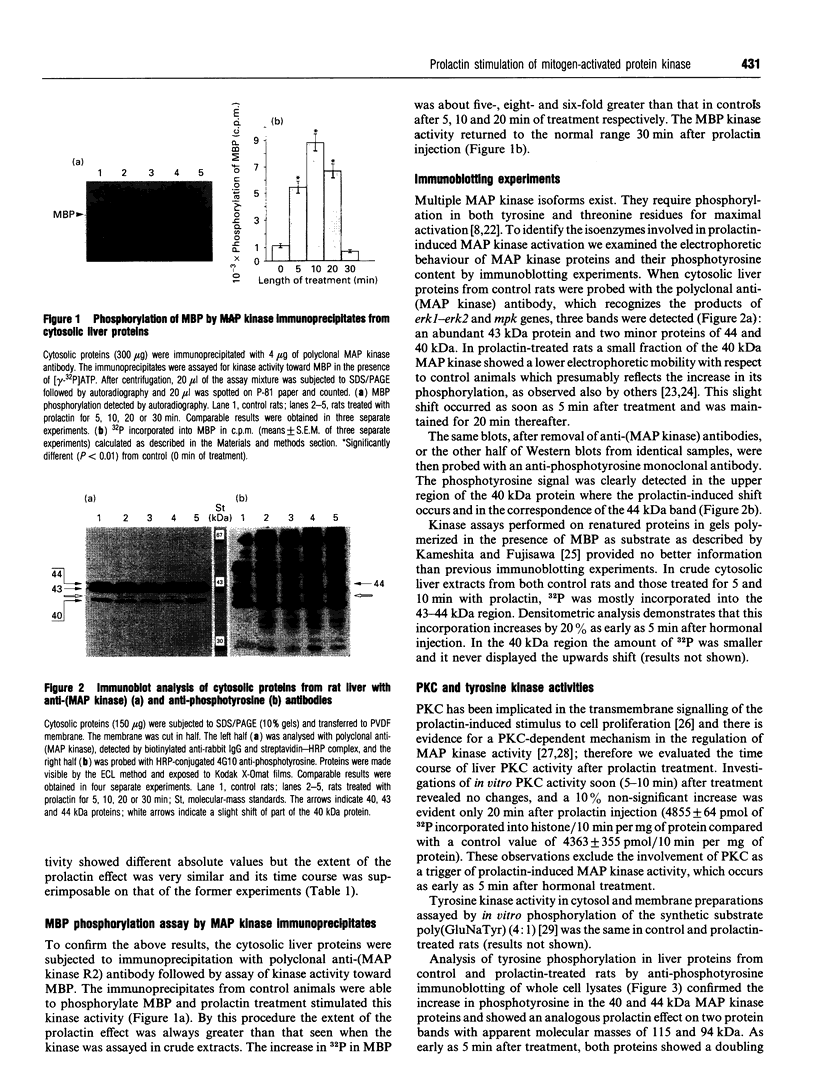
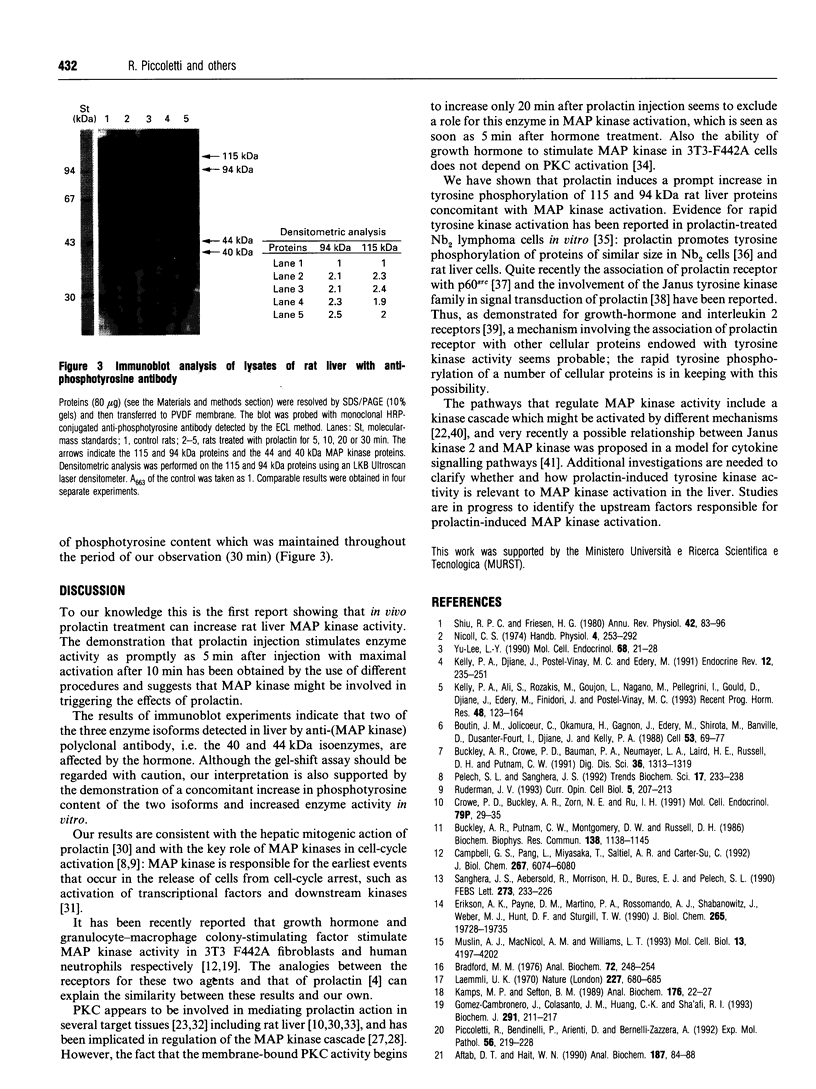
Intraperitoneal prolactin administration to female rats caused a rapid and transient stimulation of hepatic mitogen-activated kinase (MAP kinase) activity measured in vitro as cytosolic phosphotransferase capacity towards two specific substrates. Myelin basic protein kinase activity of MAP kinase immunoprecipitates confirmed the specificity and magnified the prolactin effect. Immunoblot experiments with anti-(MAP kinase) and anti-phosphotyrosine antibodies showed changes in both electrophoretic mobility and phosphotyrosine content of 40 and 44 kDa isoenzymes suggesting that prolactin affects these isoforms. Concomitant with the increase in MAP kinase activity, prolactin induced tyrosine phosphorylation in a number of liver proteins, suggesting a rapid involvement of tyrosine kinases which might be correlated in some way with MAP kinase activation. Protein kinase C activity, which has been implicated in the regulation of MAP kinase and in mediating the prolactin effect, does not seem to participate in MAP kinase activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. D., Parker P. J. TPA-induced activation of MAP kinase. FEBS Lett. 1991 Sep 23;290(1-2):77–82. doi: 10.1016/0014-5793(91)81230-6. [DOI] [PubMed] [Google Scholar]
- Aftab D. T., Hait W. N. A semiautomated 96-well plate assay for protein kinase C. Anal Biochem. 1990 May 15;187(1):84–88. doi: 10.1016/0003-2697(90)90420-e. [DOI] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin J. M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P. A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988 Apr 8;53(1):69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buckley A. R., Crowe P. D., Bauman P. A., Neumayer L. A., Laird H. E., 2nd, Russell D. H., Putnam C. W. Prolactin-provoked alterations of cytosolic, membrane, and nuclear protein kinase C following partial hepatectomy. Dig Dis Sci. 1991 Sep;36(9):1313–1319. doi: 10.1007/BF01307529. [DOI] [PubMed] [Google Scholar]
- Buckley A. R., Crowe P. D., Russell D. H. Rapid activation of protein kinase C in isolated rat liver nuclei by prolactin, a known hepatic mitogen. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8649–8653. doi: 10.1073/pnas.85.22.8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley A. R., Putnam C. W., Evans R., Laird H. E., 2nd, Shah G. N., Montgomery D. W., Russell D. H. Hepatic protein kinase C: translocation stimulated by prolactin and partial hepatectomy. Life Sci. 1987 Dec 28;41(26):2827–2834. doi: 10.1016/0024-3205(87)90429-2. [DOI] [PubMed] [Google Scholar]
- Buckley A. R., Putnam C. W., Montgomery D. W., Russell D. H. Prolactin administration stimulates rat hepatic DNA synthesis. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1138–1145. doi: 10.1016/s0006-291x(86)80401-6. [DOI] [PubMed] [Google Scholar]
- Campbell G. S., Pang L., Miyasaka T., Saltiel A. R., Carter-Su C. Stimulation by growth hormone of MAP kinase activity in 3T3-F442A fibroblasts. J Biol Chem. 1992 Mar 25;267(9):6074–6080. [PubMed] [Google Scholar]
- Crowe P. D., Buckley A. R., Zorn N. E., Rui H. Prolactin activates protein kinase C and stimulates growth-related gene expression in rat liver. Mol Cell Endocrinol. 1991 Aug;79(1-3):29–35. doi: 10.1016/0303-7207(91)90092-7. [DOI] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Dubois M. F., Bensaude O. MAP kinase activation during heat shock in quiescent and exponentially growing mammalian cells. FEBS Lett. 1993 Jun 14;324(2):191–195. doi: 10.1016/0014-5793(93)81391-c. [DOI] [PubMed] [Google Scholar]
- Erickson A. K., Payne D. M., Martino P. A., Rossomando A. J., Shabanowitz J., Weber M. J., Hunt D. F., Sturgill T. W. Identification by mass spectrometry of threonine 97 in bovine myelin basic protein as a specific phosphorylation site for mitogen-activated protein kinase. J Biol Chem. 1990 Nov 15;265(32):19728–19735. [PubMed] [Google Scholar]
- Gomez-Cambronero J., Colasanto J. M., Huang C. K., Sha'afi R. I. Direct stimulation by tyrosine phosphorylation of microtubule-associated protein (MAP) kinase activity by granulocyte-macrophage colony-stimulating factor in human neutrophils. Biochem J. 1993 Apr 1;291(Pt 1):211–217. doi: 10.1042/bj2910211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Kameshita I., Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989 Nov 15;183(1):139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Ali S., Rozakis M., Goujon L., Nagano M., Pellegrini I., Gould D., Djiane J., Edery M., Finidori J. The growth hormone/prolactin receptor family. Recent Prog Horm Res. 1993;48:123–164. doi: 10.1016/b978-0-12-571148-7.50009-9. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Djiane J., Postel-Vinay M. C., Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991 Aug;12(3):235–251. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Lee R. M., Cobb M. H., Blackshear P. J. Evidence that extracellular signal-regulated kinases are the insulin-activated Raf-1 kinase kinases. J Biol Chem. 1992 Jan 15;267(2):1088–1092. [PubMed] [Google Scholar]
- Muslin A. J., MacNicol A. M., Williams L. T. Raf-1 protein kinase is important for progesterone-induced Xenopus oocyte maturation and acts downstream of mos. Mol Cell Biol. 1993 Jul;13(7):4197–4202. doi: 10.1128/mcb.13.7.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Mumberg D., Lucibello F. C. Signals and genes in the control of cell-cycle progression. Biochim Biophys Acta. 1993 Aug 23;1155(2):151–179. doi: 10.1016/0304-419x(93)90003-u. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. Mitogen-activated protein kinases: versatile transducers for cell signaling. Trends Biochem Sci. 1992 Jun;17(6):233–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- Piccoletti R., Bendinelli P., Arienti D., Bernelli-Zazzera A. State and activity of protein kinase C in postischemic reperfused liver. Exp Mol Pathol. 1992 Jun;56(3):219–228. doi: 10.1016/0014-4800(92)90038-d. [DOI] [PubMed] [Google Scholar]
- Rijksen G., Van Oirschot B. A., Staal G. E. Nonradioactive assays of protein-tyrosine kinase activity using anti-phosphotyrosine antibodies. Methods Enzymol. 1991;200:98–107. doi: 10.1016/0076-6879(91)00130-o. [DOI] [PubMed] [Google Scholar]
- Rillema J. A., Campbell G. S., Lawson D. M., Carter-Su C. Evidence for a rapid stimulation of tyrosine kinase activity by prolactin in Nb2 rat lymphoma cells. Endocrinology. 1992 Aug;131(2):973–975. doi: 10.1210/endo.131.2.1639035. [DOI] [PubMed] [Google Scholar]
- Rillema J. A. Prolactin stimulation of ornithine decarboxylase activity in the mammary gland may involve an activation of protein kinase C. Proc Soc Exp Biol Med. 1985 Mar;178(3):490–494. doi: 10.3181/00379727-178-rc2. [DOI] [PubMed] [Google Scholar]
- Rillema J. A., Waters S. B., Tarrant T. M. Studies on the possible role of protein kinase C in the prolactin regulation of cell replication in Nb2 node lymphoma cells. Proc Soc Exp Biol Med. 1989 Nov;192(2):140–144. doi: 10.3181/00379727-192-42968. [DOI] [PubMed] [Google Scholar]
- Ruderman J. V. MAP kinase and the activation of quiescent cells. Curr Opin Cell Biol. 1993 Apr;5(2):207–213. doi: 10.1016/0955-0674(93)90104-x. [DOI] [PubMed] [Google Scholar]
- Rui H., Djeu J. Y., Evans G. A., Kelly P. A., Farrar W. L. Prolactin receptor triggering. Evidence for rapid tyrosine kinase activation. J Biol Chem. 1992 Nov 25;267(33):24076–24081. [PubMed] [Google Scholar]
- Sanghera J. S., Aebersold R., Morrison H. D., Bures E. J., Pelech S. L. Identification of the sites in myelin basic protein that are phosphorylated by meiosis-activated protein kinase p44mpk. FEBS Lett. 1990 Oct 29;273(1-2):223–226. doi: 10.1016/0014-5793(90)81090-b. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Mechanism of action of prolactin in the control of mammary gland function. Annu Rev Physiol. 1980;42:83–96. doi: 10.1146/annurev.ph.42.030180.000503. [DOI] [PubMed] [Google Scholar]
- Winston L. A., Bertics P. J. Growth hormone stimulates the tyrosine phosphorylation of 42- and 45-kDa ERK-related proteins. J Biol Chem. 1992 Mar 5;267(7):4747–4751. [PubMed] [Google Scholar]
- Yu-Lee L. Y. Prolactin stimulates transcription of growth-related genes in Nb2 T lymphoma cells. Mol Cell Endocrinol. 1990 Jan 2;68(1):21–28. doi: 10.1016/0303-7207(90)90165-5. [DOI] [PubMed] [Google Scholar]





