Abstract
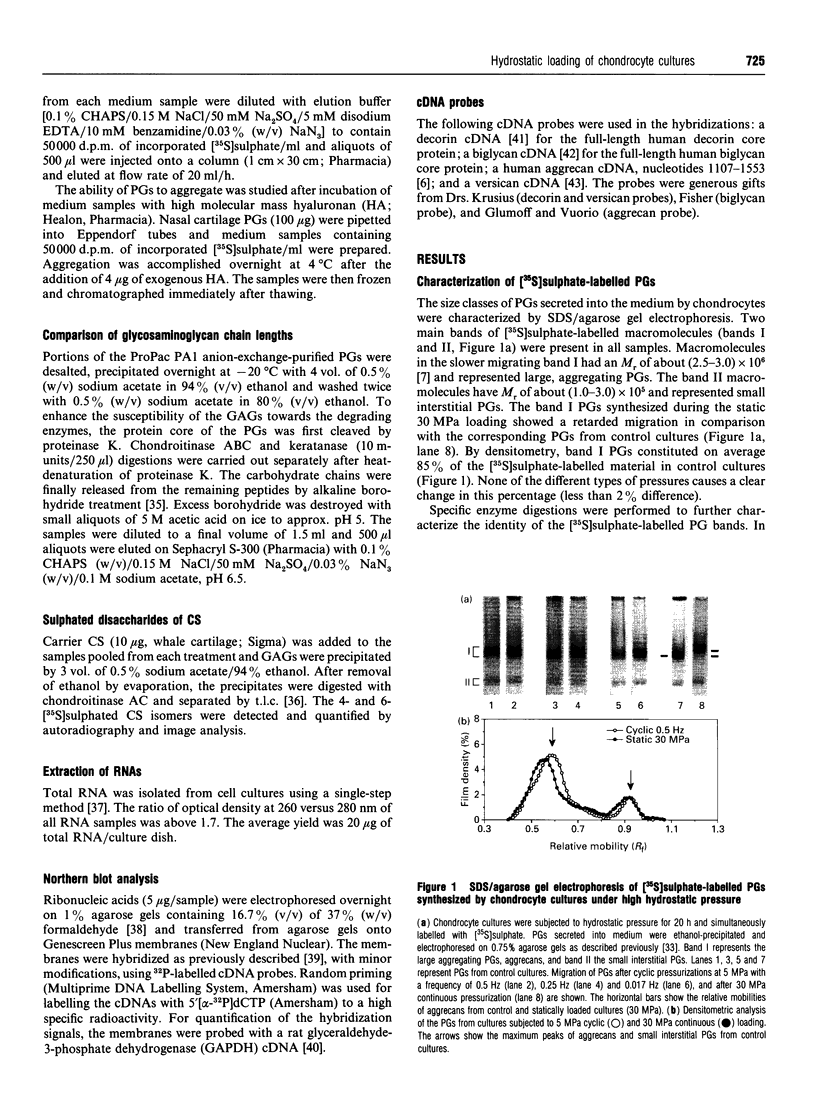
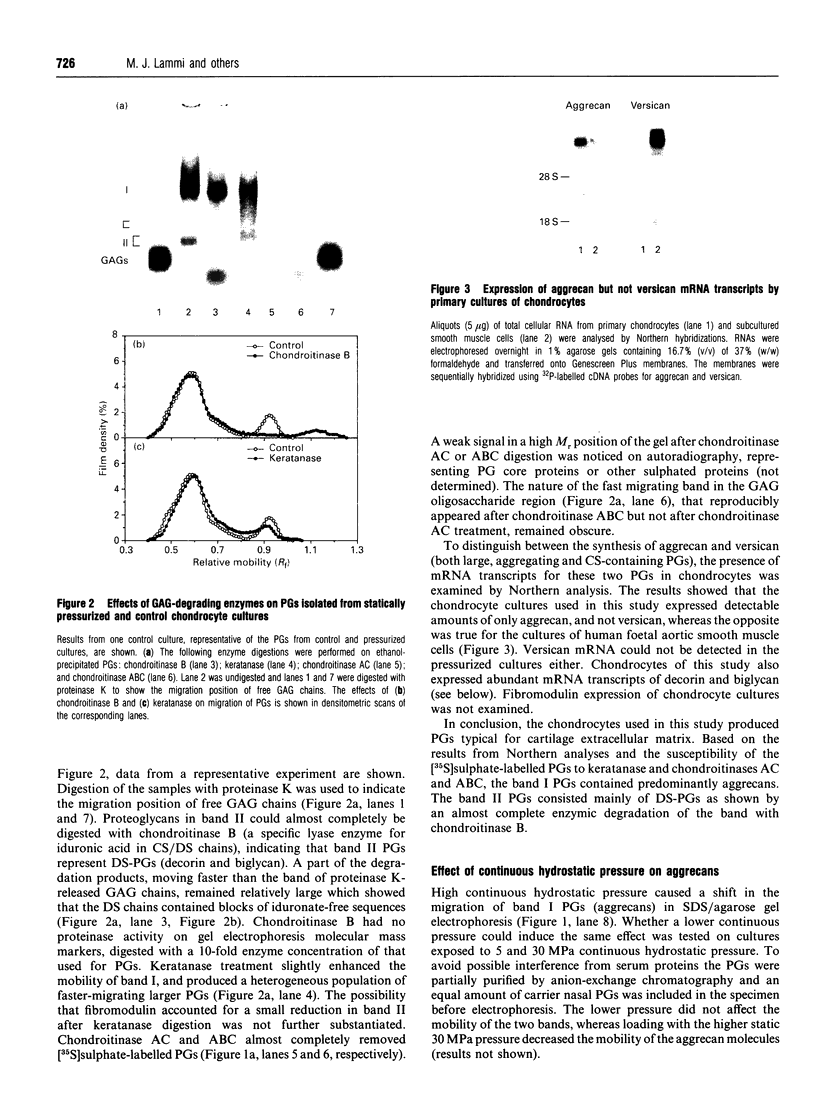
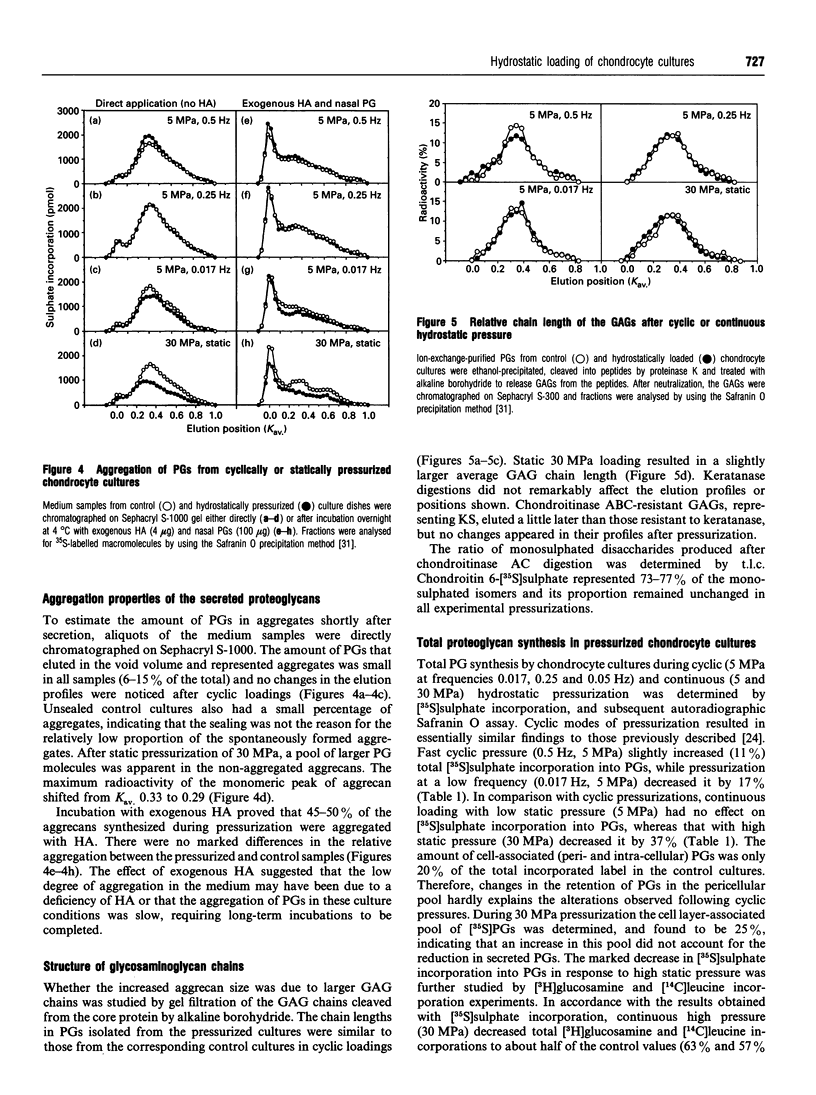
The effect of hydrostatic pressure on proteoglycan (PG) metabolism of chondrocyte cultures was examined using a specially designed test chamber. Primary cultures of bovine articular chondrocytes at confluence were exposed for 20 h to 5 and 30 MPa continuous hydrostatic pressures and 5 MPa hydrostatic pulses (0.017, 0.25 and 0.5 Hz) in the presence of [35S]sulphate. Northern blot analyses showed that chondrocyte cultures used in this study expressed abundant mRNA transcripts of aggrecan, typical of chondrocytes, but not versican. The cultures also expressed biglycan and decorin. Enzymic digestions with keratanase and chondroitinases AC, ABC and B and subsequent SDS/agarose gel electrophoresis confirmed the synthesis of aggrecans and small dermatan sulphate PGs. The continuous 30 MPa pressure reduced total PG synthesis by 37% as measured by [35S]sulphate incorporation, in contrast to the 5 MPa continuous pressure which had no effect. The high static pressure also reduced total [3H]glucosamine incorporation by 63% and total [14C]leucine incorporation by 57%. The cyclic pressures showed a frequency-dependent stimulation (0.5 Hz, 11%) or inhibition (0.017 Hz, -17%) of [35S]sulphate incorporation. Aggrecans secreted under continuous 30 MPa pressure showed a retarded migration in 0.75% SDS/agarose gel electrophoresis and they also eluted earlier on Sephacryl S-1000 gel filtration, indicative of a larger molecular size. The increased size was consistent with an increase of average glycosaminoglycan chain length as determined by Sephacryl S-300 gel filtration. No change in aggrecan size was observed with the lower (5 MPa) static or cyclic pressures. Continuous 30 MPa hydrostatic pressure slightly reduced the steady-state mRNA level of aggrecan, in parallel with the decline in PG synthesis measured by [35S]sulphate incorporation. The results demonstrated that high hydrostatic pressure could influence the synthesis of PGs, especially of aggrecans, in chondrocytes both at the transcriptional and translational/post-translational levels.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi T., Larson D. E., Sells B. H. Cytoskeletal association of muscle-specific mRNAs in differentiating L6 rat myoblasts. Exp Cell Res. 1987 Jan;168(1):160–172. doi: 10.1016/0014-4827(87)90425-3. [DOI] [PubMed] [Google Scholar]
- Baker J. R., Caterson B. The isolation and characterization of the link proteins from proteoglycan aggregates of bovine nasal cartilage. J Biol Chem. 1979 Apr 10;254(7):2387–2393. [PubMed] [Google Scholar]
- Begg D. A., Salmon E. D., Hyatt H. A. The changes in structural organization of actin in the sea urchin egg cortex in response to hydrostatic pressure. J Cell Biol. 1983 Dec;97(6):1795–1805. doi: 10.1083/jcb.97.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F., Périn J. P., Jollès P. Isolation and chemical characterization of two distinct "link proteins" from bovine nasal cartilage proteoglycan complex. Biochim Biophys Acta. 1978 Feb 15;532(2):242–248. doi: 10.1016/0005-2795(78)90578-0. [DOI] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curtis A. J., Devenish R. J., Handley C. J. Modulation of aggrecan and link-protein synthesis in articular cartilage. Biochem J. 1992 Dec 15;288(Pt 3):721–726. doi: 10.1042/bj2880721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Witt M. T., Handley C. J., Oakes B. W., Lowther D. A. In vitro response of chondrocytes to mechanical loading. The effect of short term mechanical tension. Connect Tissue Res. 1984;12(2):97–109. doi: 10.3109/03008208408992775. [DOI] [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E. H., Grodzinsky A. J., Koob T. J., Eyre D. R. Streaming potentials: a sensitive index of enzymatic degradation in articular cartilage. J Orthop Res. 1987;5(4):497–508. doi: 10.1002/jor.1100050405. [DOI] [PubMed] [Google Scholar]
- Gray M. L., Pizzanelli A. M., Grodzinsky A. J., Lee R. C. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6(6):777–792. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- Hall A. C., Urban J. P., Gehl K. A. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res. 1991 Jan;9(1):1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Jones I. L., Klämfeldt A., Sandström T. The effect of continuous mechanical pressure upon the turnover of articular cartilage proteoglycans in vitro. Clin Orthop Relat Res. 1982 May;(165):283–289. [PubMed] [Google Scholar]
- Järveläinen H. T., Kinsella M. G., Wight T. N., Sandell L. J. Differential expression of small chondroitin/dermatan sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J Biol Chem. 1991 Dec 5;266(34):23274–23281. [PubMed] [Google Scholar]
- Kajiwara T., Tanzer M. L. Undersulfated proteoglycans are induced by the ionophore monensin: study of possible mechanisms. Arch Biochem Biophys. 1982 Mar;214(1):51–55. doi: 10.1016/0003-9861(82)90007-8. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Microtubules, membrane traffic, and cell organization. Cell. 1990 Apr 6;61(1):5–7. doi: 10.1016/0092-8674(90)90206-t. [DOI] [PubMed] [Google Scholar]
- Koob T. J., Clark P. E., Hernandez D. J., Thurmond F. A., Vogel K. G. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch Biochem Biophys. 1992 Oct;298(1):303–312. doi: 10.1016/0003-9861(92)90127-i. [DOI] [PubMed] [Google Scholar]
- Korver T. H., van de Stadt R. J., Kiljan E., van Kampen G. P., van der Korst J. K. Effects of loading on the synthesis of proteoglycans in different layers of anatomically intact articular cartilage in vitro. J Rheumatol. 1992 Jun;19(6):905–912. [PubMed] [Google Scholar]
- Krusius T., Gehlsen K. R., Ruoslahti E. A fibroblast chondroitin sulfate proteoglycan core protein contains lectin-like and growth factor-like sequences. J Biol Chem. 1987 Sep 25;262(27):13120–13125. [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafeber F., Veldhuijzen J. P., Vanroy J. L., Huber-Bruning O., Bijlsma J. W. Intermittent hydrostatic compressive force stimulates exclusively the proteoglycan synthesis of osteoarthritic human cartilage. Br J Rheumatol. 1992 Jul;31(7):437–442. doi: 10.1093/rheumatology/31.7.437. [DOI] [PubMed] [Google Scholar]
- Lammi M. J., Häkkinen T. P., Parkkinen J. J., Hyttinen M. M., Jortikka M., Helminen H. J., Tammi M. I. Adaptation of canine femoral head articular cartilage to long distance running exercise in young beagles. Ann Rheum Dis. 1993 May;52(5):369–377. doi: 10.1136/ard.52.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammi M. J., Tammi M. Autoradiographic quantitation of radiolabeled proteoglycans. J Biochem Biophys Methods. 1991 May-Jun;22(4):301–310. doi: 10.1016/0165-022x(91)90036-v. [DOI] [PubMed] [Google Scholar]
- Lammi M., Tammi M. Densitometric assay of nanogram quantities of proteoglycans precipitated on nitrocellulose membrane with Safranin O. Anal Biochem. 1988 Feb 1;168(2):352–357. doi: 10.1016/0003-2697(88)90329-6. [DOI] [PubMed] [Google Scholar]
- Larsson T., Aspden R. M., Heinegård D. Effects of mechanical load on cartilage matrix biosynthesis in vitro. Matrix. 1991 Dec;11(6):388–394. doi: 10.1016/s0934-8832(11)80193-9. [DOI] [PubMed] [Google Scholar]
- Lee R. C., Rich J. B., Kelley K. M., Weiman D. S., Mathews M. B. A comparison of in vitro cellular responses to mechanical and electrical stimulation. Am Surg. 1982 Nov;48(11):567–574. [PubMed] [Google Scholar]
- Lippiello L., Kaye C., Neumata T., Mankin H. J. In vitro metabolic response of articular cartilage segments to low levels of hydrostatic pressure. Connect Tissue Res. 1985;13(2):99–107. doi: 10.3109/03008208509152388. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Shinomura T., Hascall V. C., Kimura J. H. Xylosyl transfer to the core protein precursor of the rat chondrosarcoma proteoglycan. J Biol Chem. 1989 Nov 5;264(31):18775–18780. [PubMed] [Google Scholar]
- Lohmander S., Moskalewski S., Madsen K., Thyberg J., Friberg U. Influence of colchicine on the synthesis and secretion of proteoglycans and collagen by fetal guinea pig chondrocytes. Exp Cell Res. 1976 May;99(2):333–345. doi: 10.1016/0014-4827(76)90591-7. [DOI] [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Robinson H. C., Ng K., Tzaicos C., Brooks P. R., Lowther D. A. The relation of protein synthesis to chondroitin sulphate biosynthesis in cultured bovine cartilage. Biochem J. 1984 Dec 15;224(3):977–988. doi: 10.1042/bj2240977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D., Hardingham T. Monensin inhibits synthesis of proteoglycan, but not of hyaluronate, in chondrocytes. Biochem J. 1982 Jan 15;202(1):249–254. doi: 10.1042/bj2020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D., Hardingham T. The effects of cycloheximide on the biosynthesis and secretion of proteoglycans by chondrocytes in culture. Biochem J. 1981 May 15;196(2):521–529. doi: 10.1042/bj1960521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Lindblom K., Heinegård D. A collagen-binding 59-kd protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG-S1 and PG-S2 (decorin). EMBO J. 1989 Sep;8(9):2601–2604. doi: 10.1002/j.1460-2075.1989.tb08399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Effects of static and cyclic compressive loading on articular cartilage plugs in vitro. Arthritis Rheum. 1984 Jun;27(6):675–681. doi: 10.1002/art.1780270611. [DOI] [PubMed] [Google Scholar]
- Parkkinen J. J., Ikonen J., Lammi M. J., Laakkonen J., Tammi M., Helminen H. J. Effects of cyclic hydrostatic pressure on proteoglycan synthesis in cultured chondrocytes and articular cartilage explants. Arch Biochem Biophys. 1993 Jan;300(1):458–465. doi: 10.1006/abbi.1993.1062. [DOI] [PubMed] [Google Scholar]
- Parkkinen J. J., Lammi M. J., Helminen H. J., Tammi M. Local stimulation of proteoglycan synthesis in articular cartilage explants by dynamic compression in vitro. J Orthop Res. 1992 Sep;10(5):610–620. doi: 10.1002/jor.1100100503. [DOI] [PubMed] [Google Scholar]
- Parkkinen J. J., Lammi M. J., Pelttari A., Helminen H. J., Tammi M., Virtanen I. Altered Golgi apparatus in hydrostatically loaded articular cartilage chondrocytes. Ann Rheum Dis. 1993 Mar;52(3):192–198. doi: 10.1136/ard.52.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaas A. H., Ison A. L., Ackland J. Synthesis of small proteoglycans substituted with keratan sulfate by rabbit articular chondrocytes. J Biol Chem. 1989 Aug 25;264(24):14447–14454. [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Rönnemaa T., Doherty N. S. Effect of serum and liver extracts from hypercholesterolemic rats on the synthesis of collagen by isolated aortas and cultured aortic smooth muscle cells. Atherosclerosis. 1977 Mar;26(3):261–272. doi: 10.1016/0021-9150(77)90079-x. [DOI] [PubMed] [Google Scholar]
- Sah R. L., Kim Y. J., Doong J. Y., Grodzinsky A. J., Plaas A. H., Sandy J. D. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7(5):619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- Salmon E. D. Pressure-induced depolymerization of brain microtubules in vitro. Science. 1975 Sep 12;189(4206):884–886. doi: 10.1126/science.1171523. [DOI] [PubMed] [Google Scholar]
- Salmon E. D. Pressure-induced depolymerization of spindle microtubules. II. Thermodynamics of in vivo spindle assembly. J Cell Biol. 1975 Jul;66(1):114–127. doi: 10.1083/jcb.66.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman R., Keret D., Maroudas A. Effects of mechanical and osmotic pressure on the rate of glycosaminoglycan synthesis in the human adult femoral head cartilage: an in vitro study. J Orthop Res. 1986;4(4):393–408. doi: 10.1002/jor.1100040402. [DOI] [PubMed] [Google Scholar]
- Symington A. L., Zimmerman S., Stein J., Stein G., Zimmerman A. M. Hydrostatic pressure influences histone mRNA. J Cell Sci. 1991 Jan;98(Pt 1):123–129. doi: 10.1242/jcs.98.1.123. [DOI] [PubMed] [Google Scholar]
- Sämänen A. M., Tammi M., Jurvelin J., Kiviranta I., Helminen H. J. Proteoglycan alterations following immobilization and remobilization in the articular cartilage of young canine knee (stifle) joint. J Orthop Res. 1990 Nov;8(6):863–873. doi: 10.1002/jor.1100080612. [DOI] [PubMed] [Google Scholar]
- Tesch G. H., Handley C. J., Cornell H. J., Herington A. C. Effects of free and bound insulin-like growth factors on proteoglycan metabolism in articular cartilage explants. J Orthop Res. 1992 Jan;10(1):14–22. doi: 10.1002/jor.1100100103. [DOI] [PubMed] [Google Scholar]
- Uchida A., Yamashita K., Hashimoto K., Shimomura Y. The effect of mechanical stress on cultured growth cartilage cells. Connect Tissue Res. 1988;17(4):305–311. doi: 10.3109/03008208809017480. [DOI] [PubMed] [Google Scholar]
- Wright M. O., Stockwell R. A., Nuki G. Response of plasma membrane to applied hydrostatic pressure in chondrocytes and fibroblasts. Connect Tissue Res. 1992;28(1-2):49–70. doi: 10.3109/03008209209014227. [DOI] [PubMed] [Google Scholar]
- Zambetti G., Schmidt W., Stein G., Stein J. Subcellular localization of histone messenger RNAs on cytoskeleton-associated free polysomes in HeLa S3 cells. J Cell Physiol. 1985 Nov;125(2):345–353. doi: 10.1002/jcp.1041250225. [DOI] [PubMed] [Google Scholar]
- van Kampen G. P., Veldhuijzen J. P., Kuijer R., van de Stadt R. J., Schipper C. A. Cartilage response to mechanical force in high-density chondrocyte cultures. Arthritis Rheum. 1985 Apr;28(4):419–424. doi: 10.1002/art.1780280410. [DOI] [PubMed] [Google Scholar]






