Abstract
1. Suspensions of cultured Ehrlich-Lettre tumour cells were loaded with the pH-sensitive fluorescent indicator 2',7'-bis(carboxyethyl)-5(6)-carboxyfluorescein (BCECF), and changes in intracellular pH upon addition of L-lactate and other monocarboxylates were continuously monitored by fluorimetry using dual-wavelength excitation (450/500 nm) and single-wavelength emission (> 520 nm). 2. The rapid fluorescence changes were analysed by first-order regression analysis, and with suitable calibration procedures this enabled calculation of initial rates of proton uptake associated with monocarboxylate transport. 3. The stoichiometry was shown to be one proton per lactate molecule transported. 4. The kinetics of carrier-mediated transport of a wide range of monocarboxylates were determined at 25 degrees C. The Km values for L-lactate, pyruvate and D-lactate were found to be 4.54, 0.72 and 27.5 mM respectively, similar to values found previously for rat erythrocytes. This similarity was shared with a wide range of variously substituted C2, C3 and C4 monocarboxylates, all of which were transported with similar Vmax. No stereoselectivity was found in the Km values for D- and L-2-chloropropionate (0.75 mM) or D- and L-3-hydroxybutyrate (11 mM), but in the latter case the Vmax. of the D-isomer was twice that of the L-isomer. 5. The temperature-dependence of L-lactate transport demonstrated a transition point, with activation energies of 60 and 109 kJ.mol-1 above and below 19 degrees C respectively The Km for L-lactate below the transition temperature was about half that above it. 6. Inhibition of lactate transport into tumour cells by a wide range of compounds known to inhibit the erythrocyte monocarboxylate carrier was analysed. Patterns of inhibition were similar to those seen in the erythrocyte, but the Ki values were 2-4-fold higher in the tumour cells. 7. It is concluded that tumour cells contain an isoform of the monocarboxylate carrier with functional properties almost identical with that found in erythrocytes. This is probably identical with MCT1, which was recently cloned and sequenced from Chinese Hamster Ovary cells [Kim Garcia, Goldstein, Pathak, Anderson and Brown (1994) Cell 76, 865-873].
Full text
PDF

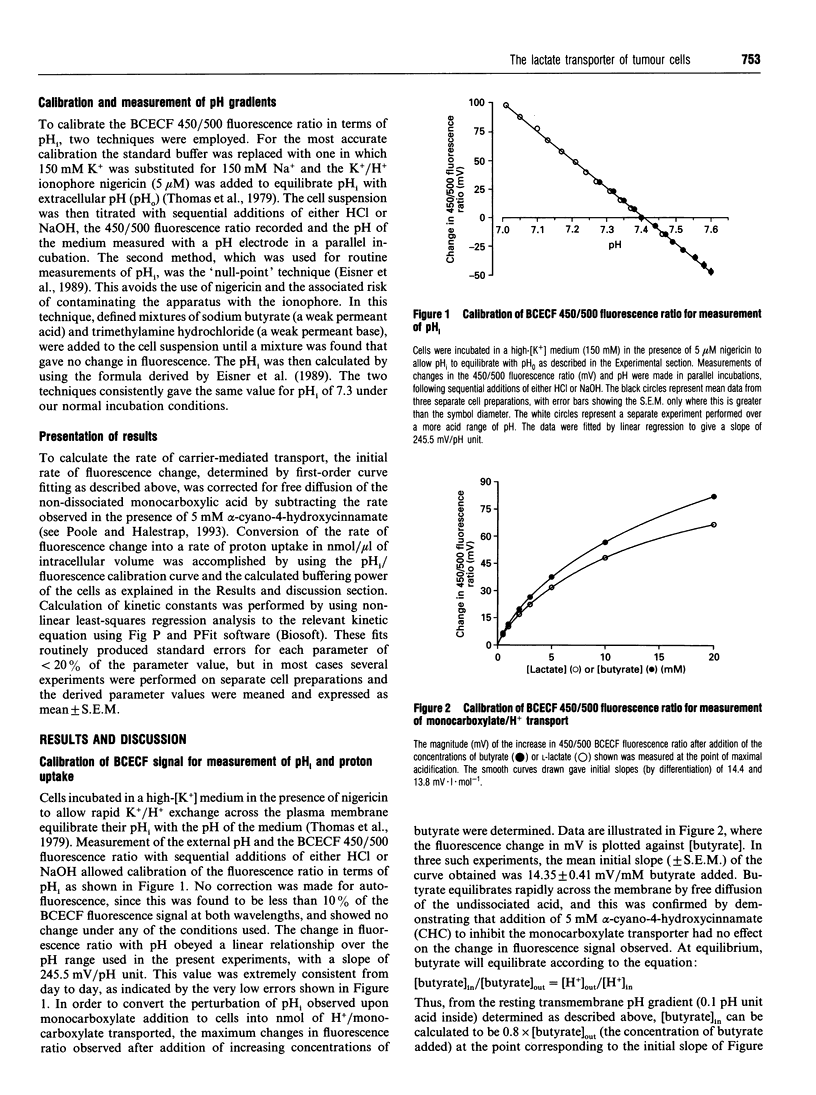
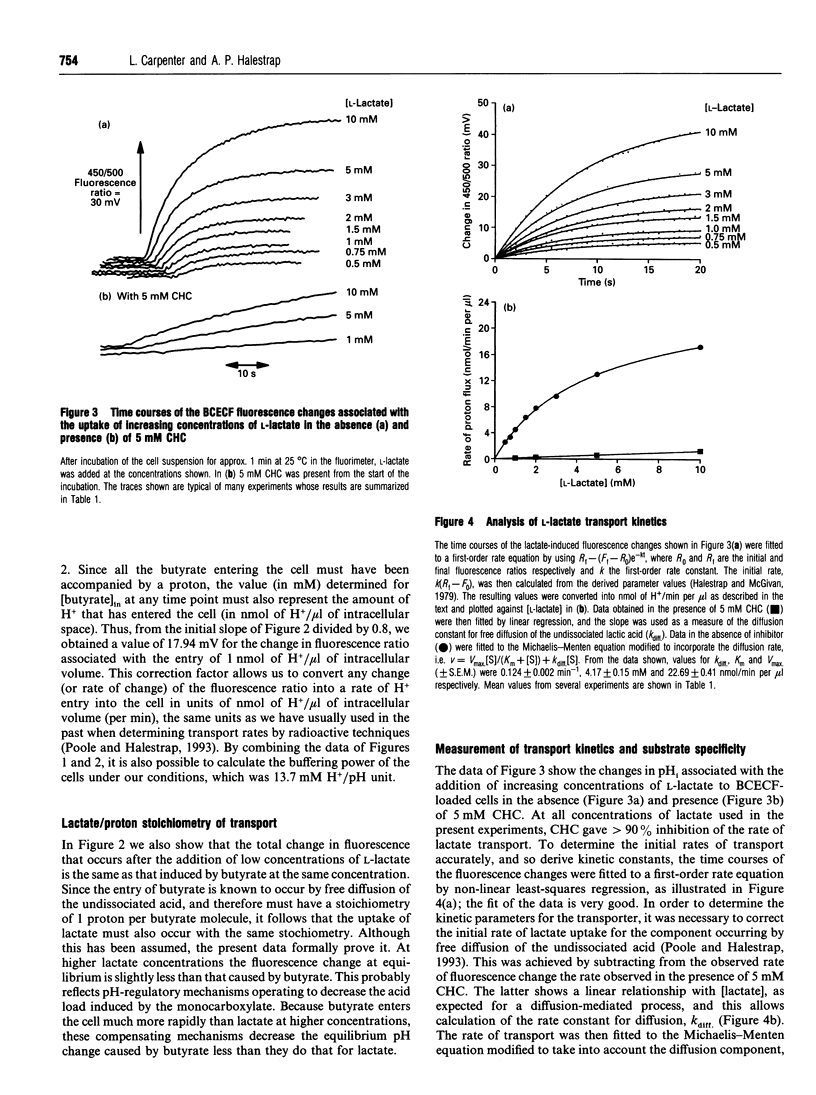






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. L., Tarpley H. T., Regen D. M. Characterization of beta-hydroxybutyrate transport in rat erythrocytes and thymocytes. Biochim Biophys Acta. 1978 Apr 20;508(3):525–538. doi: 10.1016/0005-2736(78)90097-4. [DOI] [PubMed] [Google Scholar]
- Baur H., Heldt H. W. Transport of hexoses across the liver-cell membrane. Eur J Biochem. 1977 Apr 1;74(2):397–403. doi: 10.1111/j.1432-1033.1977.tb11404.x. [DOI] [PubMed] [Google Scholar]
- Belt J. A., Thomas J. A., Buchsbaum R. N., Racker E. Inhibition of lactate transport and glycolysis in Ehrlich ascites tumor cells by bioflavonoids. Biochemistry. 1979 Aug 7;18(16):3506–3511. doi: 10.1021/bi00583a011. [DOI] [PubMed] [Google Scholar]
- Best L., Trebilcock R., Tomlinson S. Lactate transport in insulin-secreting beta-cells: contrast between rat islets and HIT-T15 insulinoma cells. Mol Cell Endocrinol. 1992 Jul;86(1-2):49–56. doi: 10.1016/0303-7207(92)90174-5. [DOI] [PubMed] [Google Scholar]
- Bonanno J. A. Lactate-proton cotransport in rabbit corneal epithelium. Curr Eye Res. 1990 Jul;9(7):707–712. doi: 10.3109/02713689008999587. [DOI] [PubMed] [Google Scholar]
- Cairns S. P., Westerblad H., Allen D. G. Changes in myoplasmic pH and calcium concentration during exposure to lactate in isolated rat ventricular myocytes. J Physiol. 1993 May;464:561–574. doi: 10.1113/jphysiol.1993.sp019651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuticke B., Beyer E., Forst B. Discrimination of three parallel pathways of lactate transport in the human erythrocyte membrane by inhibitors and kinetic properties. Biochim Biophys Acta. 1982 Jan 4;684(1):96–110. doi: 10.1016/0005-2736(82)90053-0. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Monocarboxylate transport in erythrocytes. J Membr Biol. 1982;70(2):89–103. doi: 10.1007/BF01870219. [DOI] [PubMed] [Google Scholar]
- Deuticke B., Rickert I., Beyer E. Stereoselective, SH-dependent transfer of lactate in mammalian erythrocytes. Biochim Biophys Acta. 1978 Feb 2;507(1):137–155. doi: 10.1016/0005-2736(78)90381-4. [DOI] [PubMed] [Google Scholar]
- Devés R., Krupka R. M. A simple test for the sidedness of binding of transport inhibitors. Biochim Biophys Acta. 1990 Nov 30;1030(1):24–31. doi: 10.1016/0005-2736(90)90234-f. [DOI] [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Inhibition kinetics of carrier systems. Methods Enzymol. 1989;171:113–132. doi: 10.1016/s0076-6879(89)71008-9. [DOI] [PubMed] [Google Scholar]
- Edlund G. L., Halestrap A. P. The kinetics of transport of lactate and pyruvate into rat hepatocytes. Evidence for the presence of a specific carrier similar to that in erythrocytes. Biochem J. 1988 Jan 1;249(1):117–126. doi: 10.1042/bj2490117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Kenning N. A., O'Neill S. C., Pocock G., Richards C. D., Valdeolmillos M. A novel method for absolute calibration of intracellular pH indicators. Pflugers Arch. 1989 Mar;413(5):553–558. doi: 10.1007/BF00594188. [DOI] [PubMed] [Google Scholar]
- Garcia C. K., Goldstein J. L., Pathak R. K., Anderson R. G., Brown M. S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994 Mar 11;76(5):865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- González-Mateos F., Gómez M. E., García-Salguero L., Sánchez V., Aragón J. J. Inhibition of glycolysis by amino acids in ascites tumor cells. Specificity and mechanism. J Biol Chem. 1993 Apr 15;268(11):7809–7817. [PubMed] [Google Scholar]
- Grunze M., Forst B., Deuticke B. Dual effect of membrane cholesterol on simple and mediated transport processes in human erythrocytes. Biochim Biophys Acta. 1980 Aug 14;600(3):860–869. doi: 10.1016/0005-2736(80)90489-7. [DOI] [PubMed] [Google Scholar]
- Halder J., Ray M., Ray S. Inhibition of glycolysis and mitochondrial respiration of Ehrlich ascites carcinoma cells by methylglyoxal. Int J Cancer. 1993 May 28;54(3):443–449. doi: 10.1002/ijc.2910540315. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Brand M. D., Denton R. M. Inhibition of mitochondrial pyruvate transport by phenylpyruvate and alpha-ketoisocaproate. Biochim Biophys Acta. 1974 Oct 10;367(1):102–108. doi: 10.1016/0005-2736(74)90140-0. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974 Feb;138(2):313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J. 1976 May 15;156(2):193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. H., Belt J. A., Dubinsky W. P., Zimniak A., Racker E. Inhibition of lactate transport in Ehrlich ascites tumor cells and human erythrocytes by a synthetic anhydride of lactic acid. Biochemistry. 1980 Aug 5;19(16):3836–3840. doi: 10.1021/bi00557a029. [DOI] [PubMed] [Google Scholar]
- Juel C. Muscle lactate transport studied in sarcolemmal giant vesicles. Biochim Biophys Acta. 1991 May 31;1065(1):15–20. doi: 10.1016/0005-2736(91)90004-r. [DOI] [PubMed] [Google Scholar]
- Lambert I. H., Hoffmann E. K., Jørgensen F. Membrane potential, anion and cation conductances in Ehrlich ascites tumor cells. J Membr Biol. 1989 Oct;111(2):113–131. doi: 10.1007/BF01871776. [DOI] [PubMed] [Google Scholar]
- Leeks D. R., Halestrap A. P. Chloride-independent transport of pyruvate and lactate across the erythrocyte membrane [proceedings]. Biochem Soc Trans. 1978;6(6):1363–1366. doi: 10.1042/bst0061363. [DOI] [PubMed] [Google Scholar]
- Lettré R., Paweletz N., Werner D., Granzow C. Sublines of the Ehrlich-Lettré mouse ascites tumour. A new tool for experimental cell research. Naturwissenschaften. 1972 Feb;59(2):59–63. doi: 10.1007/BF00593464. [DOI] [PubMed] [Google Scholar]
- McGivan J. D., Bradford N. M., Mendes-Mourão J. The transport of branched-chain amino acids into isolated rat liver cells. FEBS Lett. 1977 Aug 15;80(2):380–384. doi: 10.1016/0014-5793(77)80481-x. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L. Glycolysis, glutaminolysis and cell proliferation. Cell Biol Int Rep. 1982 Jul;6(7):635–650. doi: 10.1016/0309-1651(82)90125-4. [DOI] [PubMed] [Google Scholar]
- Nedergaard M., Goldman S. A. Carrier-mediated transport of lactic acid in cultured neurons and astrocytes. Am J Physiol. 1993 Aug;265(2 Pt 2):R282–R289. doi: 10.1152/ajpregu.1993.265.2.R282. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Ardawi M. S. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985 May;5(5):393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- Poole R. C., Cranmer S. L., Halestrap A. P., Levi A. J. Substrate and inhibitor specificity of monocarboxylate transport into heart cells and erythrocytes. Further evidence for the existence of two distinct carriers. Biochem J. 1990 Aug 1;269(3):827–829. doi: 10.1042/bj2690827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. C., Cranmer S. L., Holdup D. W., Halestrap A. P. Inhibition of L-lactate transport and band 3-mediated anion transport in erythrocytes by the novel stilbenedisulphonate N,N,N',N'-tetrabenzyl-4,4'-diaminostilbene-2,2'-disulpho nat e (TBenzDS). Biochim Biophys Acta. 1991 Nov 18;1070(1):69–76. doi: 10.1016/0005-2736(91)90147-z. [DOI] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. N-terminal protein sequence analysis of the rabbit erythrocyte lactate transporter suggests identity with the cloned monocarboxylate transport protein MCT1. Biochem J. 1994 Nov 1;303(Pt 3):755–759. doi: 10.1042/bj3030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P., Price S. J., Levi A. J. The kinetics of transport of lactate and pyruvate into isolated cardiac myocytes from guinea pig. Kinetic evidence for the presence of a carrier distinct from that in erythrocytes and hepatocytes. Biochem J. 1989 Dec 1;264(2):409–418. doi: 10.1042/bj2640409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. Reconstitution of the L-lactate carrier from rat and rabbit erythrocyte plasma membranes. Biochem J. 1988 Sep 1;254(2):385–390. doi: 10.1042/bj2540385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. Reversible and irreversible inhibition, by stilbenedisulphonates, of lactate transport into rat erythrocytes. Identification of some new high-affinity inhibitors. Biochem J. 1991 Apr 15;275(Pt 2):307–312. doi: 10.1042/bj2750307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993 Apr;264(4 Pt 1):C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Regen D. M., Tarpley H. L. Effects of pH on beta-hydroxybutyrate transport in rat erythrocytes and thymocytes. Biochim Biophys Acta. 1978 Apr 20;508(3):539–550. doi: 10.1016/0005-2736(78)90098-6. [DOI] [PubMed] [Google Scholar]
- Roth D. A., Brooks G. A. Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys. 1990 Jun;279(2):377–385. doi: 10.1016/0003-9861(90)90505-s. [DOI] [PubMed] [Google Scholar]
- Sekine N., Cirulli V., Regazzi R., Brown L. J., Gine E., Tamarit-Rodriguez J., Girotti M., Marie S., MacDonald M. J., Wollheim C. B. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem. 1994 Feb 18;269(7):4895–4902. [PubMed] [Google Scholar]
- Spencer T. L., Lehninger A. L. L-lactate transport in Ehrlich ascites-tumour cells. Biochem J. 1976 Feb 15;154(2):405–414. doi: 10.1042/bj1540405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Tienhaara R., Meany J. E. The lactate dehydrogenase catalyzed reduction of pyruvate. Active substrate and substrate inhibition. Biochemistry. 1973 May 22;12(11):2067–2070. doi: 10.1021/bi00735a007. [DOI] [PubMed] [Google Scholar]
- Wang X., Poole R. C., Halestrap A. P., Levi A. J. Characterization of the inhibition by stilbene disulphonates and phloretin of lactate and pyruvate transport into rat and guinea-pig cardiac myocytes suggests the presence of two kinetically distinct carriers in heart cells. Biochem J. 1993 Feb 15;290(Pt 1):249–258. doi: 10.1042/bj2900249. [DOI] [PMC free article] [PubMed] [Google Scholar]


