ABSTRACT
Background:
Vectorial transmission through hematophagous triatomine insects remains the primary mode of Chagas Disease contagion. These insects have become increasingly common in urban environments. Therefore, this study aimed to report an encounter of triatomines with trypanosomatid infection in a vertical residential condominium in Rio Branco, the capital of the state of Acre, in the western Brazilian Amazon.
Methods:
Triatomines were collected from residents and sent to the municipality's Entomological Surveillance sector. Trypanosomatid positivity was evaluated using optical microscopy, followed by species and genotype identification using molecular biology techniques.
Results:
Twenty-five adult triatomine specimens were collected from two of three condominium buildings invading apartments from the 2nd to 13th floors. Six specimens were identified as Rhodnius sp. and 19 as R. montenegrensis. Among these, molecular tests were conducted on seven specimens, with five testing positive for Trypanosoma cruzi, all belonging to genotype TcI.
Conclusions:
These findings underscore the need for further studies to better understand the invasive capacity of these insects in these environments and the mechanisms involved in this process.
Keywords: Chagas disease, Vectors, Amazon
INTRODUCTION
Chagas disease (CD), or American trypanosomiasis, is considered one of the most important public health issues in Latin America, especially in Brazil, a country estimated to have one million individuals infected with Trypanosoma cruzi, the etiological agent of this disease 1 , 2 . This parasite is genetically diverse, grouped into six genotypes, known as Discrete Typing Units (DTUs). Each of these T. cruzi strains may lead to different clinical manifestations depending on the location and the infected host 3 , 4 .
In Brazil, the Amazon region stands out in cases of this disease, where the predominant transmission routes are oral, through the ingestion of food contaminated with T. cruzi, and vector-borne, through the feces of infected hematophagous triatomine bugs 1 , 5 , 6 . Triatomine bugs belong to the family Reduviidae and subfamily Triatominae, with the latter currently comprising 18 genera and 159 described species, all of which (with the exception of three fossils) have the potential to transmitting T. cruzi 7 - 9 .
In the Brazilian Amazon alone, 8 genera and 22 species of triatomine bugs have been recorded, with species of the genera Rhodnius and Panstrongylus being of greater epidemiological importance in the region 8 , 10 . The distribution of triatomine species and the environmental degradation associated with the migratory flow that the Amazon Basin has been experiencing over the years have intensified the movement of these insects into areas increasingly closer to human contact, with growing reports of intradomiciliary and peridomiciliary invasions 11 - 13 . Modification of the forest landscape into a more urbanized landscape influences these invasions, especially species of the genus Rhodnius, which have already demonstrated the ability to adapt to deforested areas with palm trees near residences 14 .
This dispersal of triatomine bugs is epidemiologically significant in the transmission of Chagas disease and can occur in two ways: passively, when they are carried through objects or some host animal, and actively, through the terrestrial movement of nymphs and adults, mainly by flight of adults 15 , 16 . However, their ability to fly remains poorly understood. Some researchers have suggested in their studies that environmental factors and nutritional status influence triatomine dispersal 17 , 18 . A laboratory experiment concluded that R. brethesi initiated take-off approximately 15 days after the last blood meal, suggesting that fasting boosted its flying ability 18 . However, it has also been observed that despite favorable conditions, some species of triatomine bugs do not engage in flights 18 .
Nevertheless, this dispersal, albeit less discussed, is important for maintaining the cycle of Chagas disease transmission, considering that many triatomine bugs are naturally infected by T. cruzi and show greater vectorial infection abundance in deforested areas than in preserved forest areas 19 - 20 . In line with this, the present study aimed to record the occurrence of triatomine bugs in apartments and their infection by trypanosomatids in the municipality of Rio Branco, Acre, Brazil, and, to the best of our knowledge, the presence of triatomine bugs on the 13th floor of a residential building.
METHODS
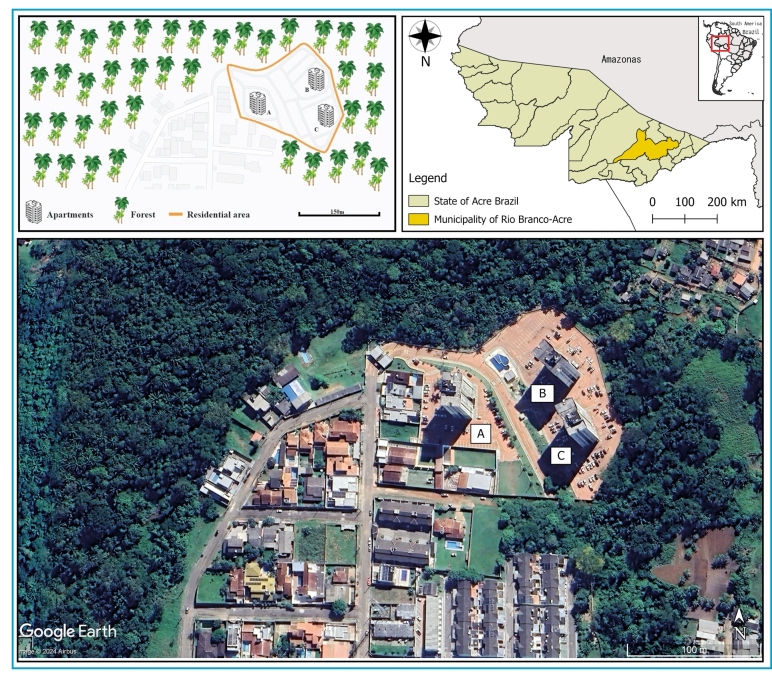
Triatomine bugs were collected in the municipality of Rio Branco, Acre, Brazil, from July 2022 to June 2023 by direct capture by apartment residents. The specimens were delivered to the Entomological Surveillance Sector of Rio Branco, Acre, Brazil. The condominium comprises three buildings A, B and C (A: 9°57'26.58"S, 67°50'40.09"W; B: 9°57'25.89"S, 67°50'40.06"W; and C: 9°57'27.35"S, 67°50'39.73"W), each with 1 ground floor plus 16 floors, each floor 2.8 m high, so the total height is 47.6 m (Figure 1). Both buildings were located near forest fragments filled with palm trees. The main balcony of Building A is at least 100 m from Buildings B and C, which are 20 m apart.
FIGURE 1: Characteristics of the residential condominium with triatomine invasion, Rio Branco, Acre, Brazil, 2023. A, B, and C represent the buildings housing the apartments.
The insects were sent in thermal boxes, kept at room temperature, to the Laboratory of Tropical Medicine (LABMEDT) at the Federal University of Acre (UFAC), where species identification was performed considering the morphological characteristics described by Lent and Wygodzinsky 21 and Rosa et al. 22 Some of the insects found in the apartments were not captured by the residents because of fear of contact with the insect; however, as the residents took photographic records, these reports were included in the study.
From the captured triatomine bugs, the contents of the rectal ampoule were extracted, diluted in saline solution (0.9%), and evaluated using optical microscopy (400x × magnification) to verify the presence of trypanosomatids. Trypanosomatid DNA was extracted from the rectal ampoules for subsequent molecular species and genotype identification.
DNA extraction followed the protocol described by Adams et al. 23 , using Digsol solution (50 mM Tris, 20 mM EDTA, 117 mM NaCl, and 1% SDS) to digest the feces and digestive tract overnight at 37°C. Subsequently, it was precipitated with ammonium acetate solution, centrifuged, and washed with 99.8% and 70% ethanol. The tubes were dried, with DNA resuspended in 40 μl of TE buffer (Tris-EDTA), and stored at -20°C.
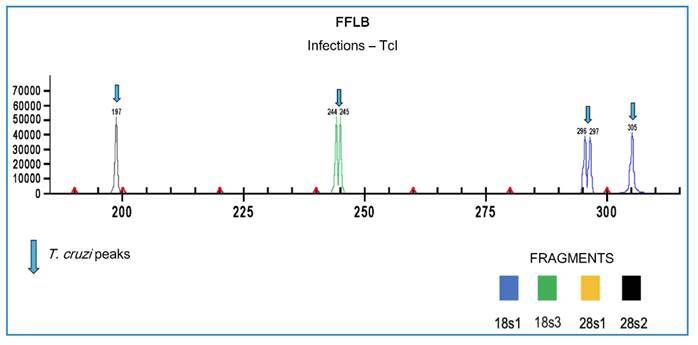
After DNA extraction, the concentration and purity of each extracted sample were verified using NanoDrop™️. To confirm the trypanosomatid species, the fluorescent fragment length coding (FFLB) method was employed, allowing for the simultaneous identification of trypanosome species or genotypes, including mixed infections 24 , 25 . The technique was based on the amplification of four variable regions of the 18S and 28Sα rRNA genes to identify trypanosome species based on the observed polymorphisms between them 24 , 26 . DNA samples from triatomine bugs were subjected to four PCR using fluorescent primers 24 , 25 , 27 , 28 described by Hamilton et al. 27 .
RESULTS
A total of 25 adult triatomine bugs were found on different floors of buildings B (two specimens) and C (23 specimens) (Figure 1), which were closest to the forest fragment, approximately 20m away. The triatomines were collected from the 2nd (5.6 to 8.4m high) to the 13th floor (36.4 to 39.2m high). The floors with the highest occurrence were the 7th with nine specimens and the 2nd with six specimens, both in Building C, in a single apartment per floor. The remaining triatomines were distributed across the different apartments.
Of the 25 triatomines found, six were only recorded through photographs, and 19 were collected and sent to LABMEDT. In seven of the samples, it was extracted the DNA of the samples for molecular identification. DNA extraction and molecular analysis were not possible in 12 samples because of the poor conditions of the collected samples. The results of species identification, trypanosomatid infection, and the floor on which they were collected are described in Table 1, Figure 2 and Figure 3.
TABLE 1: Triatomine bugs captured in the residential condominium and positivity for trypanosomatids in Rio Branco, Acre, Brazil - July 2022 to July 2023.
| Species | Specimen | Floor | Positive for trypanosomatids / T. cruzi |
|---|---|---|---|
| Rhodnius sp. (montenegrensis/robustus standard)* | 1 | 4º | NA |
| 2 | 4º | NA | |
| 3 | 10º | NA | |
| 4 | 10º | NA | |
| 5 | 10º | NA | |
| 6 | 10º | NA | |
| Rhodnius montenegrensis** | 7 | 4º | Positive for trypanosomatids# |
| 8 | 4º | Positive for trypanosomatids# | |
| 9 | 7º | Positive for trypanosomatids# | |
| 10 | 7º | Negative# | |
| 11 | 7º | Negative# | |
| 12 | 7º | Positive for trypanosomatids# | |
| 13 | 7º | Positive for trypanosomatids# | |
| 14 | 7º | Positive for trypanosomatids# | |
| 15 | 7º | Positive for trypanosomatids# | |
| 16 | 7º | Positive for trypanosomatids# | |
| 17 | 11º | Negative# | |
| 18 | 13º | Positive for trypanosomatids# | |
| 19 | 2º | Positive for T. cruzi ## (genotype TCI) | |
| 20 | 2º | Positive for T. cruzi ## (genotype TCI) | |
| 21 | 2º | Positive for T. cruzi ## (genotype TCI) | |
| 22 | 2º | Positive for T. cruzi## (genotype TCI) | |
| 23 | 2º | Positive for T. cruzi ## (genotype TCI) | |
| 24 | 2º | Negative## | |
| 25 | 7º | Negative## |
Caption: * Identification using photography. ** Identification done by morphological characteristics described by Lent and Wygodzinsky, 1979, and Rosa et al., 2012. # Analysis using optical microscopy. ## Analysis was performed using molecular biology techniques. NA: Specimens not analyzed were identified by photography. Meeting location: Specimens 1 and 2, Building B; 3-25, Building C.
FIGURE 2: Rhodnius montenegrensis captured in the residential condominium in Rio Branco, Acre, Brazil, 2023. Caption: Female: A) Dorsal view. B) Ventral view.
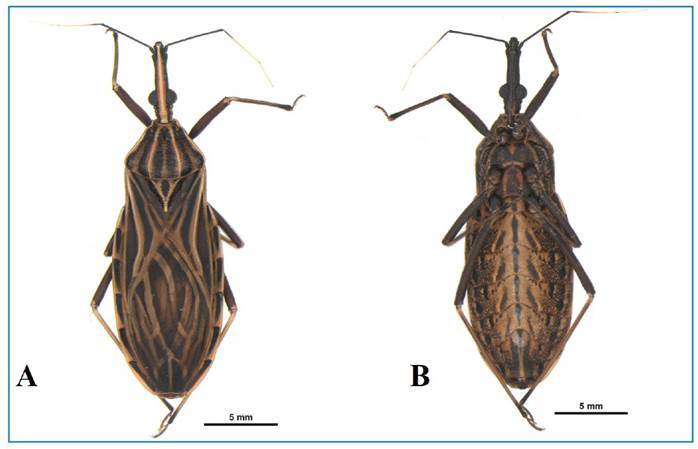
FIGURE 3: FFLB profiles obtained with DNA samples from R. montenegrensis. The image shows different peaks that form the specific profile of T. cruzi (TcI).
DISCUSSION
The occurrence of triatomine bugs in households in the Brazilian Legal Amazon has been a topic of discussion for decades 29 . However, these records have intensified in recent years, with two confirmed cases domiciled in the states of Mato Grosso and Roraima 12 , 30 .
Despite this, reports of the intrusion of these insects into human dwellings associated with high positivity for T. cruzi in specimens found are alarming, as they increase the risk of Chagas disease transmission 12 , 31 , 32 . This is because of the loss of natural ecotopes for triatomine bugs, leading them to move into residential areas attracted by artificial light or the scarcity of natural food sources 12 , 16 , 33 .
In this study, the buildings where triatomine bugs were collected were located near forest fragments filled with palm trees, which are considered the natural habitat of triatomine bugs, especially species of the genus Rhodnius 34 . Additionally, the forest fragment located near buildings may be a habitat for some mammals and a reservoir for both T. cruzi and T. rangeli, as observed in a similar study 11 . The genus Rhodnius is considered one of the most important genera because of the vectorial capacity of its species in the transmission of CD. In addition to its widespread distribution in the country, some species easily adapt to urbanized areas with frequent invasions into residences, being naturally infected with T. cruzi, such as R. montenegrensis 11 , 35 .
Rhodnius montenegrensis occurs in areas with large palm trees near houses and is naturally infected by both T. cruzi and T. rangeli 11 , 36 , which can be a problem because mixed infections can lead to errors in the differential diagnosis of Chagas disease 37 .
Of the seven specimens subjected to molecular analysis, five were diagnosed as positive for T. cruzi, all of which belonged to the TCI genotype. This Discrete Typing Unit (DTU) is widely distributed in the Americas and is frequently found in humans 3 . Its circulation is common in the Amazon region, it is present in the sylvatic cycles of the disease, and has previously been found in triatomine bugs occurring in the state of Acre 38 . Additionally, it is associated with outbreaks of Acute Chagas Disease, and is the main cause of chronic Chagas disease in Manaus, Amazonas 39 , 40 .
For the first time, this study described the occurrence of triatomine bugs on the 13th floor of a building. Similar studies reported the presence of this genus in residences on the 2nd and 5th floors 11 , 41 , 42 . Additionally, a study reported the occurrence of this genus on the 10th floor of a building in São Paulo, where colonies formed in the apartment where they were found 43 . All these cases share the proximity of residences to fragmented forest areas. However, the ability of these insects to reach high-rise buildings raises questions regarding the routes they take.
One hypothesis is that this locomotion may have occurred through the passive transport of objects, clothes, or reservoir animals. Ricardo-Silva et al. 30 raised the hypothesis that pigeons could be a means of passive transport of Triatoma maculata, favoring its domicilization in an air conditioning unit in Roraima. Additionally, Forattini et al. 44 have already detected this type of dispersion in Triatoma through birds by finding first-stage nymphs among the feathers of these animals and the colonization of these insects in their nests.
This passive dispersion is possible because some triatomines, including some species of the genus Rhodnius, secrete adhesive substances on their eggs, facilitating their adherence to reservoir animals such as birds 45 . This evidence suggests that reservoirs, such as birds and domestic animals, may have transported R. montenegrensis to the 13th floor of the building.
However, there is also the possibility of triatomines flowing directly from the palm trees to the upper floors, as species of the genus Rhodnius in the Amazon region are commonly found in palm tree canopies, indicating a good flying ability to move from one palm tree to another 43 , 46 .
Several factors may determine the induction of triatomines during flight, such as environmental (temperature) and nutritional factors 16 , 17 . In their experiments, Rocha et al. 17 concluded that, as a consequence of the decrease in wild food sources due to deforestation, R. brethesi was led to fasting and was induced to fly two weeks later, being attracted to the nearest locations with light.
Additionally, this locomotion may have occurred floor by floor, attracted by the brightness of the upper areas of the building, as they are easily drawn to artificial light in human dwellings 46 . A study conducted in Colombia observed that triatomines of the genus Rhodnius were attracted by artificial light 60-110 m away from palm trees, with the peak dispersal period occurring in the early hours after dusk 47 , reinforcing the hypothesis that these insects can cover long distances, reaching the 13th floor of a building.
However, there are no studies confirming any of these hypotheses or the factors that may have induced the dispersion of R. montenegrensis, since each triatomine species has specific characteristics regarding flight 16 .
Nevertheless, the dispersion of triatomines in urbanized environments is concerning because this expansion increases the risk of vector-borne transmission of Chagas Disease, emphasizing the need for further studies to better understand the invasive capacity of these insects in these environments and the mechanisms involved in their locomotion process.
The occurrence of triatomines on the 13th floor of a building must be elucidated, particularly regarding the occurrence of this dispersion. Clarifying these factors is crucial for the development of new surveillance and vector control strategies.
Furthermore, considering that the collection of these insects in the condominium was conducted by residents, interventions related to human contact with insects are necessary. For example, health education projects regarding the proper management of triatomines, from prevention to collection, delivery, and surveillance, are needed. Thus, with the joint efforts of the general population and healthcare professionals, it is possible to control triatomines in urban environments.
ACKNOWLEDGEMENTS
We would like to thank the Federal University of Acre (UFAC), Acre, Brazil, the Dean of Research and Graduate Studies at the Federal University of Acre (UFAC), the Graduate Program in Health Sciences in the Western Amazon at the University Federal do Acre.
Footnotes
Ethical considerations: The collections were conducted with a permanent license, issued by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA). License no. 52260-1.
Financial Support: Graduate Program in Health Sciences in the Western Amazon of the Federal University of Acre (PPGCSAO/UFAC) and Coordination for the Improvement of Higher Education Personnel (CAPES).
REFERENCES
- 1.BRASIL. Ministério da Saúde . Doença de Chagas. Boletim Epidemiológico Doença de Chagas, 2022. Secretaria de Vigilância em Saúde; 2022. [2023 Out 10]. Available from: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2022/boletim-especial-de-doenca-de-chagas-numero-especial-abril-de-2022 . [Google Scholar]
- 2.Chagas C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz. 1909;1:159–118. [Google Scholar]
- 3.Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MMG, et al. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12(2):240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Zingales B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018;184:38–52. doi: 10.1016/j.actatropica.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Madeira FP, Jesus AC, Moraes MHS, Barroso NF, Castro GVS, Ribeiro MAL, et al. Chagas Disease in the Western Brazilian Amazon: Epidemiological Overview from 2007 to 2018. J Hum Growth Dev. 2021;31(1):84–92. [Google Scholar]
- 6.Martins-Melo FR, Ramos AN, Jr, Alencar CH, Heukelbach J. Prevalence of Chagas disease in Brazil: A systematic review and metaanalysis. Acta Trop. 2014;130:167–174. doi: 10.1016/j.actatropica.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Gil-Santana HR, Chavez T, Pita S, Panzera F, Galvão C. Panstrongylus noireaui, a remarkable new species of Triatominae (Hemiptera, Reduviidae) from Bolivia. ZooKeys. 2022;1104:203–225. doi: 10.3897/zookeys.1104.81879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvão C. Vetores da doença de Chagas no Brasil. Curitiba: Sociedade Brasileira de Zoologia; 2014. [Google Scholar]
- 9.Oliveira-Correia JPS, Oliveira J, Gil-Santana HR, Rocha DS, Galvão C. Taxonomic reassessment of Rhodnius zeledoni Jurberg, Rocha & Galvão: a morphological and morphometric analysis comparing its taxonomic relationship with Rhodnius domesticus Neiva & Pinto. BMC Zoology. 2024;9(1):6–6. doi: 10.1186/s40850-024-00197-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madeira FP, Jesus AC, Moraes MHS, Oliveira AS, Oliveira J, Melchior LAK, et al. Atualidades em Medicina Tropical no Brasil: Vetores. 1ed. Rio Branco: Stricto Sensu Editora; 2020. Doença de Chagas: conceitos básicos de uma enfermidade negligenciada e seus vetores na Amazônia Ocidental brasileira. [Google Scholar]
- 11.Ribeiro MAL, Castro GVS, Souza JL, Rosa JA, Camargo LMA, Meneguetti DUO. Occurrence of triatomines in an urban residential complex in the municipality of Rio Branco, Acre, South-Western Amazon. Rev Soc Bras Med Trop. 2019;52:e20180177. doi: 10.1590/0037-8682-0177-2018. [DOI] [PubMed] [Google Scholar]
- 12.Martins MF, Moraes SC, Oliveira J, Santos JC, Santos-Silva LK, Galvão C. Triatoma williami in intradomiciliary environments of urban areas in Mato Grosso State, Brazil: domiciliation process of a wild species? Infect Dis Poverty. 2022;11:18–18. doi: 10.1186/s40249-022-00938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magalhães L, Silveira H, Prestes S, Costa Magalhães LK, Santana RA, Ramasawmy R., et al. Bioecological aspects of triatomines and marsupials as wild Trypanosoma cruzi reservoirs in urban, peri-urban and rural areas in the Western Brazilian Amazon. Med Vet Entomol. 2021;35(3):389–399. doi: 10.1111/mve.12507. [DOI] [PubMed] [Google Scholar]
- 14.Julião GR, Pimentel IF, França AK, GIL LHS, Simplício MF, Silva GS., et al. Rhodnius spp. infestation in palm trees and natural infection by Trypanosoma cruzi and Trypanosoma rangeli in periurban and rural areas of state of the Rondônia, in the Brazilian Amazon. Acta Trop. 2021:220–220. doi: 10.1016/j.actatropica.2021.105963. [DOI] [PubMed] [Google Scholar]
- 15.Abrahan LB, Gorla DE, Catalá SS. Dispersal of Triatoma infestans and other Triatominae species in tharid Chaco of Argentina - Flying, walking or passive carriage? The importance of walking females. Mem Inst Oswaldo Cruz. 2011;106(2):232–239. doi: 10.1590/s0074-02762011000200019. [DOI] [PubMed] [Google Scholar]
- 16.Rocha DS, Solano C, Jurberg J, Cunha V, Galvão C. Avaliação em laboratório da atividade de voo de Rhodnius brethesi Matta, 1919, potencial vetor silvestre do Trypanosoma cruzi na Amazônia brasileira. (Hemiptera:Reduviidae:Triatominae) Rev Pan-Amaz Saude. 2011;2(1):73–78. [Google Scholar]
- 17.Lehane MJ, Mcewan PK, Whitaker CJ, Schofield CJ. The role of temperature and nutritional status in flight initiation by Triatoma infestans. Acta Trop. 1992;52(1):27–38. doi: 10.1016/0001-706x(92)90004-h. [DOI] [PubMed] [Google Scholar]
- 18.Soares RPP, Santoro MM. α-glycerophosphate dehydrogenase activity in flight muscles of Triatominae bugs Panstrongylus megistus and Triatoma sordida. Mem Inst Oswaldo Cruz. 2000;95(5):707–709. doi: 10.1590/s0074-02762000000500016. [DOI] [PubMed] [Google Scholar]
- 19.Bilheiro AB, Rosa JA, Oliveira J, Belintani T, Fontes G, Medeiros JF, et al. Biological Aspects of Rhodnius montenegrensis (Hemiptera, Reduviidae, Triatominae) Under Laboratory Conditions. Vector Borne Zoonotic Dis. 2019;19(12):929–932. doi: 10.1089/vbz.2019.2449. [DOI] [PubMed] [Google Scholar]
- 20.Santos WS, Gurgel-Gonçalves R, Garcez LM, Abad-Franch F. Deforestation effects on Attalea palms and their resident Rhodnius, vectors of Chagas disease, in eastern Amazonia. Plos One. 2021;16(5):e0252071. doi: 10.1371/journal.pone.0252071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas' disease. Bull Am Mus Nat Hist. 1979;163(1):127–520. [Google Scholar]
- 22.Rosa JA, Rocha CS, Gardim S, Pinto MC, Mendonça VJ, Ferreira-Filho JC, et al. Description of Rhodnius montenegrensis n. sp. (Hemiptera: Reduviidae: Triatominae) from the state of Rondônia, Brazil. Zootaxa. 2012;3478(1):62–76. [Google Scholar]
- 23.Adams ER, Hamilton PB, Malele II, Gibson WC. The identification, diversity and prevalence of trypanosomes in field caught tsetse in Tanzania using ITS-1 primers and fluorescent fragment length barcoding. Infect Genet Evol. 2008;8(4):439–444. doi: 10.1016/j.meegid.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Garcia HA, Rodrigues CMF, Rodrigues AC, Pereira DL, Pereira CL, Camargo E., et al. Remarkable richness of trypanosomes in tsetse files (Glossina morsitans morsitans and Glossina pallidipes) from the Gorongosa National Park and Niassa National Reserve of Mozambique revealed by fluorescent fragment length barcoding (FFLB) Infect Genet Evol. 2018;63:370–379. doi: 10.1016/j.meegid.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Vergara-Meza JG, Brilhante AF,Valente VC Ortiz PA, Oliveira SC, et al. Trypanosoma cruzi and Trypanosoma rangeli in Acre, Brazilian Amazonia: coinfection and notable genetic diversity in an outbreak of orally acquired acute Chagas disease in a forest community, wild reservoirs and vectors. Parasitologia. 2022;2(4):350–365. [Google Scholar]
- 26.Hamilton PB, Lewis MD, Cruickshank C, Gaunt MW, Sim M, Llewellyn MS, et al. Identification and lineage genotyping of South American trypanosomes using fluorescent fragment length barcoding. Infect Genet Evol. 2011;11(1):44–51. doi: 10.1016/j.meegid.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton PB, Stevens JR. American trypanosomiasis Chagas disease. Elsevier; 2017. Classification and phylogeny of Trypanosoma cruzi; pp. 321–344. [Google Scholar]
- 28.Valença-Barbosa C, Finamore-Araújo P, Moreira OC, Vergara-Meza JG, Alvarez MVN, Nascimento J, et al. Genotypic Trypanosoma cruzi distribution and parasite load differ ecotypically andaccording to parasite genotypes in Triatoma brasiliensis from endemic and outbreak areas in Northeastern Brazil. Acta Trop. 2021;222:106054–106054. doi: 10.1016/j.actatropica.2021.106054. [DOI] [PubMed] [Google Scholar]
- 29.Coura JR, Junqueira ACV, Boia MN, Fernandes O. Chagas Disease: from Bush to Huts and Houses. Is it the Case of the Brazilian Amazon? Mem Inst Oswaldo Cruz. 1999;94:379–384. doi: 10.1590/s0074-02761999000700074. [DOI] [PubMed] [Google Scholar]
- 30.Ricardo-Silva A, Gonçalves TCM, Luitgards-Moura JF, Lopes CM, Silva SP, Bastos AQ, et al. Triatoma maculata colonises urban domiciliesin Boa Vista, Roraima, Brazil. Mem Inst Oswaldo Cruz. 2016;111(11):703–706. doi: 10.1590/0074-02760160026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribeiro MAL, Castro GVS, P SL, Júnior, Souza JL, Ávila MM, Araújo FL, et al. In: Atualidades em Medicina Tropical na América do Sul: Vetores. Oliveira J, Alevi KCC, Camargo LMA, Meneguetti DUO, editors. Rio Branco, Acre: Stricto Sensu; 2021. Ocorrência de triatomíneos e a positividade para tripanosomatídeos em residências no município de Rio Branco, Acre, Amazônia Ocidental, Brasil; pp. 164–182. [Google Scholar]
- 32.Cruz KS, Ribeiro MAL, Madeira FP, Paixão DS, Jesus AC, Camargo LMA, et al. Occurrence of triatomines in public spaces: An atypical case in the Southwestern Brazilian Amazon. Rev Soc Bras Med Trop. 2023;56:e0042-2023. doi: 10.1590/0037-8682-0042-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abad-Franch F, Monteiro FA, Jaramillo N, Gurgel-Gonçalves R, Dias FBS, Diotaiuti L. Ecology, evolution, and the long-term surveillance of vector-borne Chagas disease: A multi-scale appraisal of the tribe Rhodniini (Triatominae) Acta Trop. 2009;110:159–177. doi: 10.1016/j.actatropica.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Meneguetti DUO, Trevisan O, Camargo LMA, Rosa RM. Natural infection of triatomines (Hemiptera: Reduviidae) by trypanosomatids in two different environments in the municipality of Ouro Preto do Oeste, State of Rondônia, Brazil. Rev Soc Bras Med Trop. 2012;45(3):395–398. doi: 10.1590/s0037-86822012000300023. [DOI] [PubMed] [Google Scholar]
- 35.Moraes MHS, Jesus AC, Madeira FP, Moresco GG, Oliveira J, Rosa JA, et al. Triatominae (Hemiptera, Reduviidae) in homes: Report of their occurrence in the municipality of Cruzeiro do Sul, Acre, South Western Amazon. Rev Soc Bras Med Trop. 2021;54:e20200296. doi: 10.1590/0037-8682-0296-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meneguetti DUO, Tojal SD, Miranda PRD, Rosa JA, Camargo LMA. First report of Rhodnius montenegrensis (Hemiptera, Reduviidae, Triatominae) in the State of Acre, Brazil. Rev Soc Bras Med Trop. 2015;48:471–473. doi: 10.1590/0037-8682-0029-2015. [DOI] [PubMed] [Google Scholar]
- 37.Peterson JK, Graham AL, Elliott RJ, Dobson AP, Chávez T O. A co-infecção Trypanosoma cruzi-Trypanosoma rangeli melhora os efeitos negativos de infecções tripanossomas únicas em Rhodnius prolixus infectado experimentalmente. Parasitologia. 2016;143(9):1157–1167. [Google Scholar]
- 38.Malavazi PFNS, Daudt C, Melchior LAK, Meneguetti DUO, Xavier SCC, Jansen AM, et al. Trypanosomes of vectors and domestic dogs in Trypanosoma cruzi transmission areas from Brazilian southwestern amazon: New mammalian host for Trypanosoma janseni. Acta Trop. 2020;10:105504–105504. doi: 10.1016/j.actatropica.2020.105504. [DOI] [PubMed] [Google Scholar]
- 39.Coura JR, Junqueira AC. Vigilância, promoção da saúde e controle da doença de Chagas na Região Amazônica: atenção médica na Amazônia Brasileira: uma proposta. Mem Inst Oswaldo Cruz. 2015;110:825–830. [Google Scholar]
- 40.Santana RA, Magalhães LK, Magalhães LK, Prestes SR, Maciel RG, Silva GA, et al. Trypanosoma cruzi strain TcI is associated with chronic Chagas disease in the Brazilian Amazon. Parasit Vectors. 2014;7:267–267. doi: 10.1186/1756-3305-7-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dias-Lima AG, Sherlock IA. Sylvatic Vectors Invading Houses and the Risk of Emergence of Cases of Chagas Disease in Salvador, State of Bahia, Northeast Brazil. Mem Inst Oswaldo Cruz. 2000;95(5):611–613. doi: 10.1590/s0074-02762000000500004. [DOI] [PubMed] [Google Scholar]
- 42.Terassini FA, Stefanello C, Camargo LMA, Meneguetti DUO. First report of Panstrongylus lignarius Walker, 1873 (Hemiptera, Reduviidae, Triatominae) in the State of Rondônia, Brazil. Rev Soc Bras Med Trop. 2017;50(4):547–549. doi: 10.1590/0037-8682-0048-2017. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigues VLCC, Silva RA, Wanderley DMV, Carvalho ME, Pauliquevis JRC. Detecção de triatomíneos da espécie Rhodnius neglectus em área urbana de municípios da região de Araçatuba. Boletim Epidemiológico Paulista. 2009;6(63):20–23. [Google Scholar]
- 44.Forattini OP, Rocha-Silva EO, Ferreira AO, Rabello EX, Patolli DGB. Aspectos ecológicos da tripanosomose americana. III - Dispersão local de triatomíneos, com especial referência ao Triatoma sordida. Rev Saude Pública. 1971;5:193–205. [PubMed] [Google Scholar]
- 45.Ribeiro G, Júnior, Silva-Santos CG, Noireau F, Dias-Lima A. Potencial de dispersão de algumas espécies de Triatomíneos (Hemiptera: Reduviidae) por aves migratórias. Série Ciências Biológicas. 2006;6(4):324–328. [Google Scholar]
- 46.Castro MC, Barrett TV, Santos WS, Abad-Franch F, Rafael JA. Attraction of Chagas disease vectors (Triatominae) to artificial light sources in the canopy of primary Amazon rainforest. Mem Inst Oswaldo Cruz. 2010;105:1061–1064. doi: 10.1590/s0074-02762010000800019. [DOI] [PubMed] [Google Scholar]
- 47.Jácome-Pinilla D, Hincapie-Peñaloza E, Ortiz MI, Ramírez JD, Guhl F, Molina J. Risks associated with dispersive nocturnal flights of sylvatic Triatominae to artificial lights in a model house in the northeastern plains of Colombia. Parasit Vectors. 2015;8:600–600. doi: 10.1186/s13071-015-1209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]