Abstract
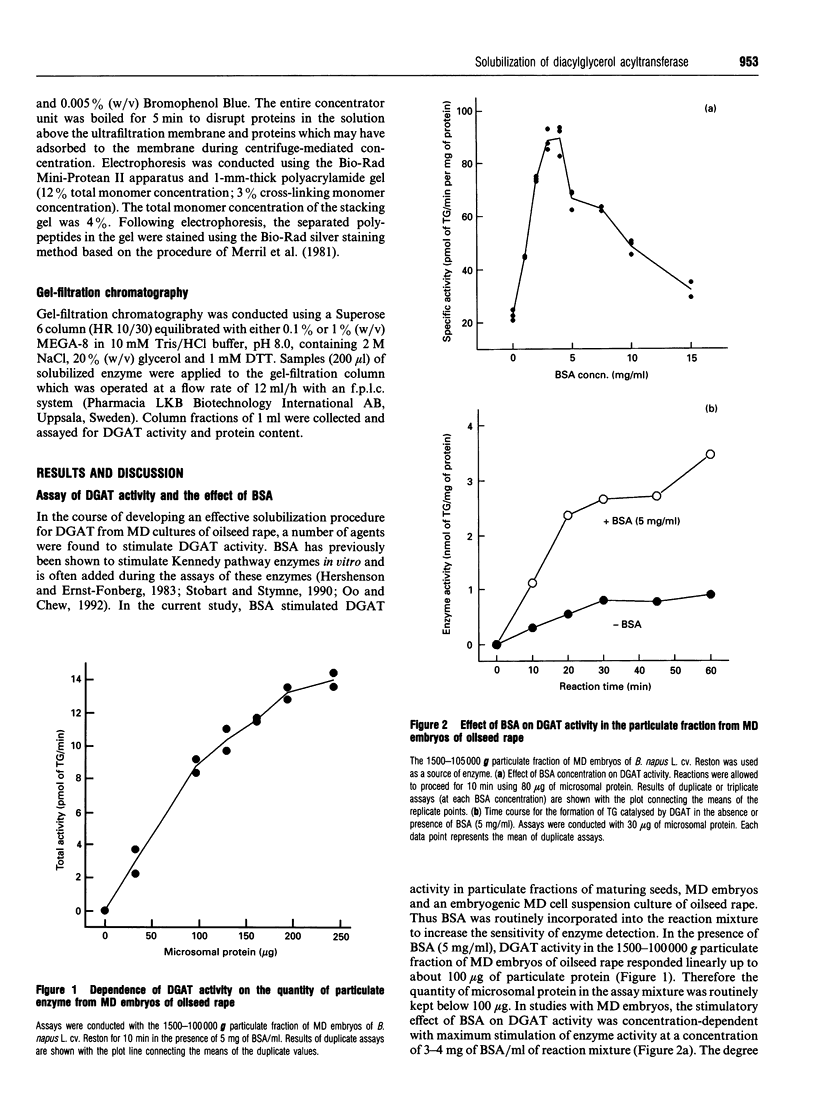
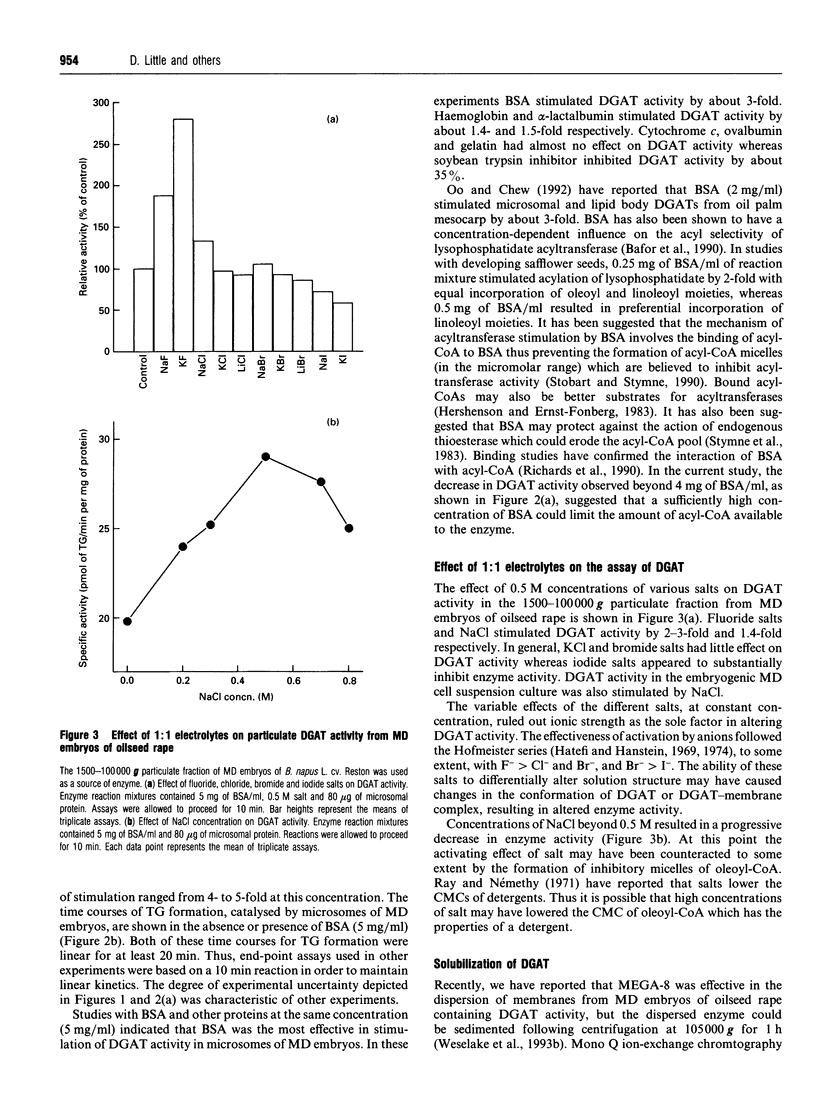
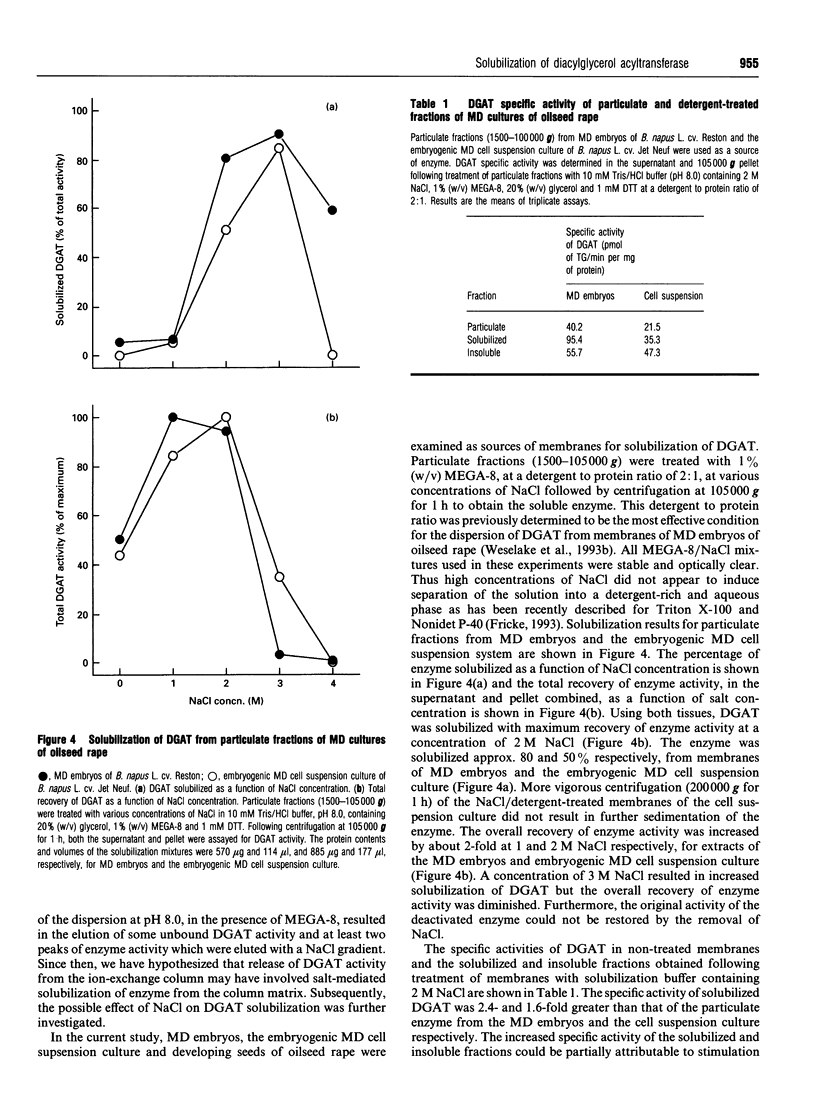
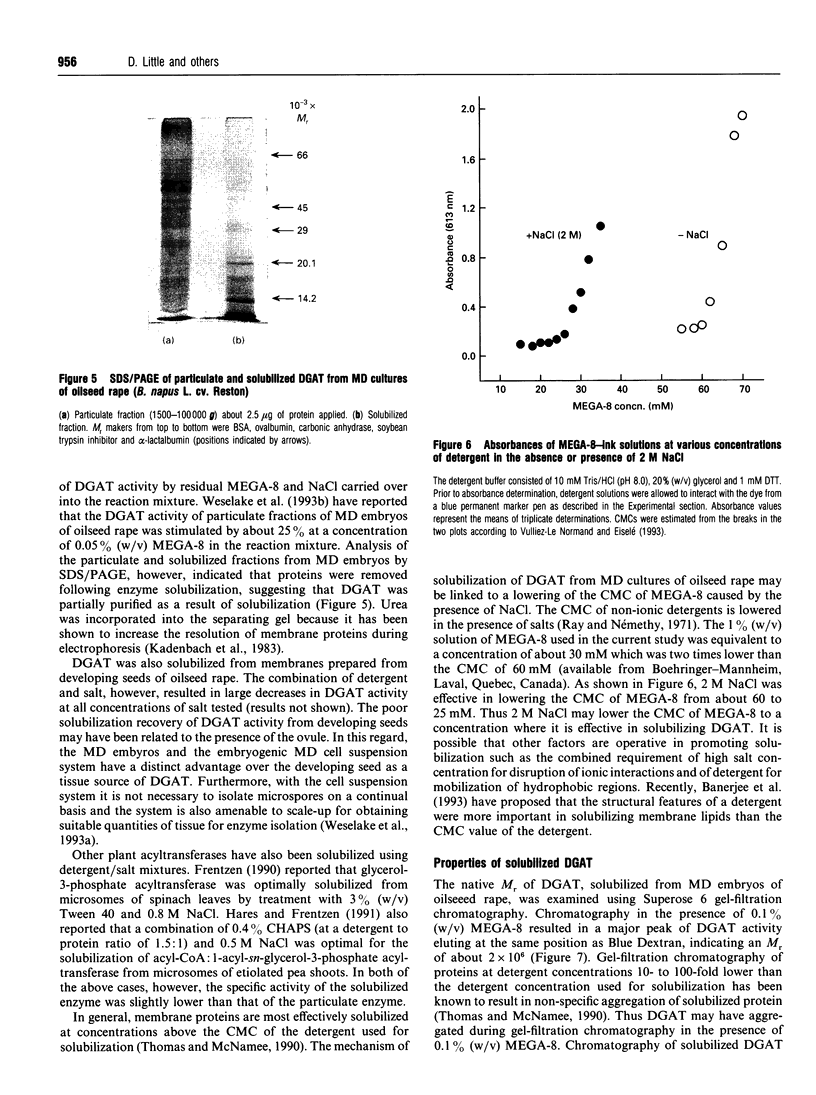
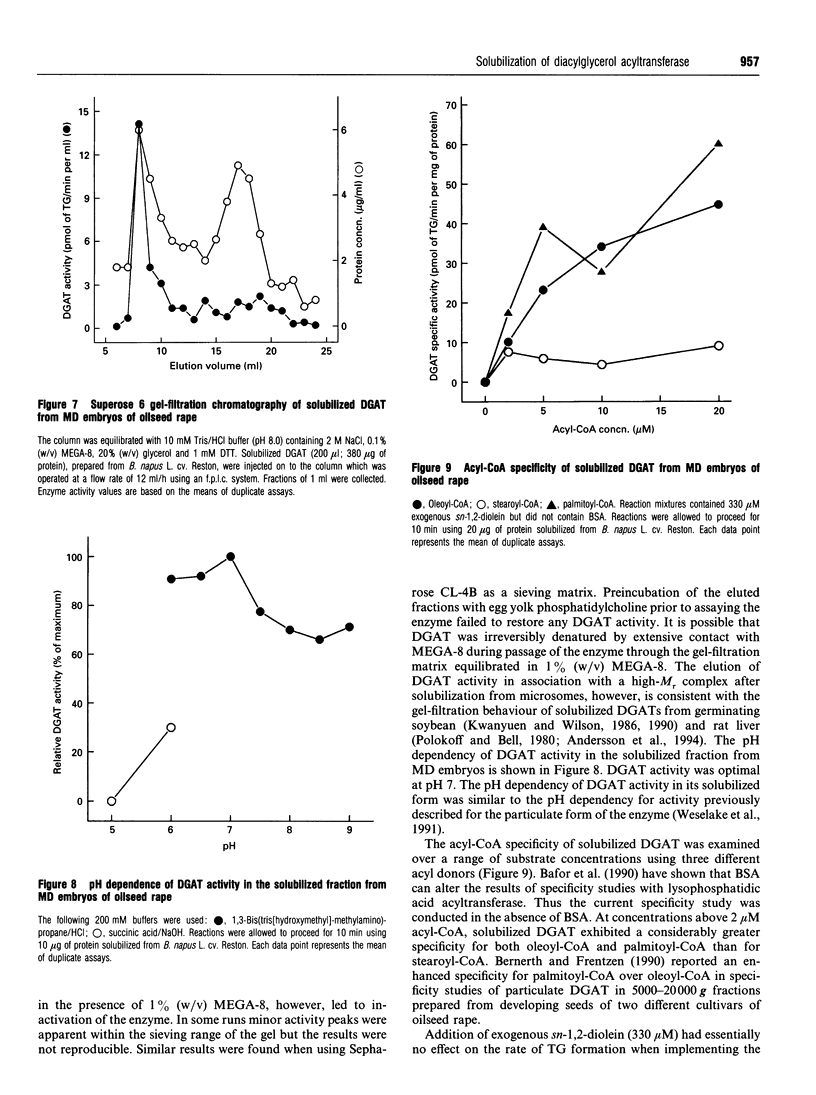
Particulate fractions prepared from microspore-derived (MD) embryos of oilseed rape (Brassica napus L. cv. Reston) and an embryogenic MD cell suspension culture of oilseed rape (B. napus L. cv. Jet Neuf) were used as a source of diacylglycerol acyltransferase (DGAT, EC 2.3.1.20) for enzyme characterization and development of a solubilization procedure. DGAT activity in the 1500-100,000 g fraction from MD embryos was stimulated 4-5-fold by 3 to 4 mg of BSA/ml of reaction mixture. DGAT activity from MD embryos was stimulated 2-3-fold by fluoride salts and 1.4-fold by NaCl, whereas iodide salts caused substantial inhibition of enzyme activity. The effect of the various 1:1 electrolytes on enzyme activity appeared to be related more to their differential effects on solution structure rather than ionic strength. DGAT was solubilized from membranes of MD embryos and the cell suspension culture by about 80 and 50% respectively, using 2 M NaCl in 1% (w/v) octanoyl-N-methyl-glucamide (MEGA-8) (pH 8.0 buffer) at a detergent to protein ratio of 2:1. The specific activity of solubilized DGAT was about 2-fold greater than that of the particulate enzyme. The mechanism of solubilization appeared to be related to the lowering of the critical micellar concentration of MEGA-8 in the presence of NaCl. DGAT, solubilized from MD embryos, eluted with an M(r) of about 2 x 10(6) during gel-filtration chromatography on a Superose 6 column equilibrated in buffer containing 0.1% (w/v) MEGA-8. The solubilized enzyme exhibited optimal activity at pH 7. At concentrations above 2 microM acyl-CoA, the specificity of solubilized DGAT for oleoyl-CoA and palmitoyl-CoA was considerably greater than for stearoyl-CoA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akao T., Kusaka T. Solubilization of diglyceride acyltransferase from the membrane of Mycobacterium smegmatis. J Biochem. 1976 Oct;80(4):723–728. doi: 10.1093/oxfordjournals.jbchem.a131332. [DOI] [PubMed] [Google Scholar]
- Andersson M., Wettesten M., Borén J., Magnusson A., Sjöberg A., Rustaeus S., Olofsson S. O. Purification of diacylglycerol:acyltransferase from rat liver to near homogeneity. J Lipid Res. 1994 Mar;35(3):535–545. [PubMed] [Google Scholar]
- Banerjee P., Dasgupta A., Chromy B. A., Dawson G. Differential solubilization of membrane lipids by detergents: coenrichment of the sheep brain serotonin 5-HT1A receptor with phospholipids containing predominantly saturated fatty acids. Arch Biochem Biophys. 1993 Aug 15;305(1):68–77. doi: 10.1006/abbi.1993.1394. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cao Y. Z., Huang A. H. Acyl coenzyme a preference of diacylglycerol acyltransferase from the maturing seeds of cuphea, maize, rapeseed, and canola. Plant Physiol. 1987 Jul;84(3):762–765. doi: 10.1104/pp.84.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke B. Phase separation of nonionic detergents by salt addition and its application to membrane proteins. Anal Biochem. 1993 Jul;212(1):154–159. doi: 10.1006/abio.1993.1306. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Schiele U., Numa S. Diacylglycerol acyltransferase from rat liver microsomes. Separation and acyl-donor specificity. Eur J Biochem. 1977 Jun 1;76(1):113–118. doi: 10.1111/j.1432-1033.1977.tb11576.x. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Takahashi T., Fujii S. Diacylglycerol acyltransferase in maturing safflower seeds: its influences on the fatty acid composition of triacylglycerol and on the rate of triacylglycerol synthesis. Biochim Biophys Acta. 1988 Jan 19;958(1):125–129. doi: 10.1016/0005-2760(88)90253-6. [DOI] [PubMed] [Google Scholar]
- KENNEDY E. P. Biosynthesis of complex lipids. Fed Proc. 1961 Dec;20:934–940. [PubMed] [Google Scholar]
- Kwanyuen P., Wilson R. F. Subunit and amino acid composition of diacylglycerol acyltransferase from germinating soybean cotyledons. Biochim Biophys Acta. 1990 May 31;1039(1):67–72. doi: 10.1016/0167-4838(90)90227-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manganaro F., Kuksis A. Rapid isolation of a triacylglycerol synthetase complex from rat intestinal mucosa. Can J Biochem Cell Biol. 1985 Feb;63(2):107–114. doi: 10.1139/o85-016. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Polokoff M. A., Bell R. M. Solubilization, partial purification and characterization of rat liver microsomal diacylglycerol acyltransferase. Biochim Biophys Acta. 1980 Apr 18;618(1):129–142. doi: 10.1016/0005-2760(80)90060-0. [DOI] [PubMed] [Google Scholar]
- Ray A., Némethy G. Effects of ionic protein denaturants on micelle formation by nonionic detergents. J Am Chem Soc. 1971 Dec 15;93(25):6787–6793. doi: 10.1021/ja00754a014. [DOI] [PubMed] [Google Scholar]
- Richards E. W., Hamm M. W., Fletcher J. E., Otto D. A. The binding of palmitoyl-CoA to bovine serum albumin. Biochim Biophys Acta. 1990 Jun 14;1044(3):361–367. doi: 10.1016/0005-2760(90)90081-8. [DOI] [PubMed] [Google Scholar]
- Stymne S., Stobart A. K., Glad G. The role of the acyl-CoA pool in the synthesis of polyunsaturated 18-carbon fatty acids and triacylglycerol production in the microsomes of developing safflower seeds. Biochim Biophys Acta. 1983 Jul 12;752(2):198–208. doi: 10.1016/0005-2760(83)90113-3. [DOI] [PubMed] [Google Scholar]
- Taylor D. C., Weber N., Barton D. L., Underhill E. W., Hogge L. R., Weselake R. J., Pomeroy M. K. Triacylglycerol Bioassembly in Microspore-Derived Embryos of Brassica napus L. cv Reston. Plant Physiol. 1991 Sep;97(1):65–79. doi: 10.1104/pp.97.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. C., Weber N., Hogge L. R., Underhill E. W. A simple enzymatic method for the preparation of radiolabeled erucoyl-CoA and other long-chain fatty acyl-CoAs and their characterization by mass spectrometry. Anal Biochem. 1990 Feb 1;184(2):311–316. doi: 10.1016/0003-2697(90)90686-4. [DOI] [PubMed] [Google Scholar]
- Thomas T. C., McNamee M. G. Purification of membrane proteins. Methods Enzymol. 1990;182:499–520. doi: 10.1016/0076-6879(90)82040-9. [DOI] [PubMed] [Google Scholar]
- Tzen JTC., Cao Yz., Laurent P., Ratnayake C., Huang AHC. Lipids, Proteins, and Structure of Seed Oil Bodies from Diverse Species. Plant Physiol. 1993 Jan;101(1):267–276. doi: 10.1104/pp.101.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliez-Le Normand B., Eiselé J. L. Determination of detergent critical micellar concentration by solubilization of a colored dye. Anal Biochem. 1993 Feb 1;208(2):241–243. doi: 10.1006/abio.1993.1039. [DOI] [PubMed] [Google Scholar]
- Weselake R. J., Pomeroy M. K., Furukawa T. L., Golden J. L., Little D. B., Laroche A. Developmental Profile of Diacylglycerol Acyltransferase in Maturing Seeds of Oilseed Rape and Safflower and Microspore-Derived Cultures of Oilseed Rape. Plant Physiol. 1993 Jun;102(2):565–571. doi: 10.1104/pp.102.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]