Abstract
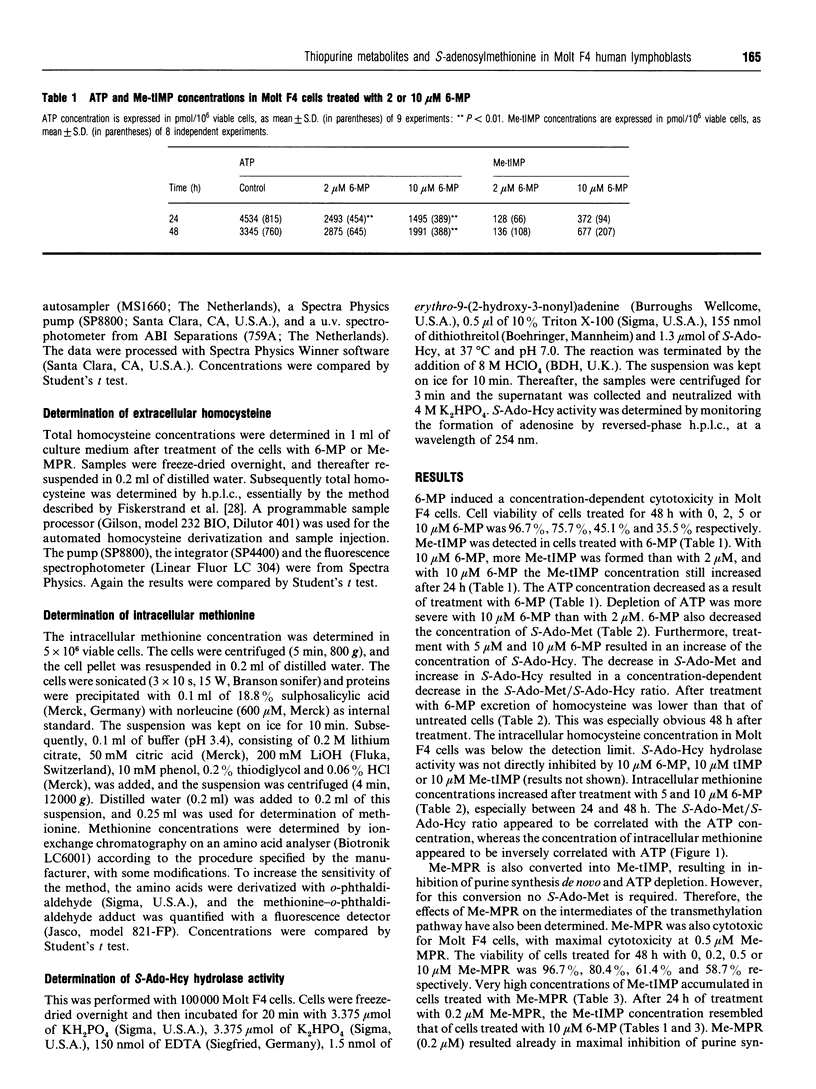
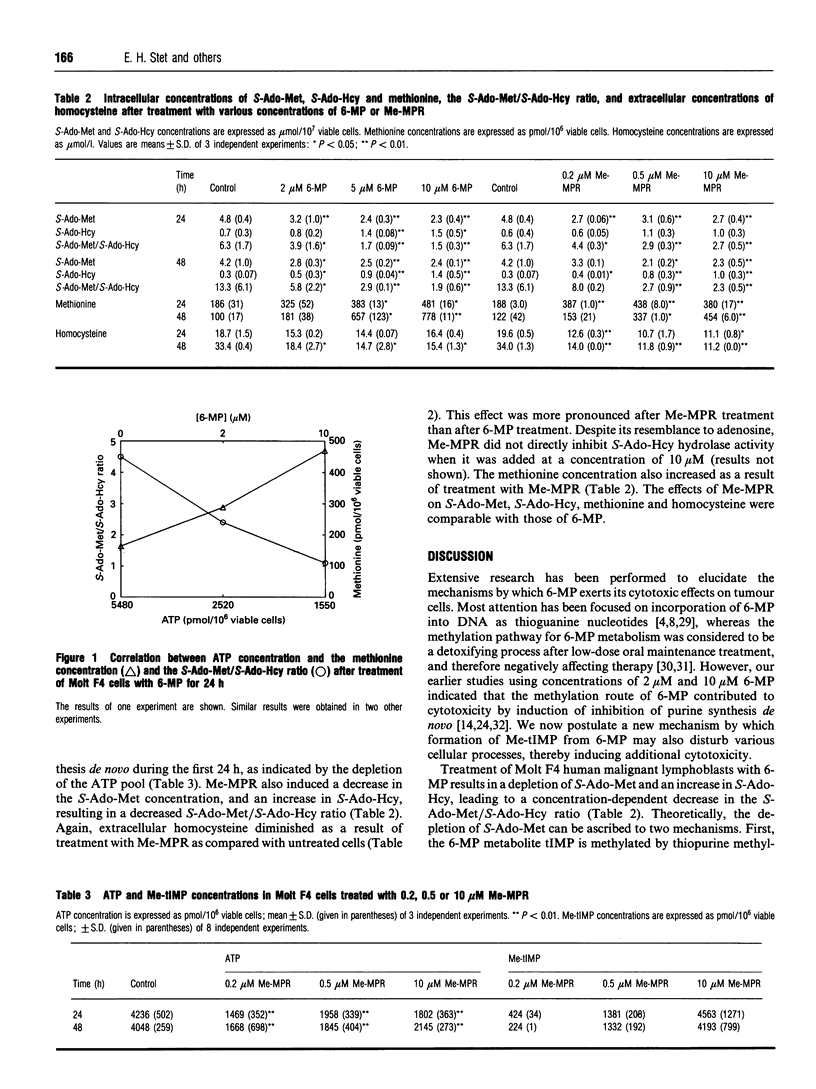
6-Mercaptopurine (6-MP) and methylmercaptopurine ribonucleoside (Me-MPR) are purine anti-metabolites which are both metabolized to methylthio-IMP (Me-tIMP), a strong inhibitor of purine synthesis de novo. Me-MPR is converted directly into Me-tIMP by adenosine kinase. 6-MP is converted into tIMP, and thereafter it is methylated to Me-tIMP by thiopurine methyltransferase, an S-adenosylmethionine (S-Ado-Met)-dependent conversion. S-Ado-Met is formed from methionine and ATP by methionine adenosyltransferase, and is a universal methyl donor, involved in methylation of several macromolecules, e.g. DNA and RNA. Therefore, depletion of S-Ado-Met could result in an altered methylation state of these macromolecules, thereby affecting their functionality, leading to dysregulation of cellular processes and cytotoxicity. In this study the effects of 6-MP and Me-MPR on S-Ado-Met, S-adenosylhomocysteine (S-Ado-Hcy), homocysteine and methionine concentrations are determined. Both drugs cause a decrease in intracellular S-Ado-Met concentrations and an increase in S-Ado-Hcy and methionine concentrations in Molt F4 human malignant lymphoblasts. The effects of both 6-MP and Me-MPR can be ascribed to a decreased conversion of methionine into S-Ado-Met, due to the ATP depletion induced by the inhibition of purine synthesis de novo by Me-tIMP. Both 6-MP and Me-MPR thus affect the methylation state of the cells, and this may result in dysregulation of cellular processes and may be an additional mechanism of cytotoxicity for 6-MP and Me-MPR.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K., Karkola K., Kajander O. Methionine adenosyltransferase activity in cultured cells and in human tissues. Biochim Biophys Acta. 1991 Sep 23;1097(2):140–144. doi: 10.1016/0925-4439(91)90098-t. [DOI] [PubMed] [Google Scholar]
- BROCKMAN R. W., BENNETT L. L., Jr, SIMPSON M. S., WILSON A. R., THOMSON J. R., SKIPPER H. E. A mechanism of resistance to 8-azaguanine. II. Studies with experimental neoplasms. Cancer Res. 1959 Sep;19:856–869. [PubMed] [Google Scholar]
- BROCKMAN R. W., CHUMLEY S. INHIBITION OF FORMYLGLYCINAMIDE RIBONUCLEOTIDE SYNTHESIS IN NEOPLASTIC CELLS BY PURINES AND ANALOGS. Biochim Biophys Acta. 1965 Mar 15;95:365–379. doi: 10.1016/0005-2787(65)90183-8. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T. S-Adenosyl-L-methionine-dependent macromolecule methyltransferases: potential targets for the design of chemotherapeutic agents. J Med Chem. 1980 Apr;23(4):347–357. doi: 10.1021/jm00178a001. [DOI] [PubMed] [Google Scholar]
- Bökkerink J. P., Bakker M. A., Hulscher T. W., De Abreu R. R., Schretlen E. D., van Laarhoven J. P., De Bruyn C. H. Sequence-, time- and dose-dependent synergism of methotrexate and 6-mercaptopurine in malignant human T-lymphoblasts. Biochem Pharmacol. 1986 Oct 15;35(20):3549–3555. doi: 10.1016/0006-2952(86)90625-8. [DOI] [PubMed] [Google Scholar]
- Bökkerink J. P., Stet E. H., De Abreu R. A., Damen F. J., Hulscher T. W., Bakker M. A., van Baal J. A. 6-Mercaptopurine: cytotoxicity and biochemical pharmacology in human malignant T-lymphoblasts. Biochem Pharmacol. 1993 Apr 6;45(7):1455–1463. doi: 10.1016/0006-2952(93)90045-x. [DOI] [PubMed] [Google Scholar]
- Caldwell I. C., Henderson J. F., Paterson A. R. The enzymic formation of 6-(methylmercapto)purine ribonucleoside 5'-phosphate. Can J Biochem. 1966 Feb;44(2):229–245. doi: 10.1139/o66-027. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Mendelsohn N., Herzog D., Schneiderman N. Effect of 5-azacytidine on differentiation and DNA methylation in human promyelocytic leukemia cells (HL-60). Cancer Res. 1983 Feb;43(2):763–769. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983 Jan 6;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Fiskerstrand T., Refsum H., Kvalheim G., Ueland P. M. Homocysteine and other thiols in plasma and urine: automated determination and sample stability. Clin Chem. 1993 Feb;39(2):263–271. [PubMed] [Google Scholar]
- HENDERSON J. F., KHOO K. Y. ON THE MECHANISM OF FEEDBACK INHIBITION OF PURINE BIOSYNTHESIS DE NOVO IN EHRLICH ASCITES TUMOR CELLS IN VITRO. J Biol Chem. 1965 Jul;240:3104–3109. [PubMed] [Google Scholar]
- Henderson J. F., Mikoshiba A., Chu S. Y., Caldwell I. C. Kinetic studies of adenosine kinase from Ehrlich ascites tumor cells. J Biol Chem. 1972 Apr 10;247(7):1972–1975. [PubMed] [Google Scholar]
- Hoffman R. M. Altered methionine metabolism, DNA methylation and oncogene expression in carcinogenesis. A review and synthesis. Biochim Biophys Acta. 1984;738(1-2):49–87. doi: 10.1016/0304-419x(84)90019-2. [DOI] [PubMed] [Google Scholar]
- Hoffman R. M. Unbalanced transmethylation and the perturbation of the differentiated state leading to cancer. Bioessays. 1990 Apr;12(4):163–166. doi: 10.1002/bies.950120404. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Martin D. V., Jr Role of S-adenosylhomocysteine in adenosinemediated toxicity in cultured mouse T lymphoma cells. Cell. 1977 Dec;12(4):931–938. doi: 10.1016/0092-8674(77)90157-x. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Pawlak K., Nguyen B. T., Robins R. K., Sadée W. Biochemical differences among four inosinate dehydrogenase inhibitors, mycophenolic acid, ribavirin, tiazofurin, and selenazofurin, studied in mouse lymphoma cell culture. Cancer Res. 1985 Nov;45(11 Pt 1):5512–5520. [PubMed] [Google Scholar]
- Lennard L., Keen D., Lilleyman J. S. Oral 6-mercaptopurine in childhood leukemia: parent drug pharmacokinetics and active metabolite concentrations. Clin Pharmacol Ther. 1986 Sep;40(3):287–292. doi: 10.1038/clpt.1986.178. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Lilleyman J. S., Weinshilboum R. M. Thiopurine pharmacogenetics in leukemia: correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther. 1987 Jan;41(1):18–25. doi: 10.1038/clpt.1987.4. [DOI] [PubMed] [Google Scholar]
- Linevsky J., Cohen M. B., Hartman K. D., Knode M. C., Glazer R. I. Effect of neplanocin A on differentiation, nucleic acid methylation, and c-myc mRNA expression in human promyelocytic leukemia cells. Mol Pharmacol. 1985 Jul;28(1):45–50. [PubMed] [Google Scholar]
- Mauer A. M. Therapy of acute lymphoblastic leukemia in childhood. Blood. 1980 Jul;56(1):1–10. [PubMed] [Google Scholar]
- Maybaum J., Mandel H. G. Unilateral chromatid damage: a new basis for 6-thioguanine cytotoxicity. Cancer Res. 1983 Aug;43(8):3852–3856. [PubMed] [Google Scholar]
- Molloy A. M., Weir D. G., Kennedy G., Kennedy S., Scott J. M. A new high performance liquid chromatographic method for the simultaneous measurement of S-adenosylmethionine and S-adenosylhomocysteine. Concentrations in pig tissues after inactivation of methionine synthase by nitrous oxide. Biomed Chromatogr. 1990 Nov;4(6):257–260. doi: 10.1002/bmc.1130040611. [DOI] [PubMed] [Google Scholar]
- Nagai M., Natsumeda Y., Weber G. Proliferation-linked regulation of type II IMP dehydrogenase gene in human normal lymphocytes and HL-60 leukemic cells. Cancer Res. 1992 Jan 15;52(2):258–261. [PubMed] [Google Scholar]
- Nelson J. A., Carpenter J. W., Rose L. M., Adamson D. J. Mechanisms of action of 6-thioguanine, 6-mercaptopurine, and 8-azaguanine. Cancer Res. 1975 Oct;35(10):2872–2878. [PubMed] [Google Scholar]
- Pan B. F., Nelson J. A. Characterization of the DNA damage in 6-thioguanine-treated cells. Biochem Pharmacol. 1990 Sep 1;40(5):1063–1069. doi: 10.1016/0006-2952(90)90494-6. [DOI] [PubMed] [Google Scholar]
- REMY C. N. Metabolism of thiopyrimidines and thiopurines. S-Methylation with S-adenosylmethionine transmethylase and catabolism in mammalian tissues. J Biol Chem. 1963 Mar;238:1078–1084. [PubMed] [Google Scholar]
- Riggs A. D., Jones P. A. 5-methylcytosine, gene regulation, and cancer. Adv Cancer Res. 1983;40:1–30. doi: 10.1016/s0065-230x(08)60678-8. [DOI] [PubMed] [Google Scholar]
- Scannell J. P., Hitchings G. H. Thioguanine in deoxyribonucleic acid from tumors of 6-mercaptopurine-treated mice. Proc Soc Exp Biol Med. 1966 Jul;122(3):627–629. doi: 10.3181/00379727-122-31210. [DOI] [PubMed] [Google Scholar]
- Silverman A. L., Park J. G., Hamilton S. R., Gazdar A. F., Luk G. D., Baylin S. B. Abnormal methylation of the calcitonin gene in human colonic neoplasms. Cancer Res. 1989 Jul 1;49(13):3468–3473. [PubMed] [Google Scholar]
- Stet E. H., De Abreu R. A., Bökkerink J. P., Vogels-Mentink T. M., Lambooy L. H., Trijbels F. J., Trueworthy R. C. Reversal of 6-mercaptopurine and 6-methylmercaptopurine ribonucleoside cytotoxicity by amidoimidazole carboxamide ribonucleoside in Molt F4 human malignant T-lymphoblasts. Biochem Pharmacol. 1993 Aug 3;46(3):547–550. doi: 10.1016/0006-2952(93)90534-4. [DOI] [PubMed] [Google Scholar]
- Stet E. H., De Abreu R. A., Janssen Y. P., Bökkerink J. P., Trijbels F. J. A biochemical basis for synergism of 6-mercaptopurine and mycophenolic acid in Molt F4, a human malignant T-lymphoblastic cell line. Biochim Biophys Acta. 1993 Jan 22;1180(3):277–282. doi: 10.1016/0925-4439(93)90050-b. [DOI] [PubMed] [Google Scholar]
- Tidd D. M., Paterson A. R. A biochemical mechanism for the delayed cytotoxic reaction of 6-mercaptopurine. Cancer Res. 1974 Apr;34(4):738–746. [PubMed] [Google Scholar]
- Ueland P. M. Pharmacological and biochemical aspects of S-adenosylhomocysteine and S-adenosylhomocysteine hydrolase. Pharmacol Rev. 1982 Sep;34(3):223–253. [PubMed] [Google Scholar]
- Vogt M. H., Stet E. H., De Abreu R. A., Bökkerink J. P., Lambooy L. H., Trijbels F. J. The importance of methylthio-IMP for methylmercaptopurine ribonucleoside (Me-MPR) cytotoxicity in Molt F4 human malignant T-lymphoblasts. Biochim Biophys Acta. 1993 Apr 30;1181(2):189–194. doi: 10.1016/0925-4439(93)90110-m. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Sladek S. L. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980 Sep;32(5):651–662. [PMC free article] [PubMed] [Google Scholar]
- Woods R. A., Henderson R. M., Henderson J. F. Consequences of inhibition of purine biosynthesis de novo by 6-methylmercaptopurine ribonucleoside in cultured lymphoma L5178Y cells. Eur J Cancer. 1978 Jul;14(7):765–770. doi: 10.1016/0014-2964(78)90007-5. [DOI] [PubMed] [Google Scholar]
- de Abreu R. A., van Baal J. M., Bakkeren J. A., de Brugn C. H., Schretlen E. D. High-performance liquid chromatographic assay for identification and quantitation of nucleotides in lymphocytes and malignant lymphoblasts. J Chromatogr. 1982 Jan 8;227(1):45–52. doi: 10.1016/s0378-4347(00)80354-0. [DOI] [PubMed] [Google Scholar]


