Abstract
Context
There has been a limited amount of research which comparatively examines the local and landscape scale ecological determinants of the community structure of both riparian and aquatic bird communities in floodplain ecosystems.
Objectives
Here, we quantified the contribution of local habitat structure, land cover and spatial configuration of the sampling sites to the taxonomical and functional structuring of aquatic and terrestrial bird communities in a relatively intact floodplain of the river Danube, Hungary.
Methods
We used the relative abundance of species and foraging guilds as response variables in partial redundancy analyses to determine the relative importance of each variable group.
Results
Local-scale characteristics of the water bodies proved to be less influential than land cover and spatial variables both for aquatic and terrestrial birds and both for taxonomic and foraging guild structures. Purely spatial variables were important determinants, besides purely environmental and the shared proportion of variation explained by environmental and spatial variables. The predictability of community structuring generally increased towards the lowest land cover measurement scales (i.e., 500, 250 or 125 m radius buffers). Different land cover types contributed at each scale, and their importance depended on aquatic vs terrestrial communities.
Conclusions
These results indicate the relatively strong response of floodplain bird communities to land cover and spatial configuration. They also suggest that dispersal dynamics and mass-effect mechanisms are critically important for understanding the structuring of floodplain bird communities, and should therefore be considered by conservation management strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10980-024-01948-3.
Keywords: Functional traits, Land cover, Habitat structure, Spatial factors, Variance partitioning
Introduction
Floodplains are essential components of natural riverine landscapes, which maintain high biodiversity due to the heterogeneity in the structure and spatial configuration of terrestrial and aquatic habitats (Ward et al. 1999; Thorp et al. 2006). Despite their crucial importance for biodiversity, floodplains are among the most endangered ecosystems globally, caused by large-scale river regulation works, that altered the spatial and temporal complexity of terrestrial, riparian, wetland and open water habitats (Ward et al. 1999; Tockner and Stanford 2002; Habersack et al. 2016). In Europe, which is the most human-dominated continent, up to 90% of former floodplains have been degraded to functional extinction (Tockner et al. 2010), with the degradation of natural hydrological dynamics and ecological processes between the land–water interface. Therefore, a detailed understanding of how both the terrestrial and aquatic environment shapes the structuring of biotic communities in still relatively intact floodplains could provide useful implications for the restoration and conservation management of floodplain ecosystems, especially in temperate regions, where most large rivers lost their floodplains (Hayes et al. 2018; Havrdová et al. 2023).
Birds are important components of the biota of floodplain ecosystems, especially since they occupy both terrestrial and aquatic habitats (Davis 1994; Kingsford et al. 2004; Lorenzón, et al. 2016a, 2019). As birds are conspicuous and mobile vertebrates, they can respond quickly to the dynamic changes of landscapes, which makes them advantageous model organisms in landscape ecological research (Sullivan et al. 2007; Gao et al. 2021). For example, among aquatic birds, different species use the mosaic of dynamically changing floodplains in various ways according to their local environmental characteristics (Kingsford et al. 2004; Lorenzón et al. 2016a). Hydrological dynamics can filter their community composition since there are species groups that prefer more secluded wetlands, silt plateaus, deeper water bodies or even running rivers (Boulton et al. 2008).
Besides the local environment, the spatial configuration of landscape patches can also affect a wide variety of ecological processes, which determine the community structure of birds (Wiens 2002; Thornton et al. 2011; Pérez-García et al. 2014). For example, some forest bird species, such as cavity-nesting species were found to be more sensitive to the surrounding land cover than to the local characteristics of habitat patches (Estades and Temple 1999; Vergara and Armesto 2009; Pérez-García et al. 2014). At the community level, local species richness can also depend on both local and regional landscape-level factors (Ekroos and Kuussaari 2012; Pérez-García et al. 2014). Therefore, a better understanding of the role of local, landscape-level and spatial variables in the structuring of floodplain bird communities could help the conservation of this important vertebrate group. However, despite the recognition of the importance of bird communities in floodplain habitats (Selwood et al. 2017; Lorenzón et al. 2019; Machar et al. 2022), there is currently insufficient knowledge on the structuring of bird communities to both spatial context and environmental characteristics of both terrestrial and aquatic components of the floodplains of large alluvial rivers (Arruda Almeida et al. 2016; Lorenzón et al. 2016b; Fluck et al. 2020).
Characterising trait-environment relationships has been emphasized to be a useful alternative approach for understanding the responses of ecological communities to the heterogeneity of the environment (Erős et al. 2009; Arruda Almeida et al. 2018; Rault et al. 2023). In this regard, functional traits, which directly inform the function of species in the environment proved to be especially important (Erős et al. 2009; Tavares et al. 2015; Arruda Almeida et al. 2018). However, taxonomic and functional characterizations of communities have been developed rather independently (Sheldon et al. 2011; Mouillot et al. 2013; Velásquez-Tibatá et al. 2013). For floodplain bird communities, it is generally unknown how, and to what extent taxonomic- and trait-based community structures show congruent patterns along major environmental gradients, and specifically, what is the role of different explanatory variable groups in predicting taxonomic and trait-based structure (but see e.g., Lorenzón et al. 2016b; Andrade et al. 2018; Aguilar et al. 2021).
Therefore, this study aimed to quantify the relative importance of local habitat structure, land cover and space in the variation of taxonomic and functional structure of both terrestrial-riparian (hereafter terrestrial) and aquatic bird communities across a river-floodplain landscape of the river Danube, Hungary. We were especially interested in determine the individual and shared effects of the above-mentioned variable groups to better understand the predictability of bird communities and the role of landscape context on predictability. In addition, we also examined the role of scale in quantifying the importance of land cover variables, since some studies found this characteristic influential (Henckel et al. 2019; Meffert and Dziock 2013).
For terrestrial bird communities, we predicted that land cover will be the most important variable group, which would determine both taxonomic and trait based structure, which have been shown to be influential determinant in other studies as well (Bossenbroek et al. 2005; Meffert and Dziock 2013; Selwood et al. 2017; Henckel et al. 2019). We also predicted that the predictive power of land cover will increase with decreasing measurement scale of land cover variables (i.e., using different radii around the study sites to characterize the contribution of land cover) since terrestrial birds show a strong affinity to land cover variables (Meffert and Dziock 2013; Henckel et al. 2019). On the contrary, for aquatic bird communities, we predicted the overarching importance of local habitat features of the waterbodies (Lorenzón et al. 2016b, 2019), and especially the importance of hydrological connectivity gradient to the main channel, since this variable can largely determine other features of habitat structure (Tockner et al. 1999; Bolland et al. 2012; Reid et al. 2016). Finally, we also predicted that both local habitat features and land cover variables will be more important predictors of bird communities than purely the spatial location of the sampling sites (i.e., space), since dispersal limitation may less influence birds than is the case for other taxa (Sullivan et al. 2007; Gao et al. 2021), at least at the floodplain scale. Rather, spatial location may interact with other features of the habitat, especially in floodplain ecosystems, where the strong influence of lateral connectivity gradients may increase the joint (i.e., shared) importance of spatial and environmental variables in community structuring. Overall, these results would indicate that landscape context is especially important for understanding the structuring of (floodplain) bird communities (Fletcher and Hutto 2008; Yuan et al. 2014; Lorenzón et al. 2016a).
Material and methods
Study area and site selection
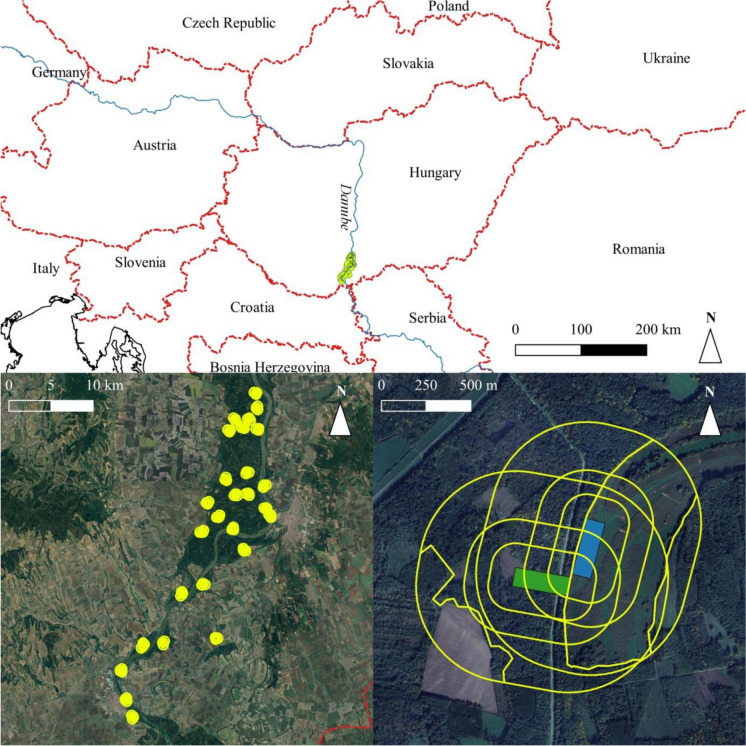
We appointed our study sites in the Middle Danube, Southern Hungary, between river km 1499–1433 (Fig. 1). The river Danube has a mean annual discharge of 2400 m3 s−1 in this region, with an average slope of about 5 cm km−1, and flow velocity of 0.8–1.2 m s−1 at mean water level (Schöll and Kiss 2008). This area consists of the largest functioning floodplain in the Middle Danube together with its transboundary Croatian counterpart (Hein et al. 2016; Funk et al. 2019). The major portion of the floodplain is part of the Danube-Dráva National Park and is also included in the list of protected sites in the Ramsar Convention on Wetlands of International Importance Especially as Waterfowl Habitat (Tardy 2007). This area has a variety of floodplain forest habitats (such as willow-poplar and oak-ash-elm floodplain forests), grasslands, agricultural fields, and a diverse array of aquatic habitats like side-arms, backwaters, wetlands, that are connected differently to the mainstream branch of the Danube River (Erős and Bányai 2020; Erős et al. 2023).
Fig. 1.
Location of the study area (top), the exact location of the study sites (bottom left) and an example of the aquatic and terrestrial bird transect arrangements with the different buffer zones for land cover measurements (bottom right). In this image the green rectangle indicates a terrestrial bird transect, while the blue one represents an aquatic bird transect
A total of 27 waterbodies were selected in the floodplains (Fig. 1) based on three major criteria: (i) to represent a hydrological connectivity gradient from the main river to the most isolated backwaters, (ii) to be located relatively evenly across the study area, (iii) to have no heavy anthropogenic degradation (e.g. oxbows with intense recreational activities were excluded from site selection). The mean distance between the study sites was 14.4 km (min–max range: 1.2–40.8 km).
Bird census
In 2022, we counted terrestrial and aquatic birds in two separate transects of 100 × 300 m as study plots at each water body. Since bird populations change over time, due to the migrating and nesting phenologies of the species, we mitigated this bias in detection probability by counting the individuals three times from late March to early July (Thompson 2002). There was at least a 1 month time lag between the three field sessions. All birds seen or heard were registered except for flyovers, and we used the maximal abundance of each species of the three visits at each transect in further analyses. To ensure that the maximum number of species was encountered, visits lasted between 1 to 5 h after sunrise (the period of highest bird activity) and were only carried out in suitable weather conditions (low wind, no rain or mist) (Dallimer et al. 2012; Andrade et al. 2018). We visited the sampling sites in alternating orders, to avoid temporal bias in detection probability related to the time of day (Blake 1992; Cornils et al. 2015).
For terrestrial birds, we delineated each rectangular transect perpendicular to the bank of each water body (Fig. 1), starting from the beginning of terrestrial vegetation to represent the horizontal gradient in terrestrial floodplain habitats (modified after Perry et al. 2011; Yabuhara et al. 2019). Transect of aquatic birds was placed parallel with the water body right on the bank (i.e. margin of the waterbody) including the silt plateau, (modified after Sulai et al. 2015; Andrade et al. 2018).The size of all transects was the same (i.e. 100 m wide 300 m long), regardless of the size and shape of the water body.
For the determination of functional structure, both terrestrial (45 species) and aquatic (33 species) birds were assigned to foraging guilds using generally accepted categories (Tables 1 and 2). Aquatic birds were categorized as dabbling ducks, vegetation gleaners, small waders, large waders, divers, fishers and raptors (Tavares et al. 2015; Shuford et al. 2016), while terrestrial birds were categorized as herbivores, ground insectivores, shrub insectivores, bark insectivores, canopy insectivores, omnivores and raptors (Pereira et al. 2014; Czeszczewik et al. 2015).
Table 1.
List of encountered species and their relative abundances along aquatic bird transects with their assigned foraging guilds
| Foraging guild | Common name | Scientific name | Mean relative abundance (%) |
|---|---|---|---|
| Dabbling ducks | Mute swan | Cygnus olor | 1.78 |
| Garganey | Spatula querquedula | 0.55 | |
| Eurasian wigeon | Mareca penelope | 1.62 | |
| Mallard | Anas platyrhynchos | 38.41 | |
| Common teal | Anas crecca | 3.56 | |
| Common pochard | Aythya ferina | 1.47 | |
| Ferruginous duck | Aythya nyroca | 0.29 | |
| Vegetation gleaners | Common moorhen | Gallinula chloropus | 0.26 |
| Common coot | Fulica atra | 2.19 | |
| Small waders | Black-winged stilt | Himantopus himantopus | 0.14 |
| Northern lapwing | Vanellus vanellus | 2.56 | |
| Common ringed plover | Charadrius hiaticula | 0.99 | |
| Black-tailed godwit | Limosa limosa | 0.03 | |
| Common sandpiper | Actitis hypoleucos | 0.49 | |
| Green sandpiper | Tringa ochropus | 3.12 | |
| Common redshank | Tringa totanus | 2.02 | |
| Common greenshank | Tringa nebularia | 0.27 | |
| Large waders | Eurasian spoonbill | Platalea leucorodia | 0.01 |
| Black-crowned night-heron | Nycticorax nycticorax | 0.74 | |
| Grey heron | Ardea cinerea | 10.53 | |
| Purple heron | Ardea purpurea | 0.12 | |
| Great white egret | Ardea alba | 2.56 | |
| Little egret | Egretta garzetta | 8.18 | |
| Divers | Great crested grebe | Podiceps cristatus | 0.42 |
| Little grebe | Tachybaptus ruficollis | 0.13 | |
| Pygmy cormorant | Microcarbo pygmaeus | 0.12 | |
| Great cormorant | Phalacrocorax carbo | 0.34 | |
| Fishers | Black-headed gull | Larus ridibundus | 0.13 |
| Common tern | Sterna hirundo | 0.21 | |
| Common kingfisher | Alcedo atthis | 1.19 | |
| Raptors | Western marsh-harrier | Circus aeruginosus | 0.25 |
| Black kite | Milvus migrans | 2.91 | |
| White-tailed sea-eagle | Haliaeetus albicilla | 1.31 |
Table 2.
List of encountered species and their relative abundances along terrestrial bird transects with their assigned foraging guilds
| Foraging guild | Common name | Scientific name | Mean relativeabundance (%) |
|---|---|---|---|
| Herbivores | Common pheasant | Phasianus colchicus | 0.42 |
| Common woodpigeon | Columba palumbus | 2.47 | |
| European turtle-dove | Streptopelia turtur | 0.33 | |
| Eurasian collared-dove | Streptopelia decaocto | 0.07 | |
| Common chaffinch | Fringilla coelebs | 9.24 | |
| Hawfinch | Coccothraustes coccothraustes | 0.90 | |
| European greenfinch | Chloris chloris | 0.67 | |
| European goldfinch | Carduelis carduelis | 0.71 | |
| Yellowhammer | Emberiza citrinella | 1.32 | |
| Eurasian tree sparrow | Passer montanus | 0.79 | |
| Ground insectivores | Eurasian wryneck | Jynx torquilla | 0.25 |
| Eurasian green woodpecker | Picus viridis | 0.73 | |
| Grey-faced woodpecker | Picus canus | 0.19 | |
| Northern wren | Troglodytes troglodytes | 2.11 | |
| Eurasian blackbird | Turdus merula | 2.50 | |
| Song thrush | Turdus philomelos | 2.52 | |
| European robin | Erithacus rubecula | 7.96 | |
| Common nightingale | Luscinia megarhynchos | 1.13 | |
| White wagtail | Motacilla alba | 0.63 | |
| Shrub insectivores | Black redstart | Phoenicurus ochruros | 0.21 |
| Eurasian blackcap | Sylvia atricapilla | 8.37 | |
| Common chiffchaff | Phylloscopus collybita | 4.22 | |
| Bark insectivores | Great spotted woodpecker | Dendrocopos major | 4.25 |
| Syrian woodpecker | Dendrocopos syriacus | 0.14 | |
| Middle spotted woodpecker | Leiopicus medius | 1.85 | |
| Lesser spotted woodpecker | Dryobates minor | 0.58 | |
| Black woodpecker | Dryocopus martius | 0.67 | |
| Eurasian nuthatch | Sitta europaea | 4.91 | |
| Short-toed treecreeper | Certhia brachydactyla | 0.40 | |
| Eurasian treecreeper | Certhia familiaris | 0.56 | |
| Canopy insectivores | Eurasian golden oriole | Oriolus oriolus | 1.95 |
| Wood warbler | Phylloscopus sibilatrix | 0.76 | |
| Marsh tit | Poecile palustris | 1.93 | |
| Eurasian blue tit | Cyanistes caeruleus | 3.71 | |
| Great tit | Parus major | 14.34 | |
| Long-tailed tit | Aegithalos caudatus | 2.47 | |
| Spotted flycatcher | Muscicapa striata | 4.51 | |
| Collared flycatcher | Ficedula albicollis | 2.79 | |
| Omnivores | Common cuckoo | Cuculus canorus | 0.78 |
| Carrion crow | Corvus corone | 0.24 | |
| Eurasian jay | Garrulus glandarius | 0.92 | |
| Common starling | Sturnus vulgaris | 4.15 | |
| Raptors | Eurasian buzzard | Buteo buteo | 0.14 |
| Black kite | Milvus migrans | 0.08 | |
| White-tailed sea-eagle | Haliaeetus albicilla | 0.12 |
Local scale habitat variables
The surface area of each waterbody was measured using a Geographical Information System (QGIS v.3.16; QGIS Development Team 2022) and Google Earth Pro.
Hydrological connectivity was defined as a percent proportion of days in a year a waterbody is connected to the main channel (river Danube) (Reckendorfer et al.; 2006; Funk et al. 2013). Mean depth and mean current velocity were measured using a digital terrain model and a water velocity meter, respectively. Within waterbodies, habitat structure was further characterized using visually estimated percentage composition of the following variables at the place of the samplings: emergent, submerged and floating vegetation, floating algae, open water habitat and woody debris. The bank structure was similarly characterized using the following variables: percentage cover of woody (i.e., tree or large bushes) and herbaceous vegetation, canopy cover and cover of artificial surfaces (concrete, rip-rap). The percentage cover of substratum types was visually estimated using the following categories: silt, sand, gravel, and rock. For the general characteristics of the environmental variables, see Table 3.
Table 3.
Descriptive statistics of local habitat structure variables and their correlation with the first three principal components of the local habitat variables PCA
| Unit | Mean | SD | Min | Max | PC1 (42.05%) | PC2 (12.70%) | PC3 (12.31%) | |
|---|---|---|---|---|---|---|---|---|
| Area | ha | 62.63 | 115.47 | 0.30 | 464.37 | 0.79 | 0.44 | − 0.25 |
| Depth | m | 1.31 | 1.25 | 0.40 | 6.00 | 0.87 | 0.39 | − 0.15 |
| Emergent plants | % | 9.25 | 15.64 | 0.00 | 75.00 | − 0.54 | − 0.08 | − 0.13 |
| Submerged plants | % | 6.48 | 12.33 | 0.00 | 57.50 | − 0.5 | 0.46 | − 0.31 |
| Floating plants | % | 16.56 | 23.09 | 0.00 | 80.00 | − 0.59 | 0.47 | − 0.03 |
| Surface algae | % | 0.29 | 1.44 | 0.00 | 7.50 | − 0.28 | 0.34 | − 0.47 |
| Open water | % | 64.38 | 33.73 | 3.00 | 100.00 | 0.79 | − 0.43 | 0.21 |
| Woody debris | % | 3.04 | 6.70 | 0.00 | 35.00 | − 0.56 | 0.15 | − 0.14 |
| Woody/trees | % | 66.54 | 28.95 | 0.00 | 100.00 | 0.16 | 0.54 | 0.78 |
| Herbaceous | % | 31.88 | 29.13 | 0.00 | 100.00 | − 0.24 | − 0.53 | − 0.76 |
| Artificial | % | 1.58 | 3.02 | 0.00 | 10.00 | 0.77 | − 0.07 | − 0.18 |
| Canopy cover | % | 21.71 | 25.84 | 0.00 | 82.50 | − 0.63 | 0.19 | − 0.33 |
| Rock | % | 3.38 | 11.78 | 0.00 | 48.00 | 0.77 | 0.43 | − 0.35 |
| Sand | % | 7.35 | 12.12 | 0.00 | 50.00 | 0.76 | − 0.31 | 0.03 |
| Silt | % | 89.27 | 18.80 | 35.00 | 100.00 | − 0.92 | 0.05 | 0.12 |
| Velocity | cm/s | 6.12 | 14.83 | 0.00 | 60.00 | 0.86 | 0.3 | − 0.29 |
| Connectivity | % | 41.4 | 34.9 | 0 | 100 | 0.22 | − 0.25 | − 0.23 |
The explained variance of each principal component is shown in parentheses
We used Principal Component Analysis (hereafter PCA) to characterize physical habitat structure and to reduce the number of explanatory variables to a small number of largely independent (orthogonal) environmental gradients (see e.g., Amoros and Bornette 2002; Peres-Neto et al. 2003; Heino et al. 2007; Legendre and Legendre 2012; Czeglédi et al. 2016, 2020; Sinha et al. 2019). The PCA was conducted on the correlation matrix of the recorded physical habitat structure variables, using the function “prcomp” in the package factoextra 1.0.7. (Kassambara and Mundt 2017). The values of the variables were square-root arcsin transformed in advance of the PCA (Legendre and Legendre 2012; Luck et al. 2013).
According to the PCA analysis, the first three most influential and interpretable environmental gradients with their eigenvalue over 1 and their explained variance over 10% were as follows (for details see Table 3 and Online Appendix 1). The PC1 axis characterized a gradient where relatively deep water bodies with high velocity, and relatively coarse substrate composition (dominantly sand) occupied one end, while relatively shallow water bodies with dense canopy and/or macrophyte cover, woody debris and fine substrate composition (silt) occupied the other end of the gradient. The PC2 axis represented a gradient where sites with relatively high canopy cover, and trees along the bank were situated on one end, while sites with relatively dense macrophyte cover and herbaceous bank vegetation the other end of the gradient. PC3 was determined mainly by canopy cover, herbaceous bank vegetation, woody debris, sand substrate, and the composition of macrophyte vegetation.
We used the component scores of the water bodies along these most influential first three principal components as explanatory variables in further analyses (see below at variance partitioning).
Landscape metrics
We measured the percentage cover of selected land cover types from the CORINE Land Cover 2018 database GIS layer (European Environmental Agency 2020, http://www.eea.europa.eu; Mag et al. 2011; Portaccio et al. 2021) in 125, 250 and 500 m buffers around each rectangular study plot (modified after Akasaka et al. 2010; Milder et al. 2010; Yabuhara et al. 2019). The studied land cover types were as follows: agricultural, artificial, forest, natural grassland, transitional woodland-shrub, water body and wetland. We measured the percentage cover of each land cover type in Quantum GIS version 3.4.12-Madeira (QGIS 2022).
Spatial metrics
We used only positively autocorrelated Moran’s Eigenvector Matrices (MEM) from the geocoordinates of the sites as explanatory variables of spatial structuring (Peres-Neto and Legendre 2010; Sattler et al. 2010; Ferenc et al. 2014). We used the “dbmem” function of the adespatial package in R for the calculations (version 0.3–20; Dray et al. 2018).
Variance partitioning analyses
We conducted redundancy (RDA) and associated variance partitioning analyses (Borcard et al. 1992, 2011, 2018; Legendre and Legendre 2012) to quantify the pure and shared effects of the three predictor variable groups (local scale habitat structure, land cover and spatial positioning) on the structure of aquatic and terrestrial bird communities. We used the Hellinger-transformed relative abundance of taxa and foraging guilds separately in the analyses. Consequently, both the relatively short gradients we obtained using preliminary detrended correspondence analyses and Hellinger transformation of the data justify the applicability of linear ordination, such as RDA (Legendre and Gallagher 2001; Peres-Neto et al. 2006; Legendre and De Cáceres 2013; Lorenzón et al. 2016b; Borcard et al. 2018; Henckel et al. 2019; Anderson et al. 2011) Statistical significance of the unique contributions of the three sets of predictors was tested using the “anova.cca” function with 1000 runs in package vegan (version 2.6–4; Oksanen et al. 2017). In advance of variance partitioning, separate forward selection of the physical habitat PCA components, the land cover and spatial variables were computed using a permutation-based test with the “ordistep” function of the package vegan with 1000 runs (Rush et al. 2014; Hill et al. 2019). Only variables that significantly (alpha = 0.05) contributed to community variability were retained in the final models (Lorenzón et al. 2016b; Hill et al. 2019; Sultana et al. 2022). All analyses were conducted in the R environment 4.2.2 (R Core Team 2022).
Results
Aquatic birds
Throughout the transects, we recorded 778 aquatic birds of 33 species (see Table 1 for the species list and relative abundance data). The mallard (Anas platyrhynchos), grey heron (Ardea cinerea) and little egret (Egretta garzetta) were the three most abundant species and had a relative abundance of 38%, 11% and 8% respectively. The three foraging guilds with the highest relative abundance were dabbling ducks (54%), large wading birds (25%) and small wading birds (11%). For the relative abundance data of aquatic species, the total explained variance was 10%, 14% and 15% for the 500, 250 and 125 m spatial scales, respectively. All three variable groups contributed to the explained variance at the 500 m scale, with both pure and shared components (Table 4a), while at the 250 m scale pure local habitat structure, land cover, spatial and the joint (i.e., spatially structured local habitat structure) components were influential. On the other hand, pure land cover, spatial variables, locally structured land cover—the shared component of local and land cover factors—and the intersection of all three variable groups contributed to the explained variance at the 125 m scale. Considering local habitat structure, only the first principal component contributed to the explained variance at each scale. Land cover types contributed differently at the different scales (Table 5): at the 500 m scale the cover of transitional woodland-shrub areas, at the 250 m scale, the cover of wetlands and at the 125 m scale the cover of forests and wetlands affected taxonomic structure significantly. The importance of land cover increased with decreasing scale. The shared components had only a marginal contribution to the explained variance.
Table 4.
Results of variance partitioning analyses, which shows the proportions of explained variance of pure local (lo), land cover (la), spatial (sp) variables and their shared components at different landscape scales (500 m, 250 m, 125 m)
| Aquatic species relative abundance | Local (%) | lo+la (%) | Land cover (%) | Spatial (%) | lo + sp (%) | lo + la + sp (%) |
|---|---|---|---|---|---|---|
| a) | ||||||
| 500 m | 18 | 9 | 9 | 36 | 9 | 9 |
| 250 m | 20 | 0 | 33 | 33 | 13 | 0 |
| 125 m | 0 | 13 | 40 | 33 | 0 | 13 |
| Aquatic foraging guild relative abundance | Local (%) | lo + la (%) | Land cover (%) | Spatial (%) | lo + sp (%) | lo + la + sp (%) |
|---|---|---|---|---|---|---|
| b) | ||||||
| 500 m | 0 | 7 | 0 | 43 | 14 | 21 |
| 250 m | 7 | 0 | 0 | 57 | 36 | 0 |
| 125 m | 7 | 0 | 0 | 57 | 36 | 0 |
| Terrestrial species relative abundance | Land cover (%) | la + sp (%) | Spatial (%) | |||
|---|---|---|---|---|---|---|
| c) | ||||||
| 500 m | 43 | 43 | 14 | |||
| 250 m | 63 | 38 | 0 | |||
| 125 m | 67 | 33 | 0 | |||
| Terrestrial foraging guild relative abundance | Land cover (%) | la+sp (%) | Spatial (%) | |||
|---|---|---|---|---|---|---|
| d) | ||||||
| 500 m | 48 | 0 | 52 | |||
| 250 m | 58 | 0 | 42 | |||
| 125 m | 70 | 0 | 30 | |||
Table 5.
Determinants of the taxonomic and foraging guild structure of aquatic and terrestrial bird communities. Variables that contributed significantly to each variable group in variance partitioning at different landscape scales (500 m, 250 m, 125 m) are listed. PC1 characterizes the main environmental gradient, while the different MEM vectors represent spatial gradients based on Moran’s Eigenvector Matrices
| Response | Variable group | 500 m | 250 m | 125 m |
|---|---|---|---|---|
| Aquatic species relative abundance | Local scale habitat structure | PC1 | PC1 | PC1 |
| Land cover | Transitional woodland-shrub | Wetland | Wetland, forest | |
| Spatial vector | MEM3 | MEM3 | MEM3 | |
| Aquatic guild relative abundance | Local scale habitat structure | PC1 | PC1 | PC1 |
| Land cover | Transitional woodland-shrub | – | – | |
| Spatial vector | MEM3 | MEM3 | MEM3 | |
| Terrestrial species relative abundance | Local scale habitat structure | – | – | – |
| Land cover | Agricultural | Agricultural, transitional woodland-shrub | Agricultural, transitional woodland-shrub | |
| Spatial vector | MEM8 | MEM8 | MEM8 | |
| Terrestrial guild relative abundance | Local scale habitat structure | – | – | – |
| Land cover | Agricultural | Agricultural, natural grassland | Agricultural, natural grassland, transitional woodland-shrub | |
| Spatial vector | MEM1, MEM5 | MEM1, MEM5 | MEM1, MEM5 |
For the relative abundance of aquatic foraging guilds, the total explained variance was 14% at each spatial scale. All three variable groups contributed to the explained variance at the 500 m scale, while land cover did not contribute to the explained variance at other scales (Table 4b). At the 500 m scale the only contributing pure component was the spatial variable group, while at other scales pure local, pure spatial and spatially structured local components were represented. Considering local habitat structure, only the first principal component contributed to the explained variance (Table 5). Land cover types (specifically transitional woodland-shrub) proved to be influential only at the 500 m scale (Table 5). The majority of the explained variance was contributed by the pure spatial component at each scale (57%), while the locally structured spatial component comprised the second highest proportion (36%). The pure local component had only a marginal influence on guild-based structure (Table 4b).
Terrestrial birds
We observed 1192 individuals of 45 terrestrial bird species (see Table 2 for the species list and relative abundance data). The great tit (Parus major), common chaffinch (Fringilla coelebs) and Eurasian blackcap (Sylvia atricapilla) were the three most abundant species and had a relative abundance of 14%, 9% and 8% respectively. Crown insectivores (25%), ground insectivores (18%) and herbivores (17%) were the three most abundant foraging guilds and were present in 25, 18 and 17% relative abundances. For the relative abundance data of terrestrial species, the total explained variance was 6%, 8% and 9% for the 500, 250 and 125 m buffer zones, respectively. Only land cover and spatial variables contributed to the explained variance (Table 4c). At the 500 m scale, both the two pure components and their shared component contributed to the explained variance, but at other scales, no contribution of the pure spatial component emerged. Land cover types contributed differently to the variance at the different scales (Table 5): at the 500 m scale, only the cover of agricultural surfaces contributed to the explained variance, while at other scales the importance of both agricultural and transitional woodland-shrub surfaces emerged. The importance of the pure land cover variable group increased with decreasing spatial scale (from 43% at the 500 m scale to 67% at the 125 m scale). Parallelly, the contribution of spatially structured landscape component decreased with decreasing scale (from 43 to 33%). The pure spatial component had only marginal contribution, and only at the 500 m scale.
For the relative abundance of foraging guilds, the total explained variance was 20%, 25% and 32% at the 500, 250 and 125 m scales, respectively. Only land cover and spatial variables contributed to the explained variance, and only with their pure components at each scale (Table 4d). Similarly to taxonomic structure, land cover types contributed differently to guild structure at the different spatial scales (Table 5). For example, agricultural fields were important at each scale, but natural grasslands were influential only at the 250 m and 125 m scales. In addition, the contribution of transitional woodland shrub surfaces was significant only at the 125 m scale. The importance of the pure land cover variable group increased with decreasing scale, similarly to taxonomic structure. The pure landscape component contributed significantly to the explained variance at each scale, and its contribution further increased with decreasing scale (from 48% at the 500 scale to 70% at the 125 m scale).
Discussion
Total variance
We found low total explained variances for both aquatic and terrestrial bird communities, which varied between 9 and 32%. These values could have been even lower if we had not accounted for detection probability bias in the methodology, such as by conducting three count sessions and alternating the order of visits (Thompson 2002; Cornils et al. 2015). The lowest and highest total explained variances were found for the taxonomic and functional structuring of terrestrial birds, respectively, while aquatic birds showed intermediate variance values. Several factors may contribute to the differences in the predictability of terrestrial and bird communities and between taxonomic and functional approaches, including for example the range of the underlying environmental gradient(s), the number of environmental and spatial predictors structuring the communities, or the number of taxa or functional groups used in the analysis (Heino et al. 2007, 2013).
Low total explained variance values are more general than exceptional in those community ecological studies which deal with the importance of environmental structuring and space using variance partitioning procedures (Sattler et al. 2010; Meffert et al. 2013; Meynard et al. 2013; Heino et al. 2015a). Several authors assumed that low total variance values can be due to nondeterministic factors, such as unmeasured biotic and abiotic variables, or more complex spatial structure compared to what can be characterized by field observations (Borcard et al. 1992; Sattler et al. 2010; Henckel et al. 2019; Ovaskainen et al. 2019). On the other hand, others argued that the interpretation of ‘unexplained variation’ as random variation caused by unmeasured factors is generally inappropriate (e.g., Økland 1999; Meffer and Dziock 2013). Due to the high variability in ecological data, and elusive species environmental relationships, Økland (1999) recommended concentrating on the relative contribution of variation explained by different sets of explanatory variables rather than focusing on the importance of explained and unexplained variations. Stegen and Hurlbert (2011) also argued that low explained variance does not necessarily indicate weak dispersal limitation and environmental filtering and suggested to use relative proportions of partitioned variances to characterize the relative influences of these two mechanisms. Consequently, we focussed on the interpretation of the relative importance of the different variable groups in the discussion below.
Local scale habitat structure
As hypothesised, local-scale habitat structure of the waterbodies and the riparian zone proved to be important for aquatic birds in the case of both taxonomic and foraging guild structure. However, its contribution as a pure or as a shared component varied depending on spatial scale, presumably due to its interference with land cover variables at different measurement scales. Several papers considered the importance of the habitat structure of water bodies on aquatic bird species composition (Godinho et al. 2010; Arruda Almeida et al. 2016; Lorenzón et al 2016b), but, to the best of our knowledge, this study is the first which used a variance partitioning approach to comparatively examine the importance of different variable groups on foraging guild structure.
The first principal component was the only local scale habitat gradient which significantly influenced aquatic species, including both taxonomic and foraging guild structures. This is not surprising since PC1 characterized the most substantial changes in habitat quality, embracing differences in area, depth, flow velocity, and composition of substrate, aquatic plant and riparian vegetation. Interestingly, hydrologic connectivity correlated only moderately with these habitat variables. This finding shows the complex relationship between local scale habitat structure and hydrological connectivity and also reveals that connectivity in itself cannot substitute other variables for characterizing the habitat structure of floodplain water bodies. These results on the importance of complex local scale habitat gradients correspond with the findings of former studies (e.g., Godinho et al. 2010; Lorenzón et al. 2019; Arruda Almeida et al. 2016; 2018; Fluck et al. 2020).
Land cover
To the best of our knowledge, no scientific papers aimed to compare the contribution of habitat characteristics of the water bodies and land cover elements in the structuring of terrestrial floodplain bird communities. Land cover proved to be a more important determinant of both the taxonomic and functional structure of terrestrial birds than aquatic ones. This result is not surprising since terrestrial birds use the terrestrial landscape for foraging and nesting, while aquatic species are connected more to the water bodies and wetlands. However, the zero contribution of local scale habitat structure of the waterbodies in the structuring of terrestrial bird communities is somewhat surprising, since floodplain water bodies and wetlands can influence insectivore bird populations by the density of the swarming aquatic invertebrates, known as aquatic subsidies, such as mayflies (Ephemeroptera) and stoneflies (Plecoptera), which can be important foraging sources in early spring, before the later pulse of canopy insects, such as aphids (Hemiptera) and caterpillars (Lepidoptera) (Nakano and Murakami 2001; Murakami and Nakano 2002; Iwata et al. 2003; Schilke et al. 2020; Wesner et al. 2020).
The contribution of land cover variables to the explained variance generally remained stable or increased with decreasing spatial scale of the evaluation area (here from 500 to 125 m). This finding thus supports, at least partly, our hypothesis that bird communities respond relatively strongly to the heterogeneity of land cover, especially at finer spatial scales, which may better fit their territory, foraging- and nesting area (see also Henckel et al. 2019; Meffert and Dziock 2013). According to Henckel et al. (2019), this statement can also stand for individual land cover types, as some types are more characteristic factors for fine-scale territories and feeding grounds of both terrestrial and aquatic species respectively, while others are more influential on larger scales. In our case, for aquatic birds, transitional woodland-shrub surfaces (i.e., shrublands) were only significant at the 500 m scale, while wetlands were crucial at the two smaller scales. For terrestrial birds, agricultural lands were important at each scale, while shrublands and natural grasslands were influential at the 250 and 125 m scales.
In our study, the variance of aquatic bird species structure was influenced by shrublands, wetlands and forests. The cover of shrublands was the only crucial land cover variable in the variance of aquatic bird foraging guild structure. The vertically complex but open habitat structure of shrublands can influence the structure of aquatic bird communities, for instance, numerous species groups, such as large waders prefer to nest in that particular habitat type in floodplain forests (Liang et al. 2007; Parkes et al. 2012). On the other hand, raptors favouring aquatic habitats can hunt their prey with higher success in open areas of shrublands (Davis et al. 2009). Wetlands are crucial for aquatic birds, supplying a variety of microhabitats from wet grasslands, silty beaches, across different associations of aquatic macrophytes to even open water surfaces. Such complex habitats can serve as feeding or nesting grounds for diverse aquatic bird communities (Lorenzón et al. 2016a, b; Galib et al. 2018; Htay et al. 2023). Similarly, forests provide shelter, and nesting microhabitats for ground-, canopy- and cavity-nesting water birds alike (Lemelin et al. 2010; Andrade et al. 2018; Sinha et al. 2022).
Agricultural and shrubland surfaces were generally important drivers in the structuring of terrestrial bird communities (i.e., both for species and foraging guilds), while natural grasslands only influenced the variance of foraging guild structure. The substantially simple habitat structure of monocultural agricultural lands only can harbour poor bird communities, resulting in species and foraging guilds preferring open habitats that can tolerate such a low diversity of foraging sources (Best et al. 1995; Selwood et al. 2015; Socolar and Wilcove 2019). As the vertical structure of shrublands is more complex than open grasslands but less so than closed forests, this particular habitat type can harbour species of open habitats as well as forest edge or open forest-dwelling species. Thus, the cover of this habitat type can substantially influence the explained variance of species structure in a landscape (Knutson 1995; Lorenzón et al. 2016a). The cover of natural grasslands can influence the presence of numerous foraging guilds, since for example shrub insectivores live in either shrublands or forests, while bark-foraging and canopy insectivores prefer forest habitats and hardly can be present in grasslands (Reid et al. 2016; Fourcade et al. 2018; Senner et al. 2021).
Space
We found a relatively high contribution of purely spatial variables to the explained variance in the case of both aquatic and terrestrial birds and both for taxonomic and functional structure. This suggests that dispersal limitation would be an influential factor in community structuring (Gianuca et al. 2013; Henckel et al. 2019). On the other hand, the decreasing contribution towards the smaller scales suggests the decreasing importance of dispersal limitations (Henckel et al. 2019). This result was more expressed in terrestrial birds, which in our case are mainly territorial forest species, showing only post-natal dispersion in the forest corridors on relatively small scales (Machtans et al. 1996; Laurance and Gomez 2005; Seaman and Schulze 2010). On the other hand, most aquatic birds are considered large distance dispersers, regarding their movements between foraging habitats (Reynolds et al. 2015; Coughlan et al. 2017). Henckel et al. (2019) suggested that pure spatial structuring may be explained by individual movements during the breeding season rather than dispersal limitation sensu stricto. Purely spatial variables may also indicate the random (i.e., environmentally independent) aggregation of some species and/or functional groups during their movement across the landscape by mass-effect mechanisms (Meynard and Quinn 2008; Watson and Watson 2015; de Souza Leite et al. 2022). In this regard, mass-effects may increase in importance with decreasing distances between sites and small spatial extent surveyed (Heino et al. 2015b).
Joint portions
The shared component of space with local or land cover variables proved to be also important, especially in the functional structuring of aquatic birds (here spatially structured local scale habitat structure), and in the taxonomic structure of terrestrial birds (spatially structured land cover). Spatially structured environmental components (both local habitat structure and landscape features alike) indicate the spatial distribution of important environmental gradients that influence the dispersion of bird species and foraging guilds (Sattler et al. 2010). For example, for aquatic birds, this component embraces the sorting of species and/or functions along the lateral habitat and connectivity gradients from the main river to the most secluded backwaters (see also Parkinson et al. 2002). For terrestrial species, the spatially structured land cover component mirrors the effect of spatial heterogeneity in the distribution of land cover types (e.g., shrubland surfaces) both longitudinally along the river and laterally along the floodplain (Renöfält et al. 2005). Nevertheless, the large variability in the contribution of pure and shared variance components between measurement scales suggests that the effect of environmental heterogeneity, space and neutral or stochastic mechanisms cannot be easily dissected in the case of floodplain bird communities, similarly to other ecosystems or organism groups (see e.g., Borcard et al. 1992; Sattler et al. 2010; Stegen and Hurlbert 2011).
Conclusions
In conclusion, the structuring of floodplain bird communities showed high context-dependency, similar to many other studies on the metacommunity structuring of ecological communities. Generally, local scale characteristics of the waterbodies and the riparian zone proved to be less influential in community structuring than land cover and spatial variables both for aquatic and terrestrial birds and both for taxonomic and foraging guild structure. The importance of purely spatial variables suggests that mass-effect mechanisms also shape the structuring of floodplain bird communities, besides species sorting mechanisms. Mass-effect may have contributed to the low predictability of community structuring, despite the use of a variety of environmental variables. The predictability of community structuring was also influenced by the measurement scale of land cover variables (i.e., 500, 250 or 125 m radius around the survey transect) and was generally highest at the lowest spatial extent. Overall, these results indicate the relatively strong response of floodplain bird communities to heterogeneities in land use, but also suggest that dispersal dynamics of birds across the floodplain is also critically important to understand the structuring of bird communities, which should be considered by conservation management.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix 1: The ordination plot of the PCA analysis of the local scale habitat variables. Supplementary file1 (PDF 7 KB)
Acknowledgements
We would like to express our thanks to Attila Mórocz and Endre Sztellik, rangers of the Danube-Dráva National Park for helping in the fieldwork and the suggestions of anonymous reviewers who contributed to the improvement of this material. The research presented in the article was carried out under the framework of the Széchenyi Plan Plus program with the support of the RRF 2.3.1 21 2022 00008 project, and the MERLIN project funded under the European Commission’s H2020 Programme (101036337-MERLIN—H2020-LC-GD-2020). The publication is also a part of the bilateral Austrian-Hungarian Joint Research Project RIMECO co-funded by the Austrian Science Fund (FWF) (I 5006) and the ANN-OTKA (141884) grant.
Author contributions
All authors contributed to conceptualization, methodology, data collection, analysis, visualization, writing the original draft and the finalization of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by HUN-REN Balaton Limnological Research Institute. The research presented in the article was carried out under the framework of the Széchenyi Plan Plus program with the support of the RRF 2.3.1 21 2022 00008 project, and the MERLIN project funded under the European Commission’s H2020 Programme (101036337-MERLIN—H2020-LC-GD-2020). The publication is also a part of the bilateral Austrian-Hungarian Joint Research Project RIMECO co-funded by the Austrian Science Fund (FWF) (I 5006) and the ANN-OTKA (141884) grant.
Data availability
The data presented in this study are available on request from the corresponding author.
Declarations
Competing interests
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguilar S, Mugica L, Acosta M, Aguilar K, Boscolo D, Castro R, Hernández Z, Manica LT (2021) Effect of landscape structure on waterbirds community in a conservation gradient in southwestern wetlands coast of Cuba. Wetl Ecol Manag 29:929–944. [Google Scholar]
- Akasaka T, Akasaka M, Yanagawa H (2010) Relative importance of the environmental factors at site and landscape scales for bats along the riparian zone. Landsc Ecol Eng 6(2):247–255. [Google Scholar]
- Amoros C, Bornette G (2002) Connectivity and biocomplexity in waterbodies of riverine floodplains. Freshwater Biol 47(4):761–776. [Google Scholar]
- Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG (2011) Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol Lett 14(1):19–28. [DOI] [PubMed] [Google Scholar]
- Andrade R, Bateman HL, Franklin J, Allen D (2018) Waterbird community composition, abundance, and diversity along an urban gradient. Landscape Urban Plan 170:103–111. [Google Scholar]
- Arruda Almeida BD, Silva CB, Gimenes MR, dos Anjos L (2016) Waterbirds in a floodplain: influence of spatial and environmental factors through time. Rev Bras Ornitol 24:314–322. [Google Scholar]
- Arruda Almeida BD, Green AJ, Sebastian-Gonzalez E, Dos Anjos L (2018) Comparing species richness, functional diversity and functional composition of waterbird communities along environmental gradients in the neotropics. PLoS ONE 13(7):e0200959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best LB, Freemark KE, Dinsmore JJ, Camp M (1995) A review and synthesis of habitat use by breeding birds in agricultural landscapes of Iowa. Am Midl Nat 134(1):1–29. [Google Scholar]
- Blake JG (1992) Temporal variation in point counts of birds in a lowland wet forest in Costa Rica. Condor 94(1):265–275. [Google Scholar]
- Bolland JD, Nunn AD, Lucas MC, Cowx IG (2012) The importance of variable lateral connectivity between artificial floodplain waterbodies and river channels. River Res Appl 28(8):1189–1199. [Google Scholar]
- Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, New York [Google Scholar]
- Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73(3):1045–1055. [Google Scholar]
- Borcard D, Gillet F, Legendre P (2018) Canonical ordination. In: Borcard D, Gillet F, Legendre P (eds) Numerical ecology with R, 2nd edn. Springer, Cham, pp 203–297 [Google Scholar]
- Bossenbroek JM, Wagner HH, Wiens JA (2005) Taxon-dependent scaling: beetles, birds, and vegetation at four North American grassland sites. Landscape Ecol 20:675–688. [Google Scholar]
- Boulton AJ, Boyero L, Covich AP, Dobson M, Lake PS, Pearson RG (2008) Are tropical streams ecologically different from temperate streams? In: Dudgeon D (ed) Tropical stream ecology. Academic Press, San Diego, pp 257–284 [Google Scholar]
- Cornils JS, Riedl I, Fricke J, Katz M, Schulze CH (2015) Population density and habitat preferences of the black-cheeked ant-tanager Habia atrimaxillaris. Bird Conserv Int 25(3):306–321. [Google Scholar]
- Czeglédi I, Kern B, Tóth R, Seress G, Erős T (2020) Impacts of urbanization on stream fish assemblages: the role of the species pool and the local environment. Front Ecol Evol 8:137. [Google Scholar]
- Czeglédi I, Sály P, Takács P, Dolezsai A, Nagy SA, Erős T (2016) The scales of variability of stream fish assemblages at tributary confluences. Aquat Sci 78:641–654. [Google Scholar]
- Czeszczewik D, Zub K, Stanski T, Sahel M, Kapusta A, Walankiewicz W (2015) Effects of forest management on bird assemblages in the Bialowieza forest, Poland. iForest 8(3):377. [Google Scholar]
- Dallimer M, Rouquette JR, Skinner AM, Armsworth PR, Maltby LM, Warren PH, Gaston KJ (2012) Contrasting patterns in species richness of birds, butterflies and plants along riparian corridors in an urban landscape. Divers Distrib 18(8):742–753. [Google Scholar]
- Davis JB, Leopold BD, Kaminski RM, Cox RR (2009) Wood Duck duckling mortality and habitat implications in floodplain systems. Wetlands 29:607–614. [Google Scholar]
- Davis TJ (1994) The Ramsar convention manual: a guide to the convention on wetlands of international importance especially as waterfowl habitat. Ramsar Convention Bureau, Gland [Google Scholar]
- de Souza LM, Boesing AL, Metzger JP, Prado PI (2022) Matrix quality determines the strength of habitat loss filtering on bird communities at the landscape scale. J Appl Ecol 59(11):2790–2802. [Google Scholar]
- Dray S, Blanchet G, Borcard D, Guenard G, Jombart T, Legendre P, Wagner H (2018) Package ‘adespatial’: multivariate multiscale spatial analysis. R-Project. https://cran.r-project.org/web/packages/adespatial/index.html. Accessed 05 Jan 2023
- Ekroos J, Kuussaari M (2012) Landscape context affects the relationship between local and landscape species richness of butterflies in semi-natural habitats. Ecography 35(3):232–238. [Google Scholar]
- Erős T, Bányai Z (2020) Sparing and sharing land for maintaining the multifunctionality of large floodplain rivers. Sci Total Environ 728:138441. [DOI] [PubMed] [Google Scholar]
- Erős T, Heino J, Schmera D, Rask M (2009) Characterising functional trait diversity and trait–environment relationships in fish assemblages of boreal lakes. Freshwater Biol 54(8):1788–1803. [Google Scholar]
- Erős T, Petrovszki J, Mórocz A (2023) Planning for sustainability: historical data and remote sensing-based analyses aid landscape design in one of the largest remnant European floodplains. Landscape Urban Plan 238:104837. [Google Scholar]
- Estades CF, Temple SA (1999) Deciduous-forest bird communities in a fragmented landscape dominated by exotic pine plantations. Ecol Appl 9(2):573–585 [Google Scholar]
- Ferenc M, Sedláček O, Fuchs R, Dinetti M, Fraissinet M, Storch D (2014) Are cities different? Patterns of species richness and beta diversity of urban bird communities and regional species assemblages in Europe. Global Ecol Biogeogr 23(4):479–489. [Google Scholar]
- Fletcher RJ, Hutto RL (2008) Partitioning the multi-scale effects of human activity on the occurrence of riparian forest birds. Landscape Ecol 23:727–739. [Google Scholar]
- Fluck IE, Cáceres N, Hendges CD, Brum MDN, Dambros CS (2020) Climate and geographic distance are more influential than rivers on the beta diversity of passerine birds in Amazonia. Ecography 43(6):860–868. [Google Scholar]
- Fourcade Y, Besnard AG, Beslot E, Hennique S, Mourgaud G, Berdin G, Secondi J (2018) Habitat selection in a dynamic seasonal environment: vegetation composition drives the choice of the breeding habitat for the community of passerines in floodplain grasslands. Biol Conserv 228:301–309. [Google Scholar]
- Funk A, Gschöpf C, Blaschke AP, Weigelhofer G, Reckendorfer W (2013) Ecological niche models for the evaluation of management options in an urban floodplain—conservation vs. restoration purposes. Environ Sci Policy 34:79–91. [Google Scholar]
- Funk A, Martínez-López J, Borgwardt F, Trauner D, Bagstad KJ, Balbi S, Magrach A, Villa F, Hein T (2019) Identification of conservation and restoration priority areas in the Danube River based on the multifunctionality of river-floodplain systems. Sci Total Environ 654:763–777. [DOI] [PubMed] [Google Scholar]
- Galib SM, Lucas MC, Chaki N, Fahad FH, Mohsin ABM (2018) Is current floodplain management a cause for concern for fish and bird conservation in Bangladesh’s largest wetland? Aquat Conserv 28(1):98–114. [Google Scholar]
- Gao B, Gong P, Zhang W, Yang J, Si Y (2021) Multiscale effects of habitat and surrounding matrices on waterbird diversity in the Yangtze River Floodplain. Landscape Ecol 36:179–190. [Google Scholar]
- Gianuca AT, Bastazini VAG, Dias RA, Hernández MIM (2013) Independent and shared effects of environmental features and space driving avian community beta diversity across a coastal gradient in southern Brazil. Austral Ecol 38(8):864–873. [Google Scholar]
- Godinho C, Rabaça JE, Segurado P (2010) Breeding bird assemblages in riparian galleries of the Guadiana River basin (Portugal): the effect of spatial structure and habitat variables. Ecol Res 25(2):283–294. [Google Scholar]
- Habersack H, Hein T, Stanica A, Liska I, Mair R, Jäger E, Hauer C, Bradley C (2016) Challenges of river basin management: current status of, and prospects for, the River Danube from a river engineering perspective. Sci Total Environ 543:828–845. [DOI] [PubMed] [Google Scholar]
- Havrdová A, Douda J, Doudová J (2023) Threats, biodiversity drivers and restoration in temperate floodplain forests related to spatial scales. Sci Total Environ 854:158743. [DOI] [PubMed] [Google Scholar]
- Hayes DS, Brändle JM, Seliger C, Zeiringer B, Ferreira T, Schmutz S (2018) Advancing towards functional environmental flows for temperate floodplain rivers. Sci Total Environ 633:1089–1104. [DOI] [PubMed] [Google Scholar]
- Hein T, Schwarz U, Habersack H, Nichersu I, Preiner S, Willby N, Weigelhofer G (2016) Current status and restoration options for floodplains along the Danube River. Sci Total Environ 543:778–790. [DOI] [PubMed] [Google Scholar]
- Heino J, Melo AS, Bini LM, Altermatt F, Al-Shami SA, Angeler DG, Bonada N, Brand C, Callisto M, Cottenie K, Dangles O, Dudgeon D, Encalada A, Göthe E, Grönroos M, Hamada N, Jacobsen D, Landeiro VL, Ligeiro R, Martins RT, Miserendino ML, Rawi CSM, Rodrigues ME, de Oliveira RF, Sandin L, Schmera D, Sgarbi LF, Simaika JP, Siqueira T, Thompson RM, Townsend CR (2015a) A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecol Evol 5(6):1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J, Melo AS, Siqueira T, Soininen J, Valanko S, Bini LM (2015b) Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biol 60(5):845–869. [Google Scholar]
- Heino J, Mykrä H, Kotanen J, Muotka T (2007) Ecological filters and variability in stream macroinvertebrate communities: do taxonomic and functional structure follow the same path? Ecography 30(2):217–230. [Google Scholar]
- Heino J, Schmera D, Erős T (2013) A macroecological perspective of trait patterns in stream communities. Freshwater Biol 58(8):1539–1555. [Google Scholar]
- Henckel L, Meynard CN, Devictor V, Mouquet N, Bretagnolle V (2019) On the relative importance of space and environment in farmland bird community assembly. PLoS ONE 14(3):e0213360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MJ, Heino J, White JC, Ryves DB, Wood PJ (2019) Environmental factors are primary determinants of different facets of pond macroinvertebrate alpha and beta diversity in a human-modified landscape. Biol Conserv 237:348–357. [Google Scholar]
- Htay T, Røskaft E, Ringsby TH, Ranke PS (2023) Spatio-temporal variation in avian taxonomic, functional, and phylogenetic diversity and its relevance for conservation in a wetland ecosystem in Myanmar. Biodivers Conserv 32:2841–2867. [Google Scholar]
- Iwata T, Nakano S, Murakami M (2003) Stream meanders increase insectivorous bird abundance in riparian deciduous forests. Ecography 26(3):325–337. [Google Scholar]
- Kassambara A, Mundt F (2017) Package ‘factoextra’. Extract and visualize the results of multivariate data analyses. R-Project. https://cran.r-project.org/web/packages/factoextra/index.html. Accessed 07 Jan 2023
- Knutson MG (1995) Birds of large floodplain forests: Local and regional habitat associations on the Upper Mississippi River. PhD dissertation, Iowa State University
- Kingsford RT, Jenkins KM, Porter JL (2004) Imposed hydrological stability on lakes in arid Australia and effects on waterbirds. Ecology 85(9):2478–2492. [Google Scholar]
- Laurance SG, Gomez MS (2005) Clearing width and movements of understory rainforest birds. Biotropica 37(1):149–152. [Google Scholar]
- Legendre P, De Cáceres M (2013) Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol Lett 16(8):951–963. [DOI] [PubMed] [Google Scholar]
- Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. [DOI] [PubMed] [Google Scholar]
- Legendre P, Legendre LF (2012) Numerical ecology. Elsevier, Amsterdam [Google Scholar]
- Lemelin LV, Darveau M, Imbeau L, Bordage D (2010) Wetland use and selection by breeding waterbirds in the boreal forest of Quebec, Canada. Wetlands 30:321–332. [Google Scholar]
- Liang B, Chen S, Wang Z (2007) Nest selection of Chinese egret (Egretta eulophotes) in Wuzhishan Archipelago, Zhejiang. Biodivers Sci 15(1):92–96. [Google Scholar]
- Lorenzón RE, Beltzer AH, Olguin PF, León EJ, Sovrano LV, Antoniazzi CE, Ronchi-Virgolini AL (2019) Temporal variation of bird assemblages in dynamic fluvial wetlands: seasonality and influence of water level and habitat availability. Rev Biol Trop 67(6):1131–1145. [Google Scholar]
- Lorenzón RE, Beltzer AH, Olguin PF, Ronchi-Virgolini AL (2016a) Habitat heterogeneity drives bird species richness, nestedness and habitat selection by individual species in fluvial wetlands of the Paraná River, Argentina. Austral Ecol 41(7):829–841. [Google Scholar]
- Lorenzón RE, Beltzer AH, Peltzer PM, Ronchi-Virgolini AL, Tittarelli M, Olguin P (2016b) Local conditions vs regional context: variation in composition of bird communities along the Middle Paraná River, an extensive river-floodplain system of South America. Community Ecol 17:40–47. [Google Scholar]
- Luck GW, Smallbone LT, Sheffield KJ (2013) Environmental and socio-economic factors related to urban bird communities. Austral Ecol 38(1):111–120. [Google Scholar]
- Machar I, Šimek P, Schlossárek M, Pechanec V, Petrovič F, Brus J, Špinlerová Z, Seják J (2022) Comparison of bird diversity between temperate floodplain forests and urban parks. Urban for Urban Gree 67:127427. [Google Scholar]
- Machtans CS, Villard MA, Hannon SJ (1996) Use of riparian buffer strips as movement corridors by forest birds. Conserv Biol 10(5):1366–1379. [Google Scholar]
- Mag Z, Szép T, Nagy K, Standovár T (2011) Modelling forest bird community richness using CORINE land cover data: a study at the landscape scale in Hungary. Community Ecol 12:241–248. [Google Scholar]
- Meffert PJ, Dziock F (2013) The influence of urbanisation on diversity and trait composition of birds. Landscape Ecol 28:943–957. [Google Scholar]
- Meynard CN, Lavergne S, Boulangeat I, Garraud L, Van Es J, Mouquet N, Thuiller W (2013) Disentangling the drivers of metacommunity structure across spatial scales. J Biogeogr 40(8):1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynard CN, Quinn JF (2008) Bird metacommunities in temperate South American forest: vegetation structure, area, and climate effects. Ecology 89(4):981–990. [DOI] [PubMed] [Google Scholar]
- Milder JC, DeClerck FA, Sanfiorenzo A, Sánchez DM, Tobar DE, Zuckerberg B (2010) Effects of farm and landscape management on bird and butterfly conservation in western Honduras. Ecosphere 1(1):1–22. [Google Scholar]
- Mouillot D, Graham NA, Villéger S, Mason NW, Bellwood DR (2013) A functional approach reveals community responses to disturbances. Trends Ecol Evol 28(3):167–177. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nakano S (2002) Indirect effect of aquatic insect emergence on a terrestrial insect population through by birds predation. Ecol Lett 5(3):333–337. [Google Scholar]
- Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci-Biol 98(1):166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H (2017) Vegan: community ecology package. R-Project. http://CRAN.R-project.org/package=vegan. Accessed 26 Jan 2023
- Økland RH (1999) On the variation explained by ordination and constrained ordination axes. J Veg Sci 10(1):131–136. [Google Scholar]
- Ovaskainen O, Rybicki J, Abrego N (2019) What can observational data reveal about metacommunity processes? Ecography 42(11):1877–1886. [Google Scholar]
- Parkes ML, Mora MA, Feagin RA (2012) Using scale, cover type and GIS to evaluate nuisance cattle egret colony site selection. Waterbirds 35(1):56–63. [Google Scholar]
- Parkinson A, Mac Nally R, Quinn GP (2002) Differential macrohabitat use by birds on the unregulated Ovens River floodplain of southeastern Australia. River Res Appl 18(5):495–506. [Google Scholar]
- Pereira P, Godinho C, Gomes M, Rabaça JE (2014) The importance of the surroundings: are bird communities of riparian galleries influenced by agroforestry matrices in SW Iberian Peninsula? Ann for Sci 71(1):33–41. [Google Scholar]
- Peres-Neto PR, Jackson DA, Somers KM (2003) Giving meaningful interpretation to ordination axes: assessing loading significance in principal component analysis. Ecology 84(9):2347–2363. [Google Scholar]
- Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87(10):2614–2625. [DOI] [PubMed] [Google Scholar]
- Peres-Neto PR, Legendre P (2010) Estimating and controlling for spatial structure in the study of ecological communities. Global Ecol Biogeogr 19:174–184. [Google Scholar]
- Pérez-García JM, Sebastián-González E, Alexander KL, Sánchez-Zapata JA, Botella F (2014) Effect of landscape configuration and habitat quality on the community structure of waterbirds using a man-made habitat. Eur J Wildlife Res 60(6):875–883. [Google Scholar]
- Perry RW, Wigley TB, Melchiors MA, Thill RE, Tappe PA, Miller DA (2011) Width of riparian buffer and structure of adjacent plantations influence occupancy of conservation priority birds. Biodivers Conserv 20(3):625–642. [Google Scholar]
- Portaccio A, Basile M, Favaretto A, Campagnaro T, Pettenella D, Sitzia T (2021) The role of Natura 2000 in relation to breeding birds decline on multiple land cover types and policy implications. J Nat Conserv 62:126023. [Google Scholar]
- QGIS.org (2022) QGIS geographic information system. Open Source Geospatial Foundation Project. http://qgis.org Accessed 12 Feb 2022
- Rault C, Leprieur F, Barbaro L, Kreft H, Mouquet N, Papaïx J, Şekercioğlu ÇH, Violle C, Barnagaud JY (2023) Trait-environment associations diverge between native and alien breeding bird assemblages on the world’s oceanic islands. Global Ecol Biogeogr 32(10):1773–1789. [Google Scholar]
- Reckendorfer W, Baranyi C, Funk A, Schiemer F (2006) Floodplain restoration by reinforcing hydrological connectivity: expected effects on aquatic mollusc communities. J Appl Ecol 43(3):474–484. [Google Scholar]
- Reid MA, Reid MC, Thoms MC (2016) Ecological significance of hydrological connectivity for wetland plant communities on a dryland floodplain river, MacIntyre River, Australia. Aquat Sci 78:139–158. [Google Scholar]
- Renöfält BM, Nilsson C, Jansson R (2005) Spatial and temporal patterns of species richness in a riparian landscape. J Biogeogr 32(11):2025–2037. [Google Scholar]
- Reynolds C, Miranda NA, Cumming GS (2015) The role of waterbirds in the dispersal of aquatic alien and invasive species. Divers Distrib 21(7):744–754. [Google Scholar]
- Rush SA, Romito T, Robison TL (2014) Avian diversity in a suburban park system: current conditions and strategies for dealing with anticipated change. Urban Ecosyst 17:45–60. [Google Scholar]
- Sattler T, Borcard D, Arlettaz R, Bontadina F, Legendre P, Obrist MK, Moretti M (2010) Spider, bee, and bird communities in cities are shaped by environmental control and high stochasticity. Ecology 91(11):3343–3353. [DOI] [PubMed] [Google Scholar]
- Schilke PR, Bartrons M, Gorzo JM, Vander Zanden MJ, Gratton C, Howe RW, Pidgeon AM (2020) Modeling a cross-ecosystem subsidy: forest songbird response to emergent aquatic insects. Landscape Ecol 35:1587–1604. [Google Scholar]
- Schöll K, Kiss A (2008) Spatial and temporal distribution patterns of zooplankton assemblages (Rotifera, Cladocera, Copepoda) in the water bodies of the Gemenc Floodplain (Duna-Dráva National Park, Hungary). Opuscula Zool 39:65–76 [Google Scholar]
- Seaman BS, Schulze CH (2010) The importance of gallery forests in the tropical lowlands of Costa Rica for understorey forest birds. Biol Conserv 143(2):391–398. [Google Scholar]
- Selwood KE, Clarke RH, McGeoch MA, Mac Nally R (2017) Green tongues into the arid zone: river floodplains extend the distribution of terrestrial bird species. Ecosystems 20:745–756. [Google Scholar]
- Selwood KE, Thomson JR, Clarke RH, McGeoch MA, Mac Nally R (2015) Resistance and resilience of terrestrial birds in drying climates: do floodplains provide drought refugia? Global Ecol Biogeogr 24(7):838–848. [Google Scholar]
- Senner PW, Barzen JA, Silbernagel J, Larsen B, Nguyen-Phuc H (2021) Changes in avian community composition at a restored floodplain grassland in the Mekong Delta. Waterbirds 44(4):526–538. [Google Scholar]
- Sheldon KS, Yang S, Tewksbury JJ (2011) Climate change and community disassembly: impacts of warming on tropical and temperate montane community structure. Ecol Lett 14(12):1191–1200. [DOI] [PubMed] [Google Scholar]
- Shuford WD, Reiter ME, Strum KM, Gilbert MM, Hickey CM, Golet GH (2016) The benefits of crops and field management practices to wintering waterbirds in the Sacramento-San Joaquin River Delta of California. Renew Agr Food Syst 31(6):495–506. [Google Scholar]
- Sinha A, Chatterjee N, Ormerod SJ, Adhikari BS, Krishnamurthy R (2019) River birds as potential indicators of local-and catchment-scale influences on Himalayan river ecosystems. Ecosyst People 15(1):90–101. [Google Scholar]
- Sinha A, Menzies RK, Chatterjee N, Rao M, Naniwadekar R (2022) Drivers of taxonomic, phylogenetic, and functional beta diversity of himalayan riverine birds. Front Ecol Evol 10:788184. [Google Scholar]
- Socolar JB, Wilcove DS (2019) Forest-type specialization strongly predicts avian responses to tropical agriculture. P Roy Soc B-Biol Sci 286(1913):20191724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen JC, Hurlbert AH (2011) Inferring ecological processes from taxonomic, phylogenetic and functional trait β-diversity. PLoS ONE 6(6):e20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulai P, Nurhidayu S, Aziz N, Zakaria M, Barclay H, Azhar B (2015) Effects of water quality in oil palm production landscapes on tropical waterbirds in Peninsular Malaysia. Ecol Res 30(5):941–949. [Google Scholar]
- Sullivan SMP, Watzin MC, Keeton WS (2007) A riverscape perspective on habitat associations among riverine bird assemblages in the Lake Champlain Basin, USA. Landscape Ecol 22:1169–1186. [Google Scholar]
- Sultana M, Müller M, Meyer M, Storch I (2022) Neighboring green network and landscape metrics explain biodiversity within small urban green areas—A case study on birds. Sustain-Basel 14(11):6394. [Google Scholar]
- Tardy J (2007) A magyarországi vadvizek világa. Hazánk ramsari területei (The Life of Hungarian Wetlands. Hungarian Areas in the List of Ramsar). In Hungarian. Alexandra Kiadó, Pécs
- Tavares DC, Guadagnin DL, de Moura JF, Siciliano S, Merico A (2015) Environmental and anthropogenic factors structuring waterbird habitats of tropical coastal lagoons: implications for management. Biol Conserv 186:12–21. [Google Scholar]
- Thompson WL (2002) Towards reliable bird surveys: accounting for individuals present but not detected. Auk 119(1):18–25. [Google Scholar]
- Thornton DH, Branch LC, Sunquist ME (2011) The influence of landscape, patch, and within-patch factors on species presence and abundance: a review of focal patch studies. Landscape Ecol 26:7–18. [Google Scholar]
- Thorp JH, Thoms MC, Delong MD (2006) The riverine ecosystem synthesis: biocomplexity in river networks across space and time. River Res Appl 22(2):123–147. [Google Scholar]
- Tockner K, Pusch M, Borchardt D, Lorang MS (2010) Multiple stressors in coupled river–floodplain ecosystems. Freshwater Biol 55:135–151. [Google Scholar]
- Tockner K, Schiemer F, Baumgartner C, Kum G, Weigand E, Zweimüller I, Ward JV (1999) The Danube restoration project: species diversity patterns across connectivity gradients in the floodplain system. River Res Appl 15(1–3):245–258 [Google Scholar]
- Tockner K, Stanford JA (2002) Riverine flood plains: present state and future trends. Environ Conserv 29(3):308–330. [Google Scholar]
- Velásquez-Tibatá J, Salaman P, Graham CH (2013) Effects of climate change on species distribution, community structure, and conservation of birds in protected areas in Colombia. Reg Environ Change 13:235–248. [Google Scholar]
- Vergara PM, Armesto JJ (2009) Responses of Chilean forest birds to anthropogenic habitat fragmentation across spatial scales. Landscape Ecol 24:25–38. [Google Scholar]
- Ward JV, Tockner K, Schiemer F (1999) Biodiversity of floodplain river ecosystems: ecotones and connectivity. River Res Appl 15(1–3):125–139 [Google Scholar]
- Watson DM, Watson MJ (2015) Wildlife restoration: mainstreaming translocations to keep common species common. Biol Conserv 191:830–838. [Google Scholar]
- Wesner JS, Swanson DL, Dixon MD, Soluk DA, Quist DJ, Yager LA, Warmbold JW, Oddy E, Seidel TC (2020) Loss of potential aquatic-terrestrial subsidies along the Missouri River floodplain. Ecosystems 23:111–123. [Google Scholar]
- Wiens JA (2002) Central concepts and issues of landscape ecology. In: Gutzwiller KJ (ed) Applying landscape ecology in biological conservation. Spinger-Verlag, New York, pp 3–21 [Google Scholar]
- Yabuhara Y, Yamaura Y, Akasaka T, Yamanaka S, Nakamura F (2019) Seasonal variation in patch and landscape effects on forest bird communities in a lowland fragmented landscape. Forest Ecol Manag 454:117140. [Google Scholar]
- Yuan Y, Zeng G, Liang J, Li X, Li Z, Zhang C, Huang L, Lai X, Lu L, Wu H, Yu X (2014) Effects of landscape structure, habitat and human disturbance on birds: a case study in East Dongting Lake wetland. Ecol Eng 67:67–75. [Google Scholar]
- Coughlan NE, Kelly TC, Davenport J, Jansen MA (2017) Up, up and away: bird-mediated ectozoochorous dispersal between aquatic environments. Freshwater Biol 62(4):631–648. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: The ordination plot of the PCA analysis of the local scale habitat variables. Supplementary file1 (PDF 7 KB)
Data Availability Statement
The data presented in this study are available on request from the corresponding author.