Abstract
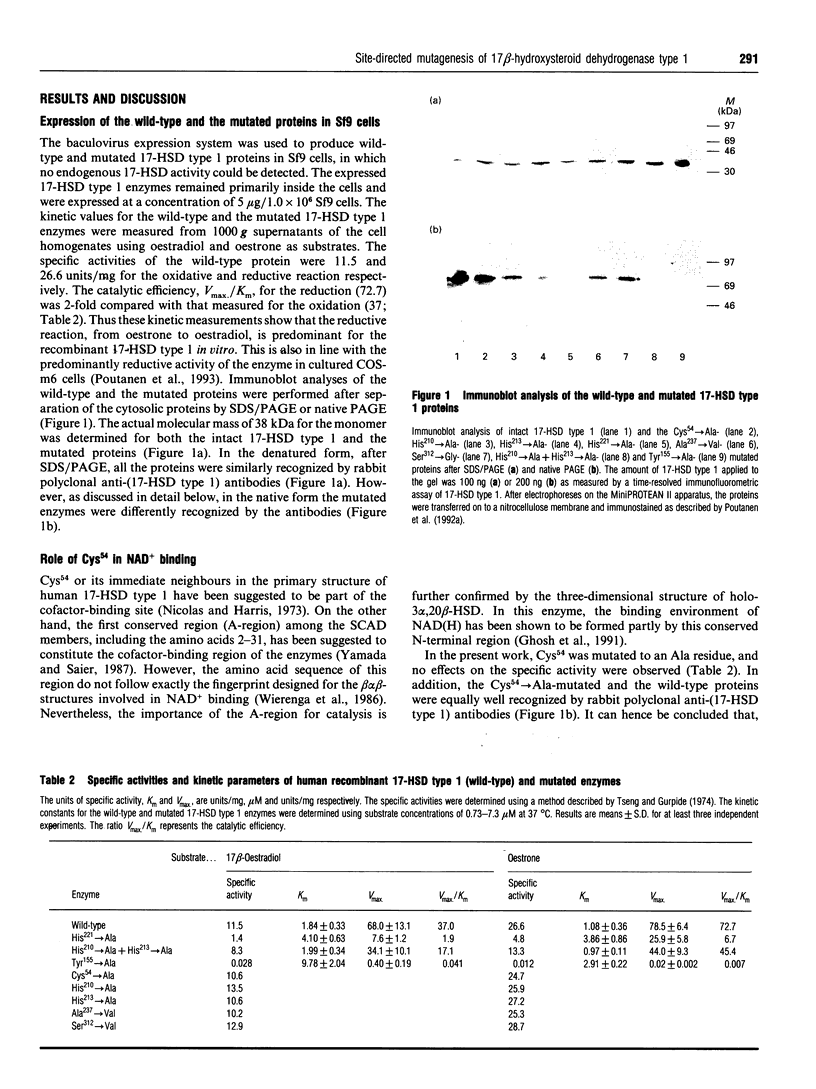
Several amino acid residues (Cys54, Tyr155, His210, His213 and His221) at a putative catalytic site of human 17 beta-hydroxysteroid dehydrogenase type 1 were mutated to Ala. Replacement of His221 by Ala remarkably reduced the catalytic activity, which resulted from a change of both the Km and the Vmax. values of the enzyme. Compared with the wild-type enzyme, the catalytic efficiency of the His221-->Ala mutant was reduced 20-fold for the oxidative reaction and 11-fold for the reductive reaction. With similar mutations at His210 or His213, no notable effects on the catalytic properties of the enzyme were detected. However, a simultaneous mutation of these amino acid residues decreased the Vmax. values of both oxidation and reduction by about 50% from those measured for the wild-type enzyme. Although Cys54 has been localized in the cofactor-binding region of the enzyme, a Cys54-->Ala mutation did not lead to changes in the enzymic activity. The most dramatic effects on the catalytic properties of the enzyme were achieved by mutating Tyr155, which resulted in an almost completely inactivation of the enzyme. The decreased enzymic activities of the Tyr155-->Ala, His210-->Ala + His213-->Ala and His221-->Ala mutations were also reflected in a reduced immunoreactivity of the enzymes. The results thus suggest that the lower catalytic efficiency of the mutant enzymes is due to an exchange of catalytically important amino acid residues and/or remarkable alterations in the three-dimensional structure of the enzyme. The recently detected polymorphisms (Ala237<-->Val and Ser312<-->Gly) were not found to affect either the catalytic or the immunological properties of the type 1 enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beranek P. A., Folkerd E. J., Newton C. J., Reed M. J., Ghilchik M. W., James V. H. The relationship between 17 beta hydroxysteroid dehydrogenase and breast tumour site and size. Int J Cancer. 1985 Dec 15;36(6):685–687. doi: 10.1002/ijc.2910360611. [DOI] [PubMed] [Google Scholar]
- Bonney R. C., Reed M. J., Beranek P. A., Ghilchik M. W., James V. H. Metabolism of [3H]oestradiol in vivo by normal breast and tumour tissue in postmenopausal women. J Steroid Biochem. 1986 Jan;24(1):361–364. doi: 10.1016/0022-4731(86)90082-8. [DOI] [PubMed] [Google Scholar]
- Burns D. J., Engel L. L., Bethune J. L. Amino acid composition and subunit structure. Human placental 17 -estradiol dehydrogenase. Biochemistry. 1972 Jul 4;11(14):2699–2703. doi: 10.1021/bi00764a023. [DOI] [PubMed] [Google Scholar]
- Cullen K. J., Lippman M. E. Estrogen regulation of protein synthesis and cell growth in human breast cancer. Vitam Horm. 1989;45:127–172. doi: 10.1016/s0083-6729(08)60394-5. [DOI] [PubMed] [Google Scholar]
- Garavito R. M., Rossmann M. G., Argos P., Eventoff W. Convergence of active center geometries. Biochemistry. 1977 Nov 15;16(23):5065–5071. doi: 10.1021/bi00642a019. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Weeks C. M., Grochulski P., Duax W. L., Erman M., Rimsay R. L., Orr J. C. Three-dimensional structure of holo 3 alpha,20 beta-hydroxysteroid dehydrogenase: a member of a short-chain dehydrogenase family. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10064–10068. doi: 10.1073/pnas.88.22.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Jany K. D., Ulmer W., Fröschle M., Pfleiderer G. Complete amino acid sequence of glucose dehydrogenase from Bacillus megaterium. FEBS Lett. 1984 Jan 2;165(1):6–10. doi: 10.1016/0014-5793(84)80003-4. [DOI] [PubMed] [Google Scholar]
- Krozowski Z. 11 beta-hydroxysteroid dehydrogenase and the short-chain alcohol dehydrogenase (SCAD) superfamily. Mol Cell Endocrinol. 1992 Mar;84(1-2):C25–C31. doi: 10.1016/0303-7207(92)90064-d. [DOI] [PubMed] [Google Scholar]
- LANGER L. J., ENGEL L. L. Human placental estradiol-17 beta dehydrogenase. I. Concentration, characterization and assay. J Biol Chem. 1958 Sep;233(3):583–588. [PubMed] [Google Scholar]
- Lin S. X., Yang F., Jin J. Z., Breton R., Zhu D. W., Luu-The V., Labrie F. Subunit identity of the dimeric 17 beta-hydroxysteroid dehydrogenase from human placenta. J Biol Chem. 1992 Aug 15;267(23):16182–16187. [PubMed] [Google Scholar]
- Luu The V., Labrie C., Zhao H. F., Couët J., Lachance Y., Simard J., Leblanc G., Côté J., Bérubé D., Gagné R. Characterization of cDNAs for human estradiol 17 beta-dehydrogenase and assignment of the gene to chromosome 17: evidence of two mRNA species with distinct 5'-termini in human placenta. Mol Endocrinol. 1989 Aug;3(8):1301–1309. doi: 10.1210/mend-3-8-1301. [DOI] [PubMed] [Google Scholar]
- Luu-The V., Labrie C., Simard J., Lachance Y., Zhao H. F., Couët J., Leblanc G., Labrie F. Structure of two in tandem human 17 beta-hydroxysteroid dehydrogenase genes. Mol Endocrinol. 1990 Feb;4(2):268–275. doi: 10.1210/mend-4-2-268. [DOI] [PubMed] [Google Scholar]
- Mannermaa A., Peltoketo H., Winqvist R., Ponder B. A., Kiviniemi H., Easton D. F., Poutanen M., Isomaa V., Vihko R. Human familial and sporadic breast cancer: analysis of the coding regions of the 17 beta-hydroxysteroid dehydrogenase 2 gene (EDH17B2) using a single-strand conformation polymorphism assay. Hum Genet. 1994 Mar;93(3):319–324. doi: 10.1007/BF00212030. [DOI] [PubMed] [Google Scholar]
- Martel C., Rhéaume E., Takahashi M., Trudel C., Couët J., Luu-The V., Simard J., Labrie F. Distribution of 17 beta-hydroxysteroid dehydrogenase gene expression and activity in rat and human tissues. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):597–603. doi: 10.1016/0960-0760(92)90390-5. [DOI] [PubMed] [Google Scholar]
- Murdock G. L., Chin C. C., Offord R. E., Bradshaw R. A., Warren J. C. Human placental estradiol 17 beta-dehydrogenase. Identification of a single histidine residue affinity-labeled by both 3-bromoacetoxyestrone and 12 beta-bromoacetoxy-4-estrene-3,17-dione. J Biol Chem. 1983 Oct 10;258(19):11460–11464. [PubMed] [Google Scholar]
- Murdock G. L., Chin C. C., Warren J. C. Human placental estradiol 17 beta-dehydrogenase: sequence of a histidine-bearing peptide in the catalytic region. Biochemistry. 1986 Feb 11;25(3):641–646. doi: 10.1021/bi00351a019. [DOI] [PubMed] [Google Scholar]
- Murdock G. L., Pineda J., Nagorsky N., Lawrence S. S., Heritage R., Warren J. C. Estradiol 17 beta-dehydrogenase: full enzymatic activity in the absence of zinc. Biochim Biophys Acta. 1991 Jan 29;1076(2):197–202. doi: 10.1016/0167-4838(91)90266-3. [DOI] [PubMed] [Google Scholar]
- Murdock G. L., Warren J. C., Sweet F. Human placental estradiol 17 beta-dehydrogenase: evidence for inverted substrate orientation ("wrong-way" binding) at the active site. Biochemistry. 1988 Jun 14;27(12):4452–4458. doi: 10.1021/bi00412a036. [DOI] [PubMed] [Google Scholar]
- Mäentausta O., Menjivar M., Vihko R. Time-resolved immunofluorometric assay of 17 beta-hydroxysteroid dehydrogenase in plasma. Clin Chem. 1991 Aug;37(8):1412–1415. [PubMed] [Google Scholar]
- Mäentausta O., Peltoketo H., Isomaa V., Jouppila P., Vihko R. Immunological measurement of human 17 beta-hydroxysteroid dehydrogenase. J Steroid Biochem. 1990 Aug 28;36(6):673–680. doi: 10.1016/0022-4731(90)90187-w. [DOI] [PubMed] [Google Scholar]
- Nicolas J. C., Harris J. I. Human placental 17 -oestradiol dehydrogenase. Sequence of a tryptic peptide containing an essential cysteine. FEBS Lett. 1973 Jan 15;29(2):173–176. doi: 10.1016/0014-5793(73)80554-x. [DOI] [PubMed] [Google Scholar]
- Normand T., Narod S., Labrie F., Simard J. Detection of polymorphisms in the estradiol 17 beta-hydroxysteroid dehydrogenase II gene at the EDH17B2 locus on 17q11-q21. Hum Mol Genet. 1993 Apr;2(4):479–483. doi: 10.1093/hmg/2.4.479. [DOI] [PubMed] [Google Scholar]
- Peltoketo H., Isomaa V., Mäentausta O., Vihko R. Complete amino acid sequence of human placental 17 beta-hydroxysteroid dehydrogenase deduced from cDNA. FEBS Lett. 1988 Oct 24;239(1):73–77. doi: 10.1016/0014-5793(88)80548-9. [DOI] [PubMed] [Google Scholar]
- Pons M., Nicolas J. C., Boussioux A. M., Descomps B., Crastes A., de Paulet A. C. Some new developments in the knowledge of human placental estradiol-17beta dehydrogenase. J Steroid Biochem. 1977 May;8(5):345–358. doi: 10.1016/0022-4731(77)90233-3. [DOI] [PubMed] [Google Scholar]
- Poutanen M., Isomaa V., Lehto V. P., Vihko R. Immunological analysis of 17 beta-hydroxysteroid dehydrogenase in benign and malignant human breast tissue. Int J Cancer. 1992 Feb 1;50(3):386–390. doi: 10.1002/ijc.2910500310. [DOI] [PubMed] [Google Scholar]
- Poutanen M., Miettinen M., Vihko R. Differential estrogen substrate specificities for transiently expressed human placental 17 beta-hydroxysteroid dehydrogenase and an endogenous enzyme expressed in cultured COS-m6 cells. Endocrinology. 1993 Dec;133(6):2639–2644. doi: 10.1210/endo.133.6.8243287. [DOI] [PubMed] [Google Scholar]
- Poutanen M., Moncharmont B., Vihko R. 17 beta-hydroxysteroid dehydrogenase gene expression in human breast cancer cells: regulation of expression by a progestin. Cancer Res. 1992 Jan 15;52(2):290–294. [PubMed] [Google Scholar]
- Rao S. T., Rossmann M. G. Comparison of super-secondary structures in proteins. J Mol Biol. 1973 May 15;76(2):241–256. doi: 10.1016/0022-2836(73)90388-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannin G. M., Agarwal A. K., Monder C., New M. I., White P. C. The human gene for 11 beta-hydroxysteroid dehydrogenase. Structure, tissue distribution, and chromosomal localization. J Biol Chem. 1991 Sep 5;266(25):16653–16658. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng L., Gurpide E. Estradiol and 20alpha-dihydroprogesterone dehydrogenase activities in human endometrium during the menstrual cycle. Endocrinology. 1974 Feb;94(2):419–423. doi: 10.1210/endo-94-2-419. [DOI] [PubMed] [Google Scholar]
- Vihko P., Kurkela R., Porvari K., Herrala A., Lindfors A., Lindqvist Y., Schneider G. Rat acid phosphatase: overexpression of active, secreted enzyme by recombinant baculovirus-infected insect cells, molecular properties, and crystallization. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):799–803. doi: 10.1073/pnas.90.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihko R., Apter D. Endogenous steroids in the pathophysiology of breast cancer. Crit Rev Oncol Hematol. 1989;9(1):1–16. doi: 10.1016/s1040-8428(89)80012-5. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Wu L., Einstein M., Geissler W. M., Chan H. K., Elliston K. O., Andersson S. Expression cloning and characterization of human 17 beta-hydroxysteroid dehydrogenase type 2, a microsomal enzyme possessing 20 alpha-hydroxysteroid dehydrogenase activity. J Biol Chem. 1993 Jun 15;268(17):12964–12969. [PubMed] [Google Scholar]
- Yamada M., Saier M. H., Jr Glucitol-specific enzymes of the phosphotransferase system in Escherichia coli. Nucleotide sequence of the gut operon. J Biol Chem. 1987 Apr 25;262(12):5455–5463. [PubMed] [Google Scholar]