Abstract
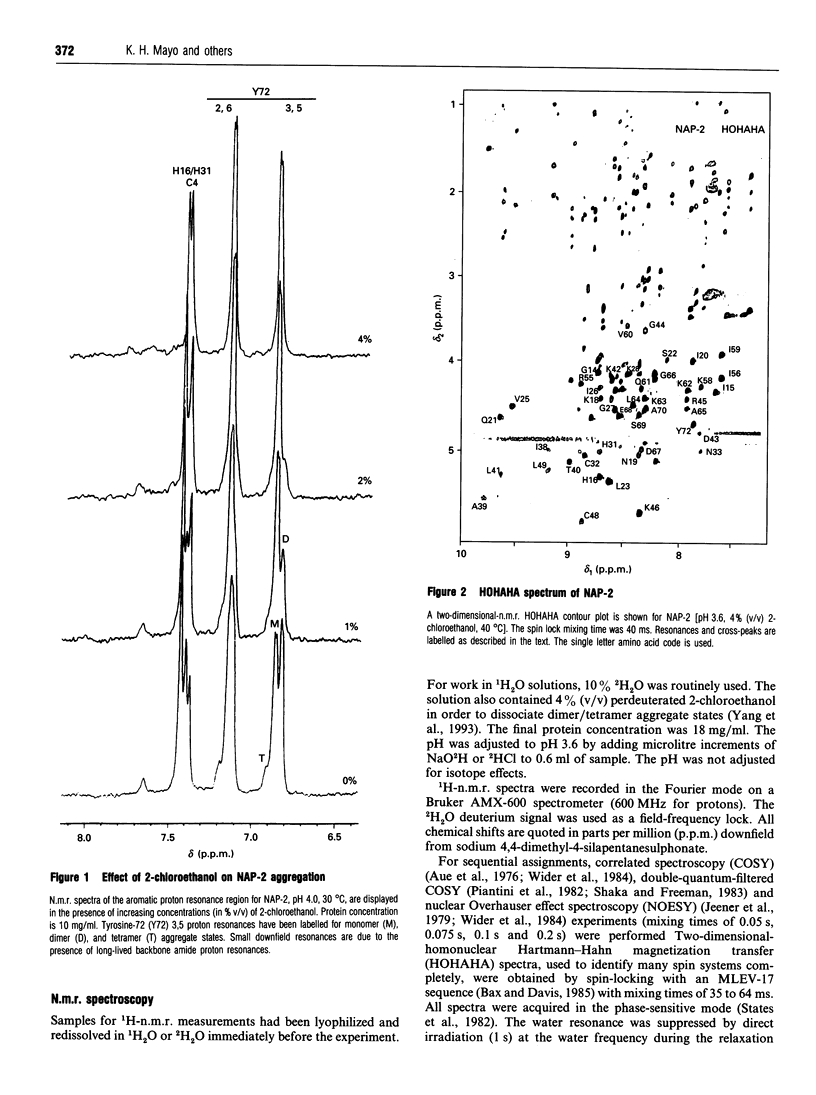
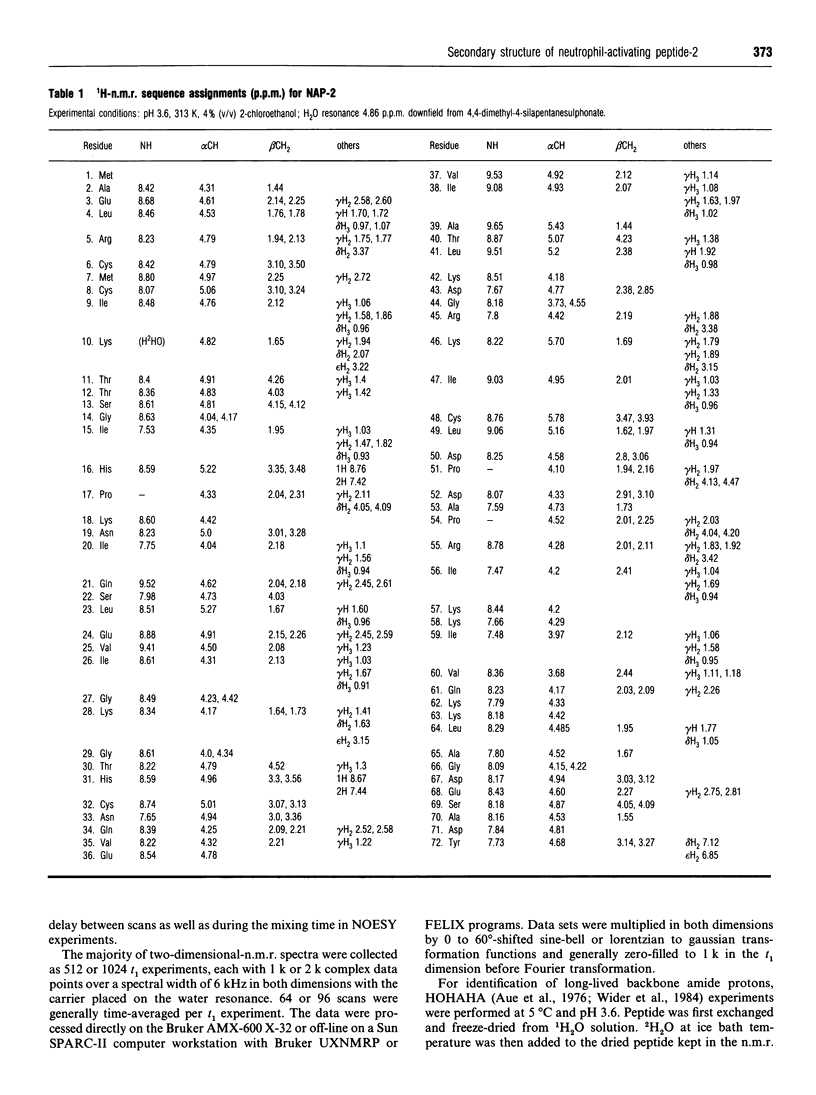
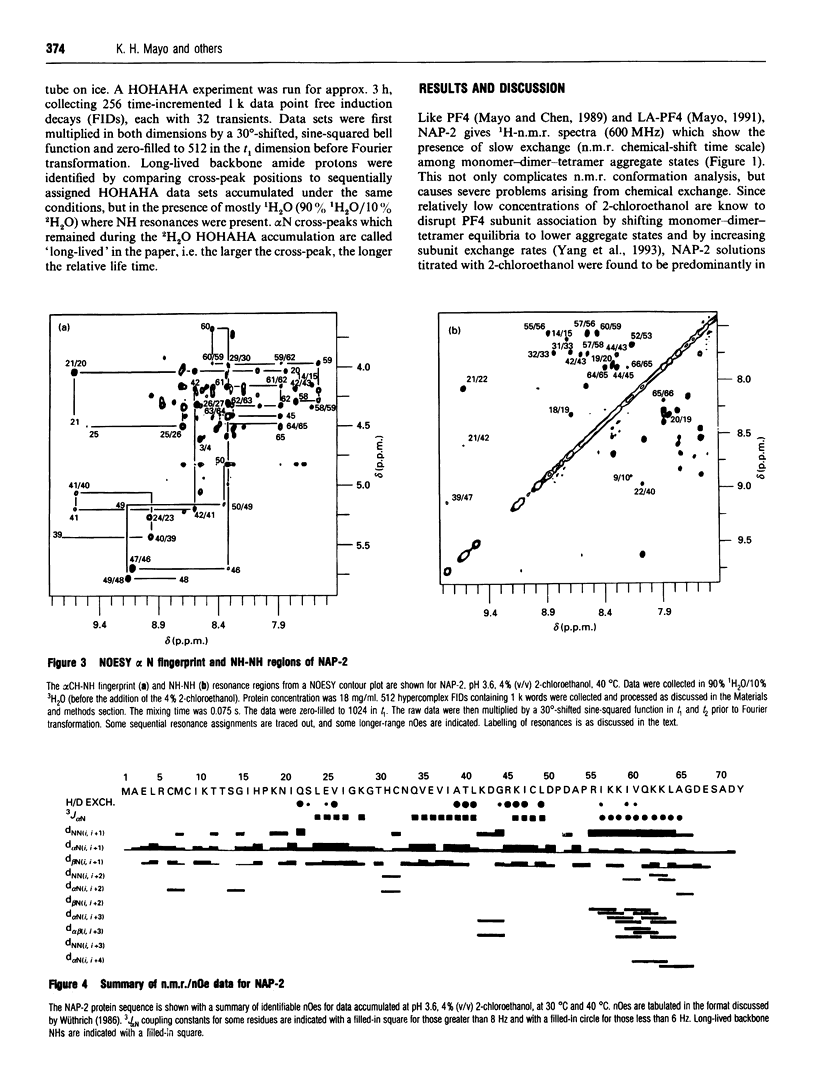
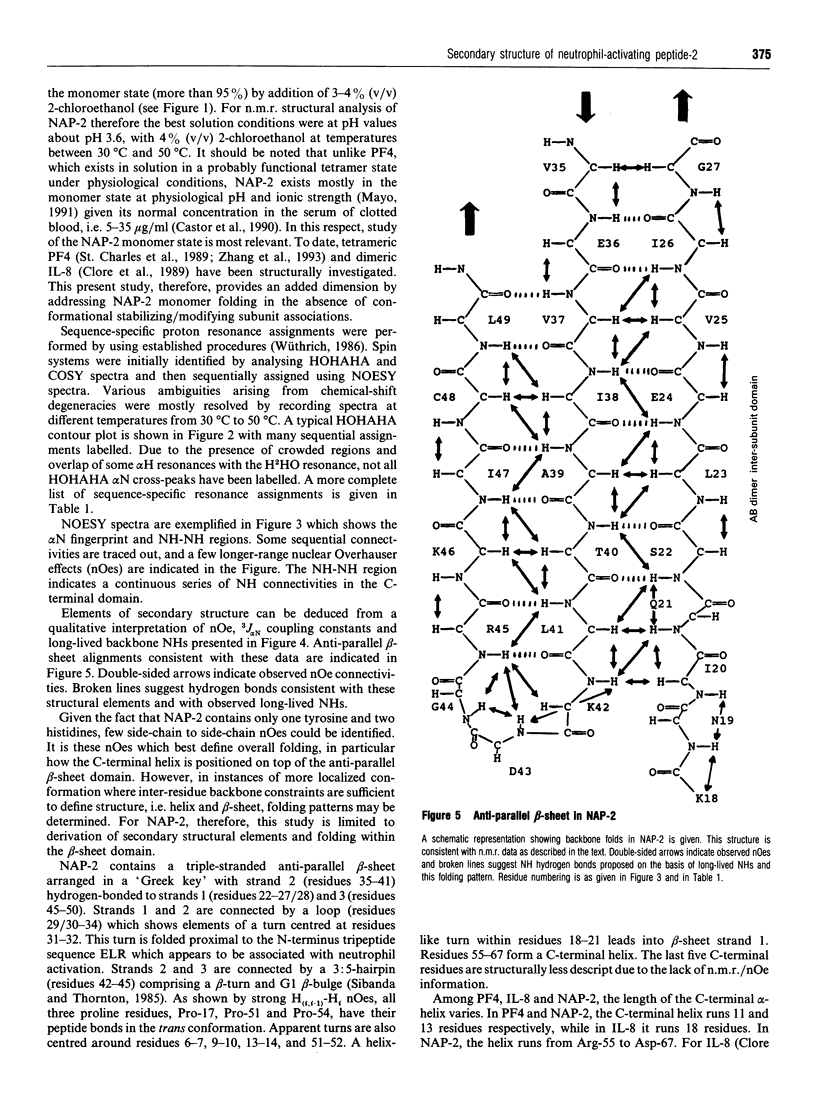
Neutrophil-activating protein-2 (NAP-2) is a 72 residue protein demonstrating a range of proinflammatory activities. The solution structure of monomeric NAP-2 has been investigated by two-dimensional 1H-n.m.r. spectroscopy. Sequence-specific proton resonance assignments have been made and secondary structural elements have been identified on the basis of nuclear Overhauser data, coupling constants and amide hydrogen/deuteron exchange. The NAP-2 monomer consists of a triple-stranded anti-parallel beta-sheet arranged in a 'Greek key' and a C-terminal helix (residues 59-70) and is very similar to that found in the n.m.r. solution conformation of dimeric interleukin-8 and the crystal structure of tetrameric bovine platelet factor-4. Results are discussed in terms of heparin binding and neutrophil-activation properties of NAP-2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anisowicz A., Bardwell L., Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg G. S., Pepper D. S., Chesterman C. N., Morgan F. J. Complete covalent structure of human beta-thromboglobulin. Biochemistry. 1978 May 2;17(9):1739–1744. doi: 10.1021/bi00602a024. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Miller J. W., Walz D. A. Structural and biological characteristics of connective tissue activating peptide (CTAP-III), a major human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1983 Feb;80(3):765–769. doi: 10.1073/pnas.80.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor C. W., Walz D. A., Johnson P. H., Hossler P. A., Smith E. M., Bignall M. C., Aaron B. P., Underhill P., Lazar J. M., Hudson D. H. Connective tissue activation. XXXIV: Effects of proteolytic processing on the biologic activities of CTAP-III. J Lab Clin Med. 1990 Oct;116(4):516–526. [PubMed] [Google Scholar]
- Clore G. M., Appella E., Yamada M., Matsushima K., Gronenborn A. M. Three-dimensional structure of interleukin 8 in solution. Biochemistry. 1990 Feb 20;29(7):1689–1696. doi: 10.1021/bi00459a004. [DOI] [PubMed] [Google Scholar]
- Cowan S. W., Bakshi E. N., Machin K. J., Isaacs N. W. Binding of heparin to human platelet factor 4. Biochem J. 1986 Mar 1;234(2):485–488. doi: 10.1042/bj2340485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Keim P. S., Farmer M., Heinrikson R. L. Amino acid sequence of human platelet factor 4. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2256–2258. doi: 10.1073/pnas.74.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. C., Harris M. E., Holt A. M., Lange E., Henschen A., Niewiarowski S. Characterization of human platelet basic protein, a precursor form of low-affinity platelet factor 4 and beta-thromboglobulin. Biochemistry. 1986 Apr 22;25(8):1988–1996. doi: 10.1021/bi00356a023. [DOI] [PubMed] [Google Scholar]
- Holt J. C., Yan Z. Q., Lu W. Q., Stewart G. J., Niewiarowski S. Isolation, characterization, and immunological detection of neutrophil-activating peptide 2: a proteolytic degradation product of platelet basic protein. Proc Soc Exp Biol Med. 1992 Feb;199(2):171–177. doi: 10.3181/00379727-199-43343. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawler J. W. Prediction of the secondary structure of platelet factor 4 and beta-thromboglobulin from their amino acid sequences. Thromb Res. 1981 Jan 1;21(1-2):121–127. doi: 10.1016/0049-3848(84)90039-2. [DOI] [PubMed] [Google Scholar]
- Mayo K. H., Chen M. J. Human platelet factor 4 monomer-dimer-tetramer equilibria investigated by 1H NMR spectroscopy. Biochemistry. 1989 Nov 28;28(24):9469–9478. doi: 10.1021/bi00450a034. [DOI] [PubMed] [Google Scholar]
- Mayo K. H. Low-affinity platelet factor 4 1H NMR derived aggregate equilibria indicate a physiologic preference for monomers over dimers and tetramers. Biochemistry. 1991 Jan 29;30(4):925–934. doi: 10.1021/bi00218a007. [DOI] [PubMed] [Google Scholar]
- Miller M. D., Krangel M. S. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12(1-2):17–46. [PubMed] [Google Scholar]
- Moser B., Schumacher C., von Tscharner V., Clark-Lewis I., Baggiolini M. Neutrophil-activating peptide 2 and gro/melanoma growth-stimulatory activity interact with neutrophil-activating peptide 1/interleukin 8 receptors on human neutrophils. J Biol Chem. 1991 Jun 5;266(16):10666–10671. [PubMed] [Google Scholar]
- Myers J. A., Gray G. S., Peters D. J., Grimaila R. J., Hunt A. J., Maione T. E., Mueller W. T. Expression and purification of active recombinant platelet factor 4 from a cleavable fusion protein. Protein Expr Purif. 1991 Apr-Jun;2(2-3):136–143. doi: 10.1016/1046-5928(91)90062-n. [DOI] [PubMed] [Google Scholar]
- Rajarathnam K., Sykes B. D., Kay C. M., Dewald B., Geiser T., Baggiolini M., Clark-Lewis I. Neutrophil activation by monomeric interleukin-8. Science. 1994 Apr 1;264(5155):90–92. doi: 10.1126/science.8140420. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Schmid J., Weissmann C. Induction of mRNA for a serine protease and a beta-thromboglobulin-like protein in mitogen-stimulated human leukocytes. J Immunol. 1987 Jul 1;139(1):250–256. [PubMed] [Google Scholar]
- Schnitzel W., Garbeis B., Monschein U., Besemer J. Neutrophil activating peptide-2 binds with two affinities to receptor(s) on human neutrophils. Biochem Biophys Res Commun. 1991 Oct 15;180(1):301–307. doi: 10.1016/s0006-291x(05)81292-6. [DOI] [PubMed] [Google Scholar]
- Schumacher C., Clark-Lewis I., Baggiolini M., Moser B. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10542–10546. doi: 10.1073/pnas.89.21.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibanda B. L., Thornton J. M. Beta-hairpin families in globular proteins. Nature. 1985 Jul 11;316(6024):170–174. doi: 10.1038/316170a0. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- St Charles R., Walz D. A., Edwards B. F. The three-dimensional structure of bovine platelet factor 4 at 3.0-A resolution. J Biol Chem. 1989 Feb 5;264(4):2092–2099. [PubMed] [Google Scholar]
- Van Osselaer N., Van Damme J., Rampart M., Herman A. G. Increased microvascular permeability in vivo in response to intradermal injection of neutrophil-activating protein (NAP-2) in rabbit skin. Am J Pathol. 1991 Jan;138(1):23–27. [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Walz A., Dewald B., von Tscharner V., Baggiolini M. Effects of the neutrophil-activating peptide NAP-2, platelet basic protein, connective tissue-activating peptide III and platelet factor 4 on human neutrophils. J Exp Med. 1989 Nov 1;170(5):1745–1750. doi: 10.1084/jem.170.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Barker S., Chen M. J., Mayo K. H. Effect of low molecular weight aliphatic alcohols and related compounds on platelet factor 4 subunit association. J Biol Chem. 1993 May 5;268(13):9223–9229. [PubMed] [Google Scholar]
- Zhang X., Chen L., Bancroft D. P., Lai C. K., Maione T. E. Crystal structure of recombinant human platelet factor 4. Biochemistry. 1994 Jul 12;33(27):8361–8366. doi: 10.1021/bi00193a025. [DOI] [PubMed] [Google Scholar]


