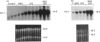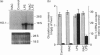Abstract
In in vitro systems haem oxygenase-1 (HO-1) mRNA increases after exposure to agents causing oxidative stress. We lowered cellular antioxidant defence systems in vivo by giving mice increasing doses (0.15 g/kg-1.6 g/kg) of DL-buthionine-(S,R)-sulphoximine (BSO), a specific inhibitor of glutathione synthesis. Maximum glutathione depletion (80%) coincided with maximum hepatic HO-1 mRNA accumulation (about 20 times), whereas with 50% depletion, accumulation was only doubled. It has been suggested that reactive oxygen and nitrogen intermediates are involved in hepatic toxicity of endotoxin (lipopolysaccharide, LPS); LPS even at low doses [0.1 mg/kg, intraperitoneally (i.p.)] induces HO-1 mRNA about 25-fold after 1 h. Hepatic glutathione depletion (respectively 40% and 80%) after a low (0.3 g/kg) or a high (1.6 g/kg) BSO dose, resulted in potentiation of the HO-1 mRNA accumulation induced by LPS (0.1 mg/kg, i.p.). In the absence of BSO, N-acetylcysteine (NAC) (1 g/kg orally) reduced LPS-induced HO-1 mRNA accumulation to one fourth. Under the same experimental conditions S-adenosylmethionine (SAM) was not effective. NAC also reduced HO-1 mRNA accumulation when administered to mice in which glutathione was depleted and its synthesis blocked by BSO (1.6 g/kg). Thus reactive oxygen intermediates are likely mediators of LPS-induced HO-1 mRNA accumulation, and glutathione content appears to be one of the factors regulating this accumulation in the liver. Our findings are compatible with the theory that HO-1 induction might have a protective function in vivo when defence mechanisms against oxidants are challenged.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Applegate L. A., Luscher P., Tyrrell R. M. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991 Feb 1;51(3):974–978. [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Hoey B. M., Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J. W., Vercellotti G. M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992 Sep 5;267(25):18148–18153. [PubMed] [Google Scholar]
- Bautista A. P., Mészáros K., Bojta J., Spitzer J. J. Superoxide anion generation in the liver during the early stage of endotoxemia in rats. J Leukoc Biol. 1990 Aug;48(2):123–128. doi: 10.1002/jlb.48.2.123. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard G. R., Lucht W. D., Niedermeyer M. E., Snapper J. R., Ogletree M. L., Brigham K. L. Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J Clin Invest. 1984 Jun;73(6):1772–1784. doi: 10.1172/JCI111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Harbrecht B. G., Stuehr D. J., Demetris A. J., Simmons R. L. Modulation of nitrogen oxide synthesis in vivo: NG-monomethyl-L-arginine inhibits endotoxin-induced nitrate/nitrate biosynthesis while promoting hepatic damage. J Leukoc Biol. 1990 Dec;48(6):565–569. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., Ferrari F. K., Simmons R. L. Evidence that activation of Kupffer cells results in production of L-arginine metabolites that release cell-associated iron and inhibit hepatocyte protein synthesis. Surgery. 1989 Aug;106(2):364–372. [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., West M. A., Bentz B. G., Simmons R. L. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J Exp Med. 1989 Apr 1;169(4):1467–1472. doi: 10.1084/jem.169.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgunder J. M., Varriale A., Lauterburg B. H. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur J Clin Pharmacol. 1989;36(2):127–131. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- Cantoni L., Rossi C., Rizzardini M., Gadina M., Ghezzi P. Interleukin-1 and tumour necrosis factor induce hepatic haem oxygenase. Feedback regulation by glucocorticoids. Biochem J. 1991 Nov 1;279(Pt 3):891–894. doi: 10.1042/bj2790891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cighetti G., Debiasi S., Paroni R. Effect of glutathione depletion on the conversion of xanthine dehydrogenase to oxidase in rat liver. Biochem Pharmacol. 1993 Jun 9;45(11):2359–2361. doi: 10.1016/0006-2952(93)90213-g. [DOI] [PubMed] [Google Scholar]
- Clancy R. M., Leszczynska-Piziak J., Abramson S. B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992 Sep;90(3):1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew R., Miners J. O. The effects of buthionine sulphoximine (BSO) on glutathione depletion and xenobiotic biotransformation. Biochem Pharmacol. 1984 Oct 1;33(19):2989–2994. doi: 10.1016/0006-2952(84)90598-7. [DOI] [PubMed] [Google Scholar]
- Ewing J. F., Maines M. D. Glutathione depletion induces heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem. 1993 Apr;60(4):1512–1519. doi: 10.1111/j.1471-4159.1993.tb03315.x. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferluga J., Allison A. C. Role of mononuclear infiltrating cells in pathogenesis of hepatitis. Lancet. 1978 Sep 16;2(8090):610–611. doi: 10.1016/s0140-6736(78)92828-3. [DOI] [PubMed] [Google Scholar]
- Freedman A. R., Sharma R. J., Nabel G. J., Emerson S. G., Griffin G. E. Cellular distribution of nuclear factor kappa B binding activity in rat liver. Biochem J. 1992 Oct 15;287(Pt 2):645–649. doi: 10.1042/bj2870645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel H. A., Goa K. L., Benfield P. S-adenosyl-L-methionine. A review of its pharmacological properties and therapeutic potential in liver dysfunction and affective disorders in relation to its physiological role in cell metabolism. Drugs. 1989 Sep;38(3):389–416. doi: 10.2165/00003495-198938030-00004. [DOI] [PubMed] [Google Scholar]
- Gemsa D., Woo C. H., Fudenberg H. H., Schmid R. Stimulation of heme oxygenase in macrophages and liver by endotoxin. J Clin Invest. 1974 Feb;53(2):647–651. doi: 10.1172/JCI107599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P., Bianchi M., Mantovani A., Spreafico F., Salmona M. Enhanced xanthine oxidase activity in mice treated with interferon and interferon inducers. Biochem Biophys Res Commun. 1984 Feb 29;119(1):144–149. doi: 10.1016/0006-291x(84)91630-9. [DOI] [PubMed] [Google Scholar]
- Ghigo D., Alessio P., Foco A., Bussolino F., Costamagna C., Heller R., Garbarino G., Pescarmona G. P., Bosia A. Nitric oxide synthesis is impaired in glutathione-depleted human umbilical vein endothelial cells. Am J Physiol. 1993 Sep;265(3 Pt 1):C728–C732. doi: 10.1152/ajpcell.1993.265.3.C728. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem J. 1988 Dec 15;256(3):861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett J. A., Schultze A. E., VanCise S., Roth R. A. Neutrophil depletion protects against liver injury from bacterial endotoxin. Lab Invest. 1992 Mar;66(3):347–361. [PubMed] [Google Scholar]
- Keyse S. M., Applegate L. A., Tromvoukis Y., Tyrrell R. M. Oxidant stress leads to transcriptional activation of the human heme oxygenase gene in cultured skin fibroblasts. Mol Cell Biol. 1990 Sep;10(9):4967–4969. doi: 10.1128/mcb.10.9.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Induction of the heme oxygenase gene in human skin fibroblasts by hydrogen peroxide and UVA (365 nm) radiation: evidence for the involvement of the hydroxyl radical. Carcinogenesis. 1990 May;11(5):787–791. doi: 10.1093/carcin/11.5.787. [DOI] [PubMed] [Google Scholar]
- Klasing K. C. Effect of inflammatory agents and interleukin 1 on iron and zinc metabolism. Am J Physiol. 1984 Nov;247(5 Pt 2):R901–R904. doi: 10.1152/ajpregu.1984.247.5.R901. [DOI] [PubMed] [Google Scholar]
- Konijn A. M., Hershko C. Ferritin synthesis in inflammation. I. Pathogenesis of impaired iron release. Br J Haematol. 1977 Sep;37(1):7–16. [PubMed] [Google Scholar]
- Kunimoto F., Morita T., Ogawa R., Fujita T. Inhibition of lipid peroxidation improves survival rate of endotoxemic rats. Circ Shock. 1987;21(1):15–22. [PubMed] [Google Scholar]
- Lautier D., Luscher P., Tyrrell R. M. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis. 1992 Feb;13(2):227–232. doi: 10.1093/carcin/13.2.227. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y., Schwartzman M. L., Abraham N. G. Novel regulatory sites of the human heme oxygenase-1 promoter region. Biochem Biophys Res Commun. 1993 Oct 15;196(1):336–341. doi: 10.1006/bbrc.1993.2253. [DOI] [PubMed] [Google Scholar]
- Levy E., Ruebner B. H. Hepatic changes produced by a single dose of endotoxin in the mouse. Light microscopy and histochemistry. Am J Pathol. 1967 Aug;51(2):269–285. [PMC free article] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxides can modulate the redox state of pyridine nucleotides and the calcium balance in rat liver mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4340–4344. doi: 10.1073/pnas.76.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988 Jul;2(10):2557–2568. [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med. 1978 Jul;89(1):122–127. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro T., Yoshida T., Numazawa S., Kuroiwa Y. Possible role of glutathione depletion in the induction of rate-limiting enzymes involved in heme degradation and polyamine biosynthesis in the liver of rats. J Pharmacobiodyn. 1990 Oct;13(10):628–636. doi: 10.1248/bpb1978.13.628. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Fairchild E. J., 2nd Evidence for lipid peroxidation in endotoxin-poisoned mice. Infect Immun. 1986 May;52(2):613–616. doi: 10.1128/iai.52.2.613-616.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peristeris P., Clark B. D., Gatti S., Faggioni R., Mantovani A., Mengozzi M., Orencole S. F., Sironi M., Ghezzi P. N-acetylcysteine and glutathione as inhibitors of tumor necrosis factor production. Cell Immunol. 1992 Apr;140(2):390–399. doi: 10.1016/0008-8749(92)90205-4. [DOI] [PubMed] [Google Scholar]
- Praaning-van Dalen D. P., Brouwer A., Knook D. L. Clearance capacity of rat liver Kupffer, Endothelial, and parenchymal cells. Gastroenterology. 1981 Dec;81(6):1036–1044. [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Reed D. J., Fariss M. W. Glutathione depletion and susceptibility. Pharmacol Rev. 1984 Jun;36(2 Suppl):25S–33S. [PubMed] [Google Scholar]
- Reed D. J. Glutathione: toxicological implications. Annu Rev Pharmacol Toxicol. 1990;30:603–631. doi: 10.1146/annurev.pa.30.040190.003131. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzardini M., Terao M., Falciani F., Cantoni L. Cytokine induction of haem oxygenase mRNA in mouse liver. Interleukin 1 transcriptionally activates the haem oxygenase gene. Biochem J. 1993 Mar 1;290(Pt 2):343–347. doi: 10.1042/bj2900343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Kanda N., Hsu C. C., Sakaguchi O. Lipid peroxide formation and membrane damage in endotoxin-poisoned mice. Microbiol Immunol. 1981;25(3):229–244. doi: 10.1111/j.1348-0421.1981.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Saunders E. L., Maines M. D., Meredith M. J., Freeman M. L. Enhancement of heme oxygenase-1 synthesis by glutathione depletion in Chinese hamster ovary cells. Arch Biochem Biophys. 1991 Aug 1;288(2):368–373. doi: 10.1016/0003-9861(91)90208-z. [DOI] [PubMed] [Google Scholar]
- Schreck R., Albermann K., Baeuerle P. A. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun. 1992;17(4):221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Müller R. M., Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem. 1987 Sep 25;262(27):12889–12892. [PubMed] [Google Scholar]
- Shibahara S., Müller R., Taguchi H., Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7865–7869. doi: 10.1073/pnas.82.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Sugimoto H., Matsuzaki S., Hamana K., Yamada S., Kobayashi S. Alpha-tocopherol and superoxide dismutase suppress and diethyldithiocarbamate and phorone enhance the lipopolysaccharide-induced increase in N1-acetylspermidine concentrations in mouse liver. Circ Shock. 1991 Mar;33(3):171–177. [PubMed] [Google Scholar]
- Sugino K., Dohi K., Yamada K., Kawasaki T. The role of lipid peroxidation in endotoxin-induced hepatic damage and the protective effect of antioxidants. Surgery. 1987 Jun;101(6):746–752. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L., Heremans J. F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974 Oct 1;140(4):1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile G. F., Tyrrell R. M. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem. 1993 Jul 15;268(20):14678–14681. [PubMed] [Google Scholar]
- Wink D. A., Hanbauer I., Krishna M. C., DeGraff W., Gamson J., Mitchell J. B. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Biro P., Cohen T., Müller R. M., Shibahara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem. 1988 Feb 1;171(3):457–461. doi: 10.1111/j.1432-1033.1988.tb13811.x. [DOI] [PubMed] [Google Scholar]