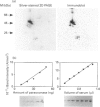
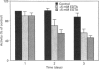
Abstract
Paraoxonase is a serum protein bound to high-density lipoproteins (HDLs). The physiological function of the enzyme is unknown, but a role in lipid metabolism has been postulated. To date, studies of the protein have had to rely on measurements of enzyme activity with various substrates. We have developed a highly specific, competitive e.l.i.s.a. using a previously characterized monoclonal antibody. The assay can detect 20 ng of paraoxonase with a working range of 75-600 ng. Intra- and interassay coefficients of variation were 6.5 and 7.9% respectively. Serum concentrations of paraoxonase in healthy subjects from Geneva and Manchester ranged from 25 to 118 micrograms/ml. There were significant differences in mean concentrations between the two groups (Geneva, 79.3 +/- 18.7 micrograms/ml; Manchester, 59.9 +/- 24.1 micrograms/ml: P < 0.001), differences also apparent when subjects were compared according to paraoxonase phenotype. These appeared to be largely a consequence of differences in apolipoprotein A-I concentrations between the two populations, suggesting that HDL particle number may be important in determining serum levels of paraoxonase. Paraoxonase specific activities were also significantly different between the two groups of subjects (Geneva, 2.08 +/- 0.96 units/mg; Manchester, 3.08 +/- 1.73 units/mg: P < 0.001), which may reflect differences in HDL particle composition. The e.l.i.s.a. should furnish the necessary complement to studies of paraoxonase enzymic activity and has already provided evidence for differences with respect to serum levels of the protein both between populations and between phenotypes within populations.
Full text
PDF

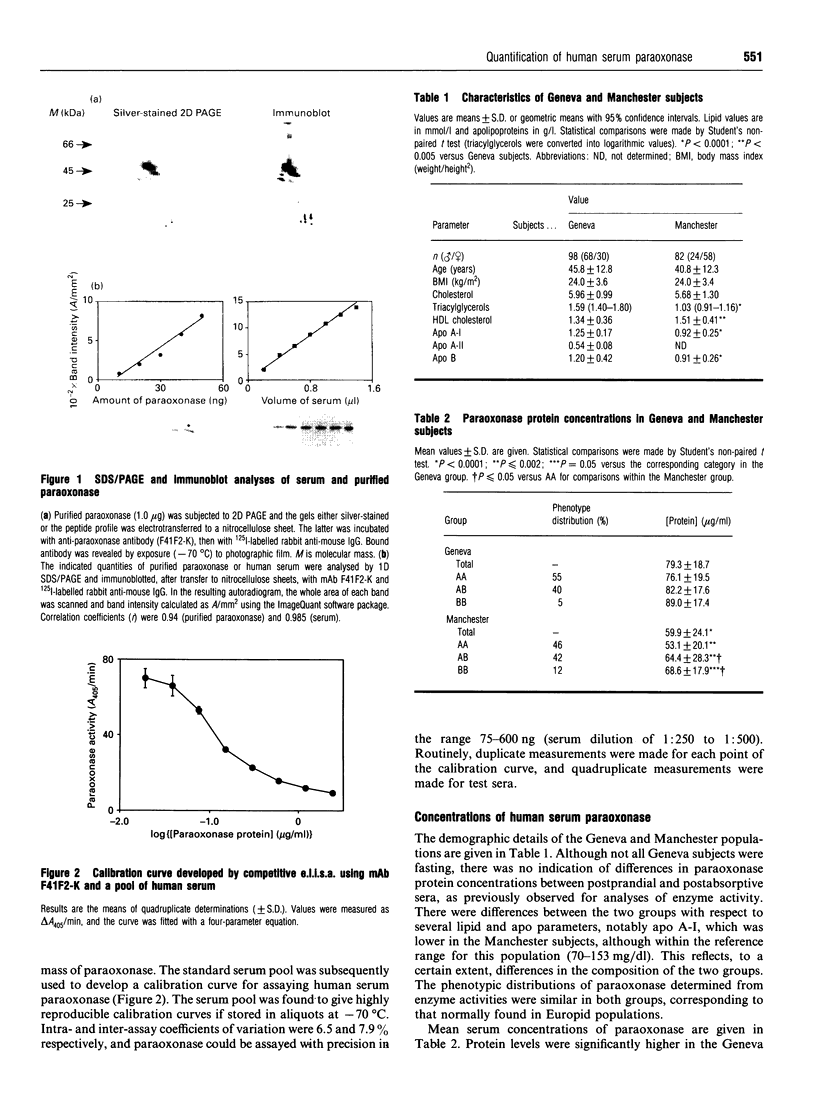
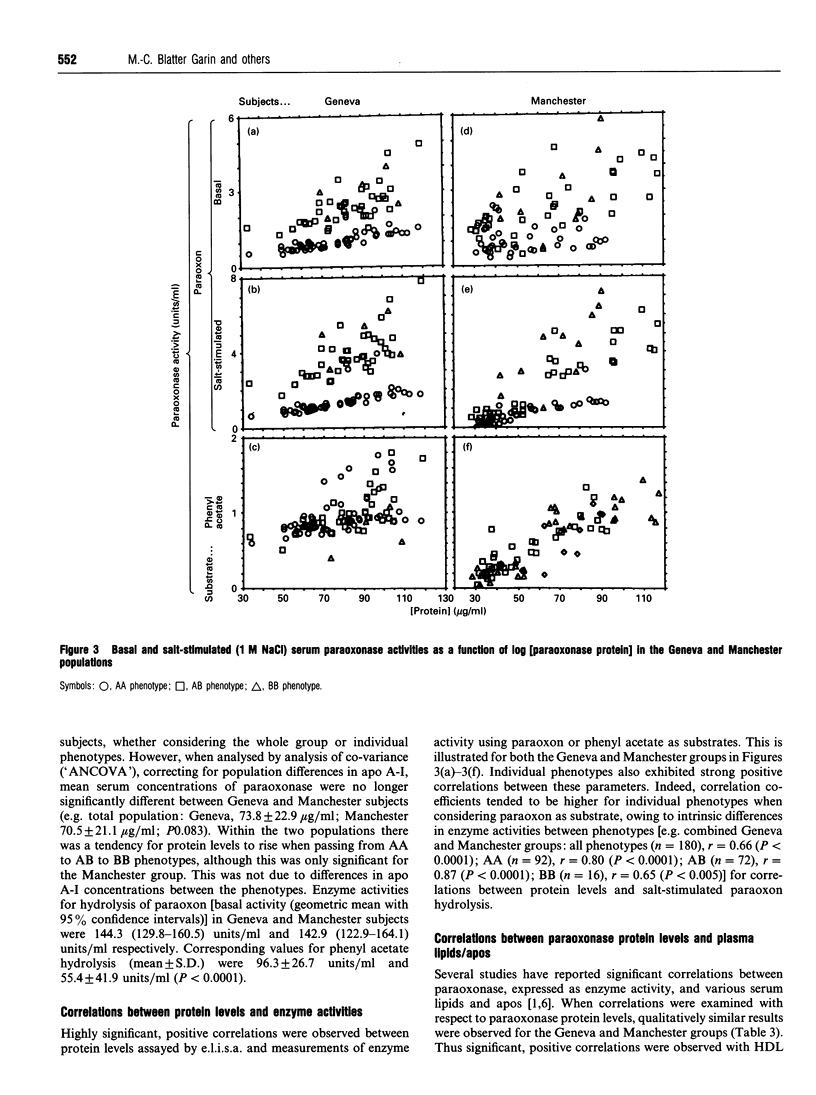

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins S., Gan K. N., Mody M., La Du B. N. Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: glutamine or arginine at position 191, for the respective A or B allozymes. Am J Hum Genet. 1993 Mar;52(3):598–608. [PMC free article] [PubMed] [Google Scholar]
- Bisgaier C. L., Sachdev O. P., Megna L., Glickman R. M. Distribution of apolipoprotein A-IV in human plasma. J Lipid Res. 1985 Jan;26(1):11–25. [PubMed] [Google Scholar]
- Blatter M. C., James R. W., Messmer S., Barja F., Pometta D. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur J Biochem. 1993 Feb 1;211(3):871–879. doi: 10.1111/j.1432-1033.1993.tb17620.x. [DOI] [PubMed] [Google Scholar]
- Boman H. Cholinesterase, arylesterase, and lipoprotein parameters in twins. Acta Genet Med Gemellol (Roma) 1980;29(4):281–287. doi: 10.1017/s0001566000007790. [DOI] [PubMed] [Google Scholar]
- Eckerson H. W., Romson J., Wyte C., La Du B. N. The human serum paraoxonase polymorphism: identification of phenotypes by their response to salts. Am J Hum Genet. 1983 Mar;35(2):214–227. [PMC free article] [PubMed] [Google Scholar]
- Eckerson H. W., Wyte C. M., La Du B. N. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983 Nov;35(6):1126–1138. [PMC free article] [PubMed] [Google Scholar]
- Furlong C. E., Richter R. J., Chapline C., Crabb J. W. Purification of rabbit and human serum paraoxonase. Biochemistry. 1991 Oct 22;30(42):10133–10140. doi: 10.1021/bi00106a009. [DOI] [PubMed] [Google Scholar]
- Gan K. N., Smolen A., Eckerson H. W., La Du B. N. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991 Jan-Feb;19(1):100–106. [PubMed] [Google Scholar]
- Humbert R., Adler D. A., Disteche C. M., Hassett C., Omiecinski C. J., Furlong C. E. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet. 1993 Jan;3(1):73–76. doi: 10.1038/ng0193-73. [DOI] [PubMed] [Google Scholar]
- James R. W., Hochstrasser D., Tissot J. D., Funk M., Appel R., Barja F., Pellegrini C., Muller A. F., Pometta D. Protein heterogeneity of lipoprotein particles containing apolipoprotein A-I without apolipoprotein A-II and apolipoprotein A-I with apolipoprotein A-II isolated from human plasma. J Lipid Res. 1988 Dec;29(12):1557–1571. [PubMed] [Google Scholar]
- James R. W., Pometta D. Differences in lipoprotein subfraction composition and distribution between type I diabetic men and control subjects. Diabetes. 1990 Oct;39(10):1158–1164. doi: 10.2337/diab.39.10.1158. [DOI] [PubMed] [Google Scholar]
- James R. W., Pometta D. Immunofractionation of high density lipoprotein subclasses 2 and 3. Similarities and differences of fractions isolated from male and female populations. Atherosclerosis. 1990 Jul;83(1):35–45. doi: 10.1016/0021-9150(90)90128-6. [DOI] [PubMed] [Google Scholar]
- James R. W., Pometta D. The distribution profiles of very low density and low density lipoproteins in poorly-controlled male, type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1991 Apr;34(4):246–252. doi: 10.1007/BF00405083. [DOI] [PubMed] [Google Scholar]
- Krisch K. Enzymatische Hydrolyse von Diäthyl-p-nitrophenylphosphat (E 600) durch menschlichen Serum. Z Klin Chem Klin Biochem. 1968 Jan;6(1):41–45. [PubMed] [Google Scholar]
- Kunitake S. T., Kane J. P. Factors affecting the integrity of high density lipoproteins in the ultracentrifuge. J Lipid Res. 1982 Aug;23(6):936–940. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- La Du B. N., Adkins S., Kuo C. L., Lipsig D. Studies on human serum paraoxonase/arylesterase. Chem Biol Interact. 1993 Jun;87(1-3):25–34. doi: 10.1016/0009-2797(93)90022-q. [DOI] [PubMed] [Google Scholar]
- La Du B. N. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1988 Sep;43(3):227–229. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackness M. I. 'A'-esterases. Enzymes looking for a role? Biochem Pharmacol. 1989 Feb 1;38(3):385–390. doi: 10.1016/0006-2952(89)90376-6. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Arrol S., Abbott C., Durrington P. N. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis. 1993 Dec;104(1-2):129–135. doi: 10.1016/0021-9150(93)90183-u. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Arrol S., Durrington P. N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991 Jul 29;286(1-2):152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Hallam S. D., Peard T., Warner S., Walker C. H. The separation of sheep and human serum "A"-esterase activity into the lipoprotein fraction by ultracentrifugation. Comp Biochem Physiol B. 1985;82(4):675–677. doi: 10.1016/0305-0491(85)90506-1. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Harty D., Bhatnagar D., Winocour P. H., Arrol S., Ishola M., Durrington P. N. Serum paraoxonase activity in familial hypercholesterolaemia and insulin-dependent diabetes mellitus. Atherosclerosis. 1991 Feb;86(2-3):193–199. doi: 10.1016/0021-9150(91)90215-o. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Peuchant E., Dumon M. F., Walker C. H., Clerc M. Absence of "A"-esterase activity in the serum of a patient with Tangier disease. Clin Biochem. 1989 Dec;22(6):475–478. doi: 10.1016/s0009-9120(89)80101-8. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Walker C. H., Carlson L. A. Low A-esterase activity in serum of patients with fish-eye disease. Clin Chem. 1987 Apr;33(4):587–588. [PubMed] [Google Scholar]
- McElveen J., Mackness M. I., Colley C. M., Peard T., Warner S., Walker C. H. Distribution of paraoxon hydrolytic activity in the serum of patients after myocardial infarction. Clin Chem. 1986 Apr;32(4):671–673. [PubMed] [Google Scholar]
- Saha N., Roy A. C., Teo S. H., Tay J. S., Ratnam S. S. Influence of serum paraoxonase polymorphism on serum lipids and apolipoproteins. Clin Genet. 1991 Oct;40(4):277–282. doi: 10.1111/j.1399-0004.1991.tb03096.x. [DOI] [PubMed] [Google Scholar]
- Steinberg K. K., Cooper G. R., Graiser S. R., Rosseneu M. Some considerations of methodology and standardization of apolipoprotein A-I immunoassays. Clin Chem. 1983 Mar;29(3):415–426. [PubMed] [Google Scholar]
- Steinmetz A., Jakobs C., Motzny S., Kaffarnik H. Differential distribution of apolipoprotein E isoforms in human plasma lipoproteins. Arteriosclerosis. 1989 May-Jun;9(3):405–411. doi: 10.1161/01.atv.9.3.405. [DOI] [PubMed] [Google Scholar]
- Szabó F., Róna K., Czinner A., Gachályi B., Káldor A. Is paraoxon hydrolytic activity in serum predictive of myocardial infarction? Clin Chem. 1987 May;33(5):742–743. [PubMed] [Google Scholar]