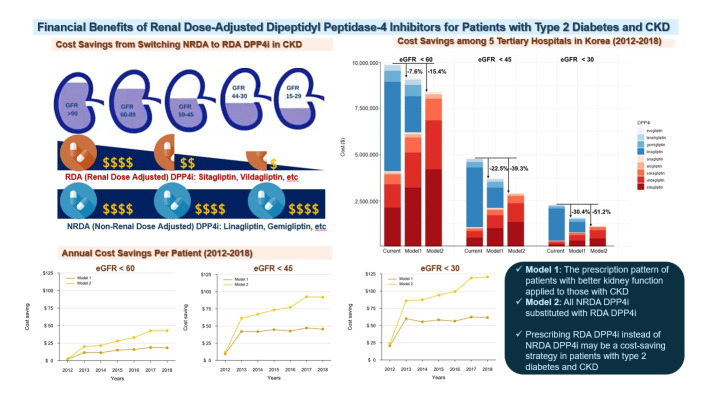
Abstract
Background
Dipeptidyl peptidase-4 (DPP4) inhibitors are frequently prescribed for patients with type 2 diabetes; however, their cost can pose a significant barrier for those with impaired kidney function. This study aimed to estimate the economic benefits of substituting non-renal dose-adjusted (NRDA) DPP4 inhibitors with renal dose-adjusted (RDA) DPP4 inhibitors in patients with both impaired kidney function and type 2 diabetes.
Methods
This retrospective cohort study was conducted from January 1, 2012 to December 31, 2018, using data obtained from common data models of five medical centers in Korea. Model 1 applied the prescription pattern of participants with preserved kidney function to those with impaired kidney function. In contrast, model 2 replaced all NRDA DPP4 inhibitors with RDA DPP4 inhibitors, adjusting the doses of RDA DPP4 inhibitors based on individual kidney function. The primary outcome was the cost difference between the two models.
Results
In total, 67,964,996 prescription records were analyzed. NRDA DPP4 inhibitors were more frequently prescribed to patients with impaired kidney function than in those with preserved kidney function (25.7%, 51.3%, 64.3%, and 71.6% in patients with estimated glomerular filtration rates [eGFRs] of ≥60, <60, <45, and <30 mL/min/1.73 m2, respectively). When model 1 was applied, the cost savings per year were 7.6% for eGFR <60 mL/min/1.73 m2 and 30.4% for eGFR <30 mL/min/1.73 m2. According to model 2, 15.4% to 51.2% per year could be saved depending on kidney impairment severity.
Conclusion
Adjusting the doses of RDA DPP4 inhibitors based on individual kidney function could alleviate the economic burden associated with medical expenses.
Keywords: Cost savings; Dipeptidyl-peptidase IV inhibitors; Renal insufficiency, chronic; Diabetes mellitus, type 2; Common data model
GRAPHICAL ABSTRACT
INTRODUCTION
Type 2 diabetes is a major contributor to chronic kidney disease (CKD). Studies indicate that about 20% of individuals with type 2 diabetes develop a reduction in kidney function, with an estimated glomerular filtration rate (eGFR) falling below 60 mL/min/1.73 m2, whether or not they have albuminuria [1,2]. Managing diabetes in conjunction with CKD is complex, as patients with reduced eGFR are highly prone to adverse drug reactions. This susceptibility often stems from renal impairment, which can alter drug responses due to decreased clearance of the drug and its metabolites.
Dipeptidyl peptidase-4 (DPP4) inhibitors serve as a therapeutic option for patients with type 2 diabetes across all stages of CKD. These inhibitors are divided into two categories: renal dose-adjusted (RDA) DPP4 inhibitors, which require a daily dose reduction in cases of renal impairment, and non-renal dose-adjusted (NRDA) DPP4 inhibitors, which do not require dosage adjustments. RDA DPP4 inhibitors are primarily excreted through the urine, with excretion rates ranging from 75% to 87% for alogliptin, saxagliptin, sitagliptin, and vildagliptin [3,4]. In contrast, linagliptin is predominantly excreted via the biliary route and does not accumulate in individuals with renal impairment [5]. Linagliptin, along with other drugs approved in Korea and Japan such as gemigliptin, teneligliptin, and evogliptin, falls into the category of NRDA DPP4 inhibitors.
Regular monitoring of kidney function and dosage adjustments are mandatory when using RDA DPP4 inhibitors to ensure safe and effective therapy. Therefore, it might be simpler for physicians to prescribe full-dose NRDA DPP4 inhibitors when treating patients with CKD. However, prescribing lower doses of RDA DPP4 inhibitors, as opposed to full, fixed doses of NRDA DPP4 inhibitors, could reduce the economic burden without compromising the efficacy needed to achieve glycemic goals. In our previous study, we analyzed the economic impact of more actively prescribing RDA DPP4 inhibitors using national insurance claims data from the Korean Health Insurance Review and Assessment Service in 2015 [6]. The study indicated that approximately 7.8% of the annual cost could be saved by substituting NRDA DPP4 inhibitors with RDA DPP4 inhibitors in patients with CKD stages 3–5. However, this approach may not accurately reflect the true condition of the patients, as eGFR values were not available in the claims data and the diagnosis of CKD stages 3–5 was based solely on the codes defined by the International Classification of Disease (ICD).
To address this limitation, we conducted a study to assess the prescription patterns of RDA and NRDA DPP4 inhibitors in patients with impaired kidney function, based on eGFR values, using a database from university hospitals in Korea. The aim of our study was to estimate the potential cost savings that could be achieved by partially or completely replacing NRDA DPP4 inhibitors with RDA DPP4 inhibitors in patients requiring renal dose adjustments.
METHODS
Study cohort
Patient information was obtained from common data models (CDMs), which provide de-identified standard data converted from electronic medical records. The study cohort included patients from five medical centers in Korea: Ajou University Hospital, Ewha Womans University Mokdong Hospital, Korea University Anam Hospital, Seoul National University Hospital, and Seoul National University Bundang Hospital, listed in alphabetical order and de-identified during analysis. Data were retrieved from the CDM datasets of these hospitals from January 1, 2012 to December 31, 2018. The data access date for research purposes was November 18, 2020. The authors did not have access to information that could identify individual participants during or after data collection. Consequently, this study was exempt from Institutional Review Board and Institutional Animal Care and Use Committee ethical approval. We screened patients aged 18 years or older who had been diagnosed with type 2 diabetes mellitus and were prescribed DPP4 inhibitors. Patients lacking valid serum creatinine levels within 90 days of the DPP4 inhibitor prescription date were excluded from the analysis.
Study design
The study utilized cost information from the national health insurance database in Korea for the corresponding years to determine the price of DPP4 inhibitors (Supplemental Table S1). It was assumed that the price of DPP4 inhibitors in fixed-dose pills containing two or more active substances would be equivalent to the price of the same dose of a DPP4 inhibitor in a single preparation. To convert the prices of the DPP4 inhibitors to US dollars, the exchange rate from each corresponding year was applied (Supplemental Table S2).
The pattern of RDA and NRDA DPP4 inhibitor prescriptions according to kidney function was analyzed, and CKD stages were defined using cutoff values of 60, 45, and 30 mL/min/1.73 m2. Participants with eGFR values above these cutoffs were considered to have preserved kidney function, whereas those with values below were considered to have impaired kidney function. Two models were applied to estimate the extent of cost reduction.
In model 1, the prescription pattern observed in participants with better kidney function was applied to those with impaired kidney function, as determined by eGFR values calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. The resulting cost reduction was estimated.
In model 2, all NRDA DPP4 inhibitors were replaced with RDA DPP4 inhibitors, and the dosages of the RDA DPP4 inhibitors were adjusted according to individual kidney function. It was assumed that the RDA DPP4 inhibitors were prescribed and dosed in line with the recommended dosage reductions outlined in the guideline (Supplemental Table S3). Our hypothesis posited that this substitution strategy would lead to a decrease in the usage of DPP4 inhibitors, thereby reducing medical costs. For instance, the standard full-dose of sitagliptin is 100 mg for individuals with normal kidney function. However, for patients with an eGFR of less than 30 mL/min/1.73 m2, the recommended daily dose of sitagliptin is 25 mg. By applying the proportion of sitagliptin used in patients with normal kidney function to those with impaired function, a cost reduction of three-quarters for sitagliptin is achieved. The extent of this cost reduction was calculated based on the cost difference between these two types of DPP4 inhibitors.
Statistical analysis
Data are presented as mean±standard deviation (SD) for continuous variables and as numbers (percentages) for categorical variables. The Cochrane formula was applied to aggregate the data on sample size, mean, and SD from five hospitals into a single dataset [7]. All analyses were conducted using PostgreSQL version 15.1 (PostgreSQL Global Development Group, Regents of the University of California, Oakland, CA, USA) and R programming version 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org). A two-sided P value of ≤0.05 was considered statistically significant.
RESULTS
Baseline characteristics
We analyzed a total of 67,964,996 prescription records from 95,567 patients during 7 years of follow-up (Fig. 1). The participants had a mean age of 64.8±11.8 years at baseline, and 57.0% were men (Table 1). Based on the number of prescriptions, 76% of participants had preserved kidney function with an eGFR of 60 mL/min/1.73 m2 or higher. Among those with an eGFR CKD-EPI of 60 mL/min/1.73 m2 or lower, 12%, 6%, and 6% were classified into CKD stages 3a (45≤ eGFR <60 mL/min/1.73 m2), 3b (30≤ eGFR <45 mL/min/1.73 m2), and 4–5 (eGFR <30 mL/min/1.73 m2), respectively (Supplemental Tables S4, S5).
Fig. 1.
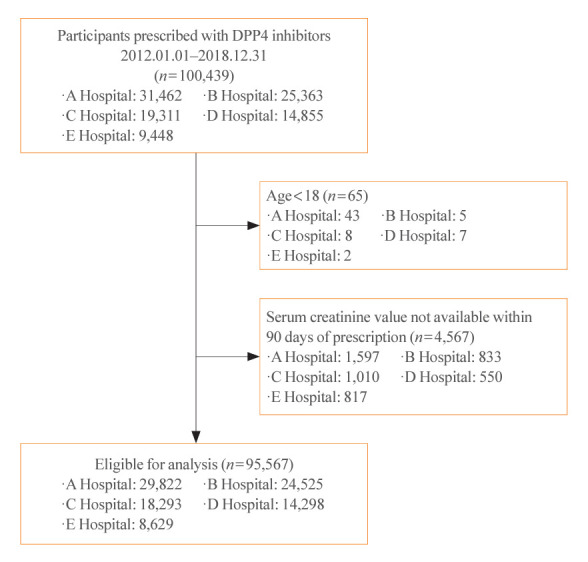
Consolidated Standards of Reporting Trials (CONSORT) diagram. Hospitals A, B, C, D, and E are Ajou University Hospital, Ewha Womans University Mokdong Hospital, Korea University Anam Hospital, Seoul National University Hospital, and Seoul National University Bundang Hospital, in an arbitrary order. DPP4, dipeptidyl peptidase-4.
Table 1.
Baseline Characteristics of Patients according to an eGFR Cutoff of 60 mL/min/1.73 m2
| Characteristic | Hospital | eGFR (full range) | eGFR ≥60 mL/min/1.73 m2 | eGFR <60 mL/min/1.73 m2 |
|---|---|---|---|---|
| No. of prescription | Total | 67,964,996 | 51,655,751 | 16,309,245 |
| A | 22,511,187 | 17,315,379 | 5,195,808 | |
| B | 17,655,782 | 13,932,457 | 3,723,325 | |
| C | 11,094,455 | 8,365,640 | 2,728,815 | |
| D | 10,850,119 | 7,653,373 | 3,196,746 | |
| E | 5,853,453 | 4,388,902 | 1,464,551 | |
| Age, yr | Total | 64.8±11.8 | 62.8±11.3 | 71.3±10.7 |
| A | 65.5±11.3 | 64.0±10.8 | 70.5±11.3 | |
| B | 65.1±11.9 | 63.2±11.5 | 72.5±10.3 | |
| C | 62.2±12.1 | 59.9±11.5 | 69.5±10.8 | |
| D | 66.3±11.5 | 63.7±11.2 | 72.3±10.0 | |
| E | 63.7±12.1 | 60.9±11.3 | 72.2±10.3 | |
| Male sex | Total | 38,755,334 (57.0) | 29,869,216 (57.8) | 8,886,118 (54.5) |
| A | 12,695,313 (56.4) | 9,765,385 (56.4) | 2,929,928 (56.4) | |
| B | 10,299,374 (58.3) | 8,143,578 (58.5) | 2,155,796 (57.9) | |
| C | 6,618,576 (59.7) | 5,213,013 (62.3) | 1,405,563 (51.5) | |
| D | 5,913,047 (54.5) | 4,215,385 (55.1) | 1,697,662 (53.1) | |
| E | 3,229,024 (55.2) | 2,531,855 (57.7) | 697,169 (47.6) | |
| eGFR, CKD-EPI, mL/min/1.73 m2 | Total | 75.3±24.5 | 86.4±14.1 | 40.1±16.1 |
| A | 75.2±24.5 | 86.0±13.7 | 39.2±17.3 | |
| B | 78.5±25.5 | 89.2±14.3 | 38.5±16.8 | |
| C | 75.2±24.0 | 86.0±14.6 | 41.8±14.5 | |
| D | 71.6±24.3 | 84.4±13.5 | 40.7±15.0 | |
| E | 72.8±21.7 | 82.8±13.1 | 43.0±13.6 |
Values are expressed as mean±standard deviation or number (%). Hospitals A, B, C, D, and E are Ajou University Hospital, Ewha Womans University Mokdong Hospital, Korea University Anam Hospital, Seoul National University Hospital, and Seoul National University Bundang Hospital, in an arbitrary order.
eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Patterns of DPP4 inhibitor prescriptions
Kidney function influenced the prescription patterns of DPP4 inhibitors. Among patients whose kidney function did not require renal dose adjustment, 25.7% were prescribed NRDA DPP4 inhibitors (Fig. 2, Supplemental Table S6). However, a higher percentage of patients with impaired kidney function, necessitating dose reductions of RDA DPP4 inhibitors, received NRDA DPP4 inhibitors (51.3%, 64.3%, and 71.6% for eGFR <60, <40, and <30 mL/min/1.73 m2, respectively). In the hospital setting, over 40% of inpatients with an eGFR of 60 mL/min/1.73 m2 or higher were prescribed NRDA DPP4 inhibitors. The proportion of patients prescribed NRDA DPP4 inhibitors increased in those with lower eGFR levels, with rates of 71.1% for eGFR <60 mL/min/1.73 m2, 79.0% for eGFR <45 mL/min/1.73 m2, and 84.7% for eGFR <30 mL/min/1.73 m2.
Fig. 2.
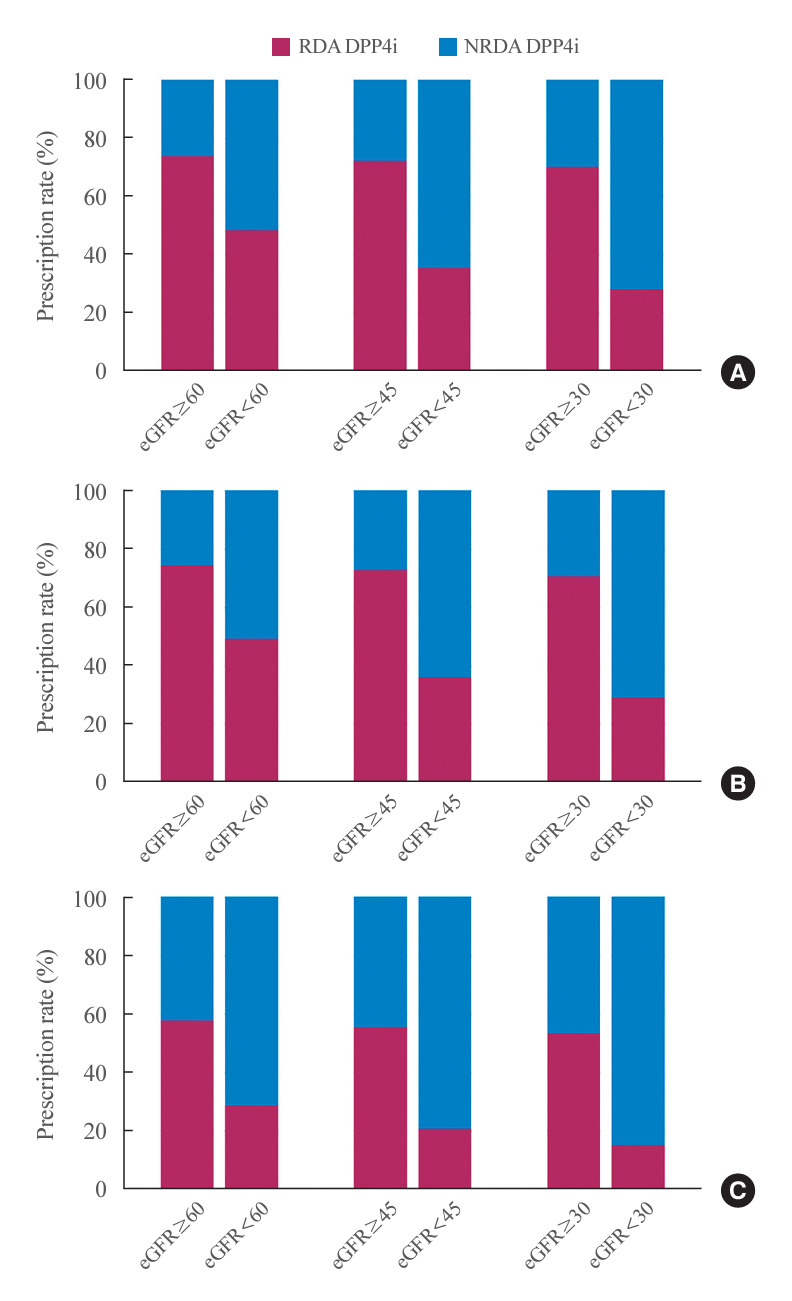
Patterns of dipeptidyl peptidase-4 inhibitor (DPP4i) prescriptions. Each bar is stacked to represent prescription rates for renal dose-adjusted (RDA) and non-renal dose-adjusted (NRDA) DPP4 inhibitors according to the estimated glomerular filtration rate (eGFR) cutoff values of 60 (left), 45 (middle), and 30 mL/min/1.73 m2 (right). (A) All patients. (B) Patients who were prescribed DPP4 inhibitors in outpatient clinics. (C) Patients who were prescribed DPP4 inhibitors during hospitalization.
Linagliptin was the most commonly prescribed NRDA DPP4 inhibitor, representing 42.7% of all DPP4 inhibitors prescribed to patients with CKD stages 3–5. The percentage of patients receiving linagliptin rose from 16.6% among those not requiring renal dose adjustment to 42.7% in those with an eGFR <60 mL/min/1.73 m2, and it increased further to 64.0% in patients with an eGFR <30 mL/min/1.73 m2. For sitagliptin, an RDA DPP4 inhibitor, the proportion of patients prescribed this medication decreased from 41.6% in those with kidney function not requiring renal dose adjustment to 25.7% in patients with an eGFR <60 mL/min/1.73 m2, and it declined further to 15.7% in those with an eGFR <30 mL/min/1.73 m2 (Supplemental Fig. S1).
Notably, an increasing trend in the prescription of NRDA DPP4 inhibitors was observed from 2012 to 2018 (Supplemental Fig. S2). Although the total number of RDA DPP4 inhibitor prescriptions was higher than that of NRDA DPP4 inhibitors from 2012 to 2018, the prescriptions for NRDA DPP4 inhibitors significantly exceeded those for RDA DPP4 inhibitors among participants with an eGFR CKD-EPI of 60 mL/min/1.73 m2 or lower starting in 2016. This trend was even more pronounced among participants with more severe kidney function impairment and in hospitalized patients. Specifically, among inpatients, prescriptions for NRDA DPP4 inhibitors significantly surpassed those for RDA DPP4 inhibitors starting in 2014 for patients with an eGFR <60 mL/min/1.73 m2, and as early as 2013 for those with an eGFR <45 mL/min/1.73 m2.
The cost-saving effect of using RDA DPP4 inhibitors
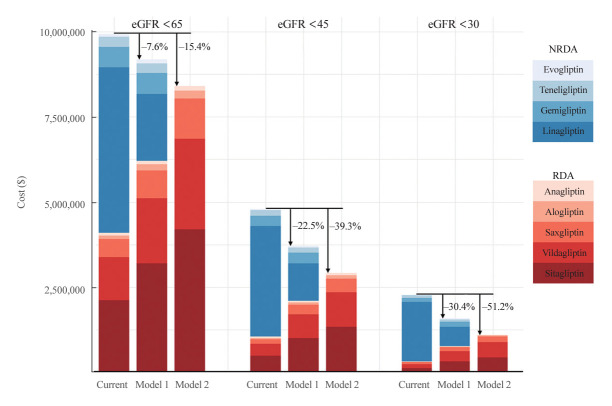
We postulated that increasing the prescription rate of RDA DPP4 inhibitors, while adjusting the dosage according to individual kidney function, could economically benefit patients with CKD stages 3–5. In model 1, we estimated the cost savings achieved by applying the prescription patterns of individuals with preserved kidney function to those with CKD stages 3–5 (Fig. 3, Supplemental Table S7). By prescribing a higher proportion of RDA DPP4 inhibitors at lower doses tailored to individual kidney function, we estimate that a total of $759,186.0 could have been saved over the 7-year follow-up period, representing 7.6% of the total cost. The cost-saving effect was more pronounced in patients with diminished kidney function, with potential savings of up to 22% in patients with an eGFR <45 mL/min/1.73 m2 and up to 30.4% in those with an eGFR <30 mL/min/1.73 m2.
Fig. 3.
Economic benefits of prescribing renal dose-adjusted (RDA) dipeptidyl peptidase-4 (DPP4) inhibitors in patients with impaired kidney function. Each bar is stacked to represent specific DPP4 inhibitors. RDA DPP4 inhibitors are colored in red shades and non-renal dose-adjusted (NRDA) DPP4 inhibitors in blue shades. The subcolumns on the left side represent the current cost ($) spent on DPP4 inhibitors according to the estimated glomerular filtration rate (eGFR) cutoff values of 60, 45, and 30 mL/min/1.73 m2. The subcolumns in the middle and on the right side represent the costs estimated by applying model 1 and model 2, respectively.
The cost-saving impact could be further amplified by applying model 2, which involved administering smaller doses of RDA DPP4 inhibitors to all patients with impaired kidney function, instead of fixed doses of NRDA DPP4 inhibitors (Fig. 3, Supplemental Table S7). We found that up to 15.4% of the total cost could be saved in patients with CKD stages 3–5, amounting to $1,529,494.9 over a 7-year period of DPP4 inhibitor usage. The cost savings reached 22.5% in patients with an eGFR <45 mL/min/1.73 m2 and exceeded 50% (51.2%) in patients with an eGFR <30 mL/min/1.73 m2 or CKD stages 4–5 when all NRDA DPP4 inhibitors were replaced with RDA DPP4 inhibitors. When calculated on a per patient basis with a 365-day prescription, the savings varied from $33.3 to $120.7, depending on the individual’s kidney function and hospitalization status (Supplemental Table S8).
Interestingly, we found that the cost-saving effect was more pronounced in hospitalized patients than in outpatients across both models, regardless of CKD stage. Using model 1, the cost reduction for hospitalized patients was 10.2%, 20.1%, and 24.3% for those with an eGFR of less than 60, 45, and 30 mL/min/1.73 m2, respectively. Similarly, when applying model 2, the cost reduction for hospitalized patients was 25.1%, 45.4%, and 52.9% for those with an eGFR of less than 60, 45, and 30 mL/min/1.73 m2, respectively.
The introduction of NRDA DPP4 inhibitors to clinical practice in Korea in 2012 has led to an increase in their prescription rate. This higher prescription rate provides an opportunity to substitute NRDA DPP4 inhibitors with lower doses of RDA DPP4 inhibitors in patients with renal impairment, resulting in cost savings. The cost-saving effect showed an increasing trend from 2012 to 2018 (Fig. 4, Supplemental Table S9). By 2018, the cost reduction was 8.2%, 21.0%, and 28.1% in patients with an eGFR of less than 60, 45, and 30 mL/min/1.73 m2, respectively. The cost reduction was even more significant when using model 2, with reductions of 19.3%, 42.6%, and 55.0% for the same eGFR categories. The annual economic benefits per patient from switching from NRDA to RDA DPP4 inhibitors for those with impaired kidney function, from 2012 to 2018, are detailed in Supplemental Fig. S3.
Fig. 4.
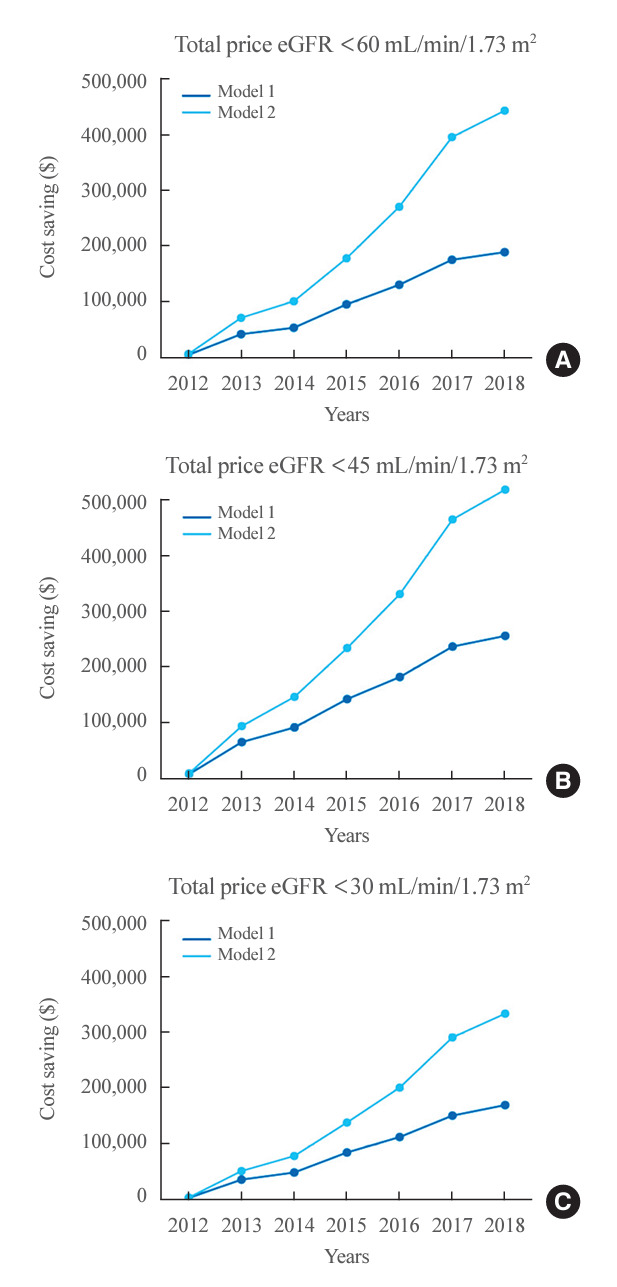
Economic benefits of prescribing renal dose-adjusted dipeptidyl peptidase-4 inhibitors in patients with impaired kidney function per year (2012 to 2018). The annual cost-saving effect estimated by applying model 1 (blue) and model 2 (light blue) in patients with estimated glomerular filtration rate (eGFRs) (A) <60, (B) <45, and (C) <30 mL/min/1.73 m2.
DISCUSSION
Chronic, non-communicable diseases, such as type 2 diabetes, significantly contribute to healthcare spending worldwide, with prescription drug costs accounting for a large portion of this expenditure. This study aimed to investigate the prescription patterns of DPP4 inhibitors in Korean patients with CKD and assess the potential cost savings from switching to optimized doses of RDA DPP4 inhibitors. The prescription of NRDA DPP4 inhibitors was more common among patients with CKD stages 3–5 compared to those with preserved kidney function. We estimated that if patients with CKD stages 3–5 were prescribed DPP4 inhibitors following the prescription patterns of those with preserved kidney function, there would be a 7.6% reduction in drug costs. Replacing all NRDA DPP4 inhibitors with RDA DPP4 inhibitors, with doses adjusted to individual kidney function, could lead to cost savings of up to 15.4%. In patients with an eGFR CKD-EPI of less than 30 mL/min/1.73 m2, switching to RDA DPP4 inhibitors could result in savings of over 50% of current medical expenditures, with even greater cost savings in hospitalized patients.
Since the introduction of sitagliptin in 2006, DPP4 inhibitors have gained significant market share in the United States [8]. In Korea, DPP4 inhibitors are by far the most commonly prescribed antidiabetic medication after metformin, with their usage increasing from 28.7% in 2012 to 63.4% in 2018 [9]. In Japan, the prescription rate of DPP4 inhibitors has surpassed both biguanides and sulfonylureas, making them the most widely used medication for managing type 2 diabetes [10]. Although DPP4 inhibitors offer the potential benefits of managing postprandial glucose excursions with minimal risk of hypoglycemia, their cost may present a significant barrier [11].
Previous studies have explored the prescription patterns of RDA DPP4 inhibitors and NRDA DPP4 inhibitors, revealing that NRDA DPP4 inhibitors are more frequently prescribed to patients with type 2 diabetes and CKD [12-15]. For example, research from the United Kingdom indicated that the prescription rate of NRDA DPP4 inhibitors was 16.6% among patients with CKD, which increased to 46.1% in those with more advanced CKD [15]. Similarly, in the United States, the prescription rates for NRDA DPP4 inhibitors were 16.4% in patients without CKD, and significantly higher at 26.7% in those with CKD [13]. However, these studies did not investigate the potential cost savings that could be achieved by substituting NRDA DPP4 inhibitors with RDA DPP4 inhibitors, assuming appropriate renal dosing is applied. To our knowledge, our previous study utilizing claims data remains the only research that has evaluated the cost-saving benefits of switching from NRDA DPP4 inhibitors to RDA DPP4 inhibitors in patients with CKD, despite the limitations posed by the unavailability of eGFR values [6].
Here, we present evidence that prescribing RDA DPP4 inhibitors is a promising strategy to maximize financial benefits for patients with impaired renal function. In fact, the cost reduction achieved by using the eGFR CKD-EPI equation was much greater than our previous estimate, which relied solely on ICD10 codes [6]. While some may argue that economic considerations should not outweigh other critical factors such as nephrotoxicity, RDA DPP4 inhibitors, with doses appropriately adjusted to individual kidney function, may also represent a more conservative drug choice for vulnerable patients with CKD stages 3–5. CKD affects drug metabolism and elimination, leading to altered pharmacokinetics and pharmacodynamics [16]. Therefore, although the glucose-lowering effect of DPP4 inhibitors may partially depend on renal clearance in patients with renal impairment, there is limited information on metrics such as drug-enzyme binding avidity, plasma protein binding, systemic retention of parent drug/metabolites, and DPP selectivity, which could increase the risk of off-target effects [17]. Thus, despite NRDA DPP4 inhibitors being an attractive option in patients with CKD, as higher than recommended doses of DPP4 inhibitors seldom cause serious adverse events and have a wide margin of safety [18-22], RDA DPP4 inhibitors may be more appropriate in terms of cost, safety, and efficacy.
The strength of this study lies in its use of over 60 million standardized prescription records from the CDM, covering a period of 7 years across five hospitals. Utilizing the CDM allowed for a more accurate representation of real-world healthcare data, facilitated by collaborative research among the hospitals.
However, this study has some limitations. First, our estimation assumed that all RDA DPP4 inhibitor doses are correctly adjusted for patients with CKD. However, it is important to acknowledge that many patients are inadvertently overtreated with RDA DPP4 inhibitors despite having reduced kidney function [23,24]. Additionally, this study did not consider physician compliance with recommended dose adjustments. Nonetheless, it is noteworthy that compliance with the current recommendations was 83.2%, which is relatively high, among patients with CKD stages 3–5 in our cohort. Second, we presumed that in renal dosing, the cost of a drug would be proportional to the price of the full dosage form. For example, we assumed that the cost of 50 mg of sitagliptin would be half that of 100 mg of sitagliptin, although this may not always be the case. Third, the relevance of DPP4 inhibitors in patients with CKD might diminish in the future. Sodium-glucose cotransporter 2 (SGLT2) inhibitors have recently emerged as a new class of antidiabetic drugs that could delay the progression of kidney disease [25,26]. While DPP4 inhibitors offer numerous benefits across a broad spectrum of CKD, the increasing use of SGLT2 inhibitors is justified, given their proven benefit in slowing the progression of kidney failure. However, it is important to note that the glucose-lowering efficacy of SGLT2 inhibitors decreases in patients with lower eGFR [27].
In conclusion, NRDA DPP4 inhibitors are commonly prescribed for individuals with diabetes and impaired kidney function. Our study indicates that prescribing RDA DPP4 inhibitors could be a cost-effective approach for managing patients with type 2 diabetes and CKD. Although NRDA DPP4 inhibitors have a favorable safety profile, it may be more suitable to prescribe RDA DPP4 inhibitors with doses adjusted based on individual kidney function, especially for patients with CKD stages 3–5. Additional research is necessary to verify our results and to assess the effects of RDA DPP4 inhibitors on clinical outcomes, including glycemic control and cardiovascular events.
Acknowledgments
This work was supported by the Technology Innovation Program (or Industrial Strategic Technology Development Program) (20004927, Upgrade of CDM based Distributed Biohealth Data Platform and Development of Verification Technology) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: Y.H.K., S.J.M., K.K., Y.M.C. Acquisition, analysis, or interpretation of data: Y.H.K., S.J.M., C.H.A., K. H.H., H.L., J.H.B., H.J.J., H.L., J.W.S., D.J.K., S.G.K. Drafting the work or revising: H.J.C. Final approval of the manuscript: H.J.C., Y.H.K., S.J.M., C.H.A., K.H.H., H.L., J.H.B., H.J.J., H.L., J.W.S., D.J.K., S.G.K., K.K., Y.M.C.
Supplementary Material
Price of DPP4 Inhibitors in Korea (Unit: ₩)
Average KRW to USD Annual Exchange Rate per Year
Recommended Dosage Adjustments of RDA DPP4 Inhibitors for Patients with Impaired Kidney Function
Baseline Characteristics of Patients according to eGFR 45 mL/min/1.73 m2
Baseline Characteristics of Patients according to eGFR 30 mL/min/1.73 m2
Prescription Patterns of DPP4 Inhibitors
Economic Benefit of Prescribing RDA DPP4 Inhibitors in Patients with Impaired Kidney Function
Annual Economic Benefit per Patient from RDA DPP4 Inhibitor Prescription for Impaired Kidney Function (2012 to 2018)
Economic Benefit of Prescribing RDA DPP4 Inhibitors in Patients with Impaired Kidney Function per Year (2012 to 2018)
Prescription patterns of renal dose-adjusted (RDA) and non-renal dose-adjusted (NRDA) dipeptidyl peptidase-4 (DPP4) inhibitors according to kidney function. Each bar stacked to represent prescription rates for specific DPP4 inhibitors according to the estimated glomerular filtration rate (eGFR) cutoff values of 60 (left), 45 (middle), and 30 mL/min/1.73 m2 (right). RDA DPP4 inhibitors are colored in reds and NRDA DPP4 inhibitors in blues. The widths of the columns are proportional to the number of patients in each eGFR group.
Prescription patterns of renal dose-adjusted (RDA) and non-renal dose-adjusted (NRDA) dipeptidyl peptidase-4 inhibitors (DPP4is) from 2012 to 2018. Prescription patterns of RDA and NRDA DPP4is from 2012 to 2018 in (A, B, C, D) patients with any estimated glomerular filtration rate (eGFR), (E, F, G, H) patients who were prescribed with DPP4is in outpatient clinics, and (I, J, K, L) patients who were prescribed with DPP4is during hospitalization.
Annual economic benefit per patient from renal dose-adjusted (RDA) dipeptidyl peptidase-4 (DPP4) inhibitors prescription in impaired kidney function (2012 to 2018). The annual cost savings per patient when prescribed RDA DPP4 inhibitors for varying stages of kidney function impairment, measured over the years 2012 to 2018. Panels correspond to patients with estimated glomerular filtration rate (eGFR) (A) <60, (B) <45, and (C) <30 mL/min/1.73 m2, respectively, displaying cost trends and savings in models 1 and 2.
REFERENCES
- 1.Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG, DEMAND investigators Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69:2057–63. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- 2.Thomas MC, Weekes AJ, Broadley OJ, Cooper ME, Mathew TH. The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study) Med J Aust. 2006;185:140–4. doi: 10.5694/j.1326-5377.2006.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 3.Furuta S, Smart C, Hackett A, Benning R, Warrington S. Pharmacokinetics and metabolism of [14C]anagliptin, a novel dipeptidyl peptidase-4 inhibitor, in humans. Xenobiotica. 2013;43:432–42. doi: 10.3109/00498254.2012.731618. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Yoo JH, Lee WJ, Park CY. Gemigliptin: an update of its clinical use in the management of type 2 diabetes mellitus. Diabetes Metab J. 2016;40:339–53. doi: 10.4093/dmj.2016.40.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graefe-Mody U, Friedrich C, Port A, Ring A, Retlich S, Heise T, et al. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin(*) Diabetes Obes Metab. 2011;13:939–46. doi: 10.1111/j.1463-1326.2011.01458.x. [DOI] [PubMed] [Google Scholar]
- 6.Moon SJ, Cho YM. Economic benefit of prescribing an adjusted renal dose of dipeptidyl peptidase IV inhibitors in type 2 diabetes patients with chronic kidney disease. J Diabetes. 2020;12:645–8. doi: 10.1111/1753-0407.13067. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4. Cochrane; 2023. Chapter 6, Choosing effect measures and computing estimates of effect [cited. 2024 May 9; [cited 2024 May 9]. Available from: https://training.cochrane.org/handbook/current/chapter-06. [Google Scholar]
- 8.Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003-2012. Diabetes Care. 2014;37:1367–74. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- 9.Bae JH, Han KD, Ko SH, Yang YS, Choi JH, Choi KM, et al. Diabetes fact sheet in Korea 2021. Diabetes Metab J. 2022;46:417–26. doi: 10.4093/dmj.2022.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura R, Kato H, Kisanuki K, Oh A, Hiroi S, Onishi Y, et al. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. BMJ Open. 2019;9:e025806. doi: 10.1136/bmjopen-2018-025806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 13. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S216–29. doi: 10.2337/dc23-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tebboth A, Lee S, Scowcroft A, Bingham-Gardiner P, Spencer W, Bolodeoku J, et al. Demographic and clinical characteristics of patients with type 2 diabetes mellitus initiating dipeptidyl peptidase 4 inhibitors: a retrospective study of UK general practice. Clin Ther. 2016;38:1825–32. doi: 10.1016/j.clinthera.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Patorno E, Gopalakrishnan C, Bartels DB, Brodovicz KG, Liu J, Schneeweiss S. Preferential prescribing and utilization trends of diabetes medications among patients with renal impairment: emerging role of linagliptin and other dipeptidyl peptidase 4 inhibitors. Endocrinol Diabetes Metab. 2017;1:e00005. doi: 10.1002/edm2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H, Shetty S, Bauer E, Lang K. Concordance with prescribing information dosage recommendations for dipeptidyl-peptidase-4 inhibitors among type 2 diabetes mellitus patients with moderate to severe chronic kidney disease. Curr Med Res Opin. 2018;34:1021–7. doi: 10.1080/03007995.2017.1416346. [DOI] [PubMed] [Google Scholar]
- 15.Spanopoulos D, Barrett B, Busse M, Roman T, Poole C. Prescription of DPP-4 inhibitors to type 2 diabetes mellitus patients with renal impairment: a UK primary care experience. Clin Ther. 2018;40:152–4. doi: 10.1016/j.clinthera.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65:757–73. doi: 10.1007/s00228-009-0678-8. [DOI] [PubMed] [Google Scholar]
- 17.Davis TM. Dipeptidyl peptidase-4 inhibitors: pharmacokinetics, efficacy, tolerability and safety in renal impairment. Diabetes Obes Metab. 2014;16:891–9. doi: 10.1111/dom.12295. [DOI] [PubMed] [Google Scholar]
- 18.Covington P, Christopher R, Davenport M, Fleck P, Mekki QA, Wann ER, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther. 2008;30:499–512. doi: 10.1016/j.clinthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Araki E, Kawamori R, Inagaki N, Watada H, Hayashi N, Horie Y, et al. Long-term safety of linagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:364–71. doi: 10.1111/dom.12039. [DOI] [PubMed] [Google Scholar]
- 20.Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376–86. doi: 10.1111/j.1463-1326.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 21.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–7. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 22.Bergman AJ, Cote J, Yi B, Marbury T, Swan SK, Smith W, et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care. 2007;30:1862–4. doi: 10.2337/dc06-2545. [DOI] [PubMed] [Google Scholar]
- 23.Hong S, Han K, Park CY. Outcomes for inappropriate renal dose adjustment of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus: population-based study. Mayo Clin Proc. 2020;95:101–12. doi: 10.1016/j.mayocp.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Franch-Nadal J, Gatius JR, Mata-Cases M, Ortega E, Valles JA, Vlacho B, et al. Compliance with the DPP-4 inhibitors dose adjustment recommendations based on renal function in a population database. Endocrinol Diabetes Nutr (Engl Ed) 2022;69:83–91. doi: 10.1016/j.endien.2022.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Heerspink HJ, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 26.The EMPA-KIDNEY Collaborative Group. Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388:117–27. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cherney DZ, Cooper ME, Tikkanen I, Pfarr E, Johansen OE, Woerle HJ, et al. Pooled analysis of phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018;93:231–44. doi: 10.1016/j.kint.2017.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Price of DPP4 Inhibitors in Korea (Unit: ₩)
Average KRW to USD Annual Exchange Rate per Year
Recommended Dosage Adjustments of RDA DPP4 Inhibitors for Patients with Impaired Kidney Function
Baseline Characteristics of Patients according to eGFR 45 mL/min/1.73 m2
Baseline Characteristics of Patients according to eGFR 30 mL/min/1.73 m2
Prescription Patterns of DPP4 Inhibitors
Economic Benefit of Prescribing RDA DPP4 Inhibitors in Patients with Impaired Kidney Function
Annual Economic Benefit per Patient from RDA DPP4 Inhibitor Prescription for Impaired Kidney Function (2012 to 2018)
Economic Benefit of Prescribing RDA DPP4 Inhibitors in Patients with Impaired Kidney Function per Year (2012 to 2018)
Prescription patterns of renal dose-adjusted (RDA) and non-renal dose-adjusted (NRDA) dipeptidyl peptidase-4 (DPP4) inhibitors according to kidney function. Each bar stacked to represent prescription rates for specific DPP4 inhibitors according to the estimated glomerular filtration rate (eGFR) cutoff values of 60 (left), 45 (middle), and 30 mL/min/1.73 m2 (right). RDA DPP4 inhibitors are colored in reds and NRDA DPP4 inhibitors in blues. The widths of the columns are proportional to the number of patients in each eGFR group.
Prescription patterns of renal dose-adjusted (RDA) and non-renal dose-adjusted (NRDA) dipeptidyl peptidase-4 inhibitors (DPP4is) from 2012 to 2018. Prescription patterns of RDA and NRDA DPP4is from 2012 to 2018 in (A, B, C, D) patients with any estimated glomerular filtration rate (eGFR), (E, F, G, H) patients who were prescribed with DPP4is in outpatient clinics, and (I, J, K, L) patients who were prescribed with DPP4is during hospitalization.
Annual economic benefit per patient from renal dose-adjusted (RDA) dipeptidyl peptidase-4 (DPP4) inhibitors prescription in impaired kidney function (2012 to 2018). The annual cost savings per patient when prescribed RDA DPP4 inhibitors for varying stages of kidney function impairment, measured over the years 2012 to 2018. Panels correspond to patients with estimated glomerular filtration rate (eGFR) (A) <60, (B) <45, and (C) <30 mL/min/1.73 m2, respectively, displaying cost trends and savings in models 1 and 2.