Abstract
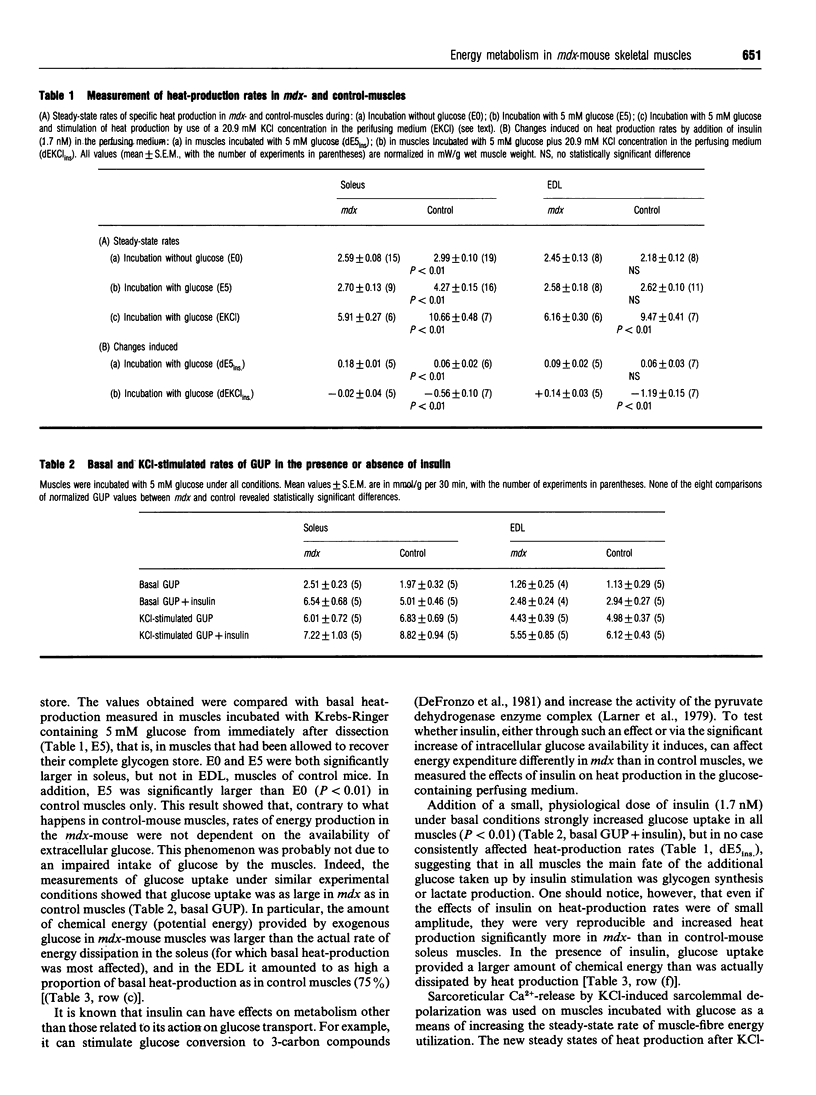
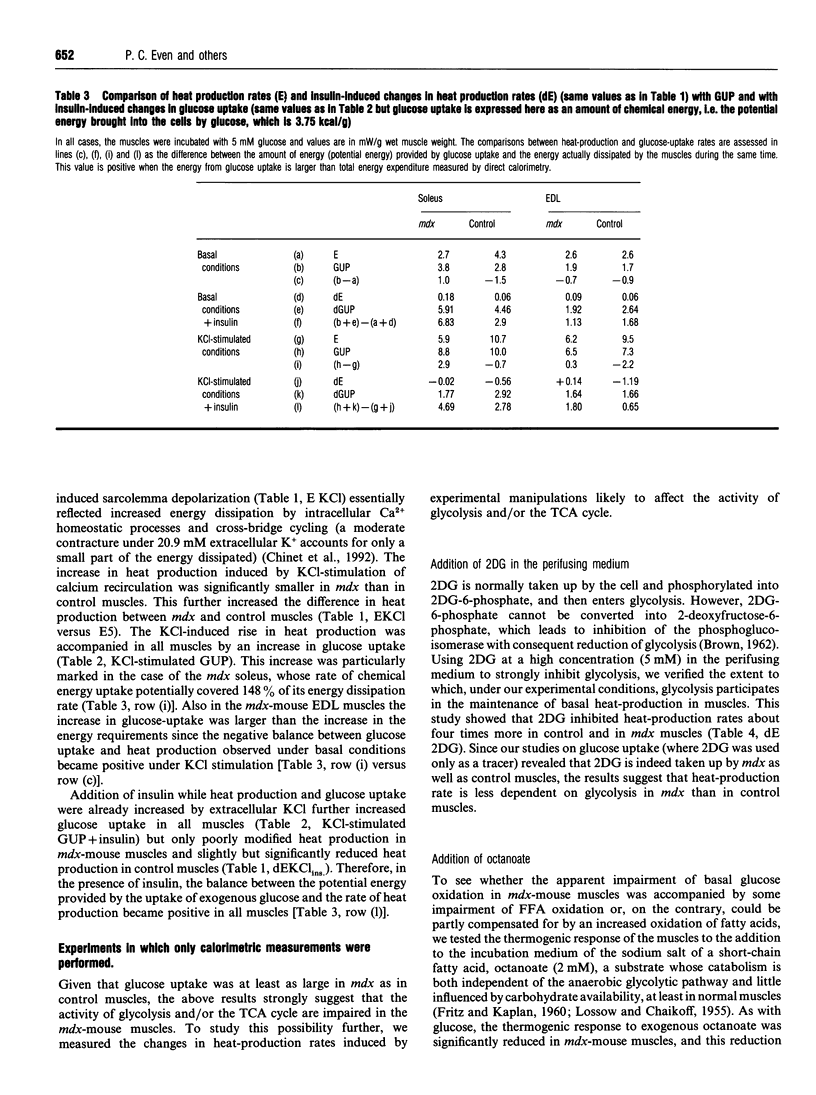
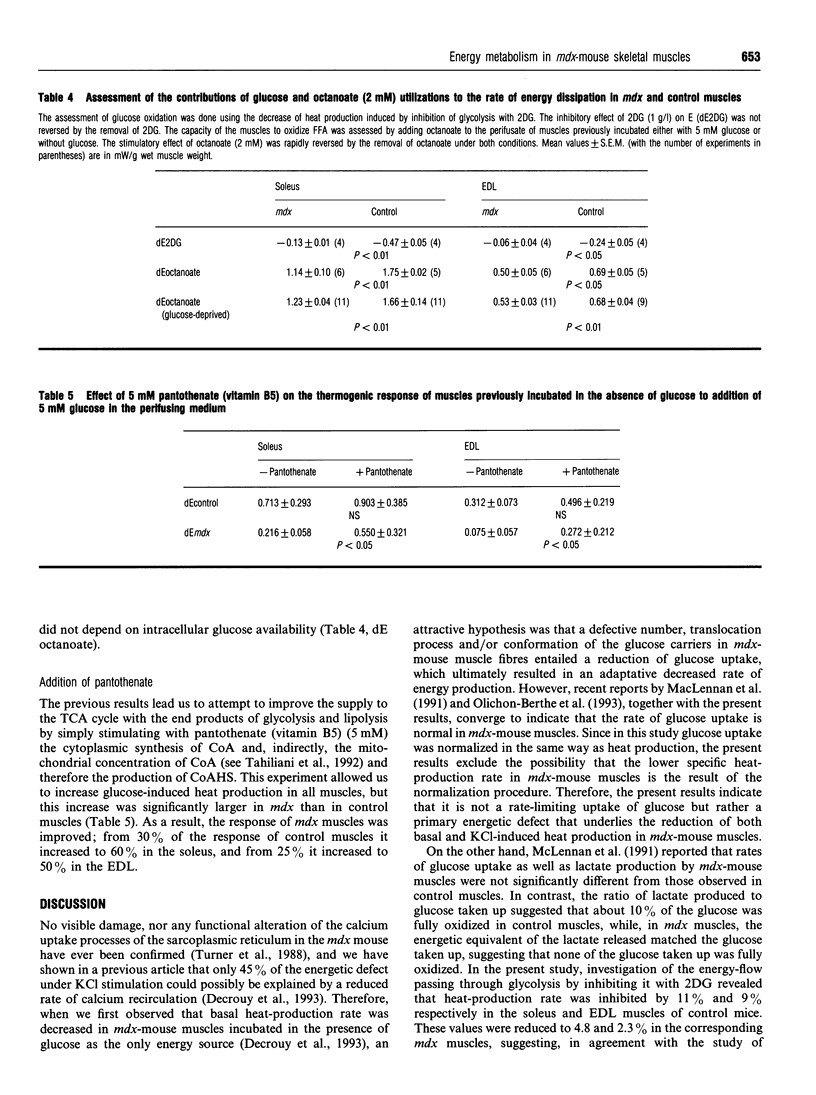
Our previous finding of a reduced energy metabolism in slow- and fast-twitch skeletal muscle fibres from the murine model of Duchenne muscular dystrophy (the mdx mouse) led us to examine the importance of intracellular glucose availability for a normal energy turnover. To this end, basal and KCl-stimulated (20.9 mM total extracellular K+) rates of glucose uptake (GUP) and heat production were measured in isolated, glucose-incubated (5 mM) soleus and extensor digitorum longus muscles from mdx and control C57B1/10 mice, in the presence and in the absence of insulin (1.7 nM). Under all conditions and for both muscle types, glucose uptake values for mdx and control muscles were similar although heat production was lower in mdx muscles. The marked stimulation of GUP by insulin in both mdx and control muscles had only minor effects on heat production. In contrast, glucose deprivation or inhibition of glycolysis with 2-deoxy-D-glucose (5 mM) significantly decreased heat production in control muscles only, which attenuated, although did not suppress, the difference in basal heat production between mdx and control muscles. Stimulation of heat production by a short-chain fatty acid salt (octanoate, 2 mM) was significantly less marked in mdx than in control muscles. Increased cytoplasmic synthesis of CoA by addition of 5 mM pantothenate (vitamin B5) increased the thermogenic response to glucose more in mdx than in control muscles. We conclude that the low energy turnover in mdx-mouse muscle fibres is not due to a decrease of intracellular glucose availability, but rather to a decreased oxidative utilization of glucose and free fatty acids. We suggest that some enzyme complex of the tricarboxylic acid cycle or inefficiency of CoA transport in the mitochondria could be involved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. E., Ovalle W. K., Bressler B. H. Electron microscopic and autoradiographic characterization of hindlimb muscle regeneration in the mdx mouse. Anat Rec. 1987 Nov;219(3):243–257. doi: 10.1002/ar.1092190305. [DOI] [PubMed] [Google Scholar]
- BROWN J. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism. 1962 Oct;11:1098–1112. [PubMed] [Google Scholar]
- Beal M. F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992 Feb;31(2):119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- Blass J. P., Sheu R. K., Cedarbaum J. M. Energy metabolism in disorders of the nervous system. Rev Neurol (Paris) 1988;144(10):543–563. [PubMed] [Google Scholar]
- Chinet A. E., Even P. C., Decrouy A. Dystrophin-dependent efficiency of metabolic pathways in mouse skeletal muscles. Experientia. 1994 Jun 15;50(6):602–605. doi: 10.1007/BF01921731. [DOI] [PubMed] [Google Scholar]
- Chinet A., Clausen T., Girardier L. Microcalorimetric determination of energy expenditure due to active sodium-potassium transport in the soleus muscle and brown adipose tissue of the rat. J Physiol. 1977 Feb;265(1):43–61. doi: 10.1113/jphysiol.1977.sp011704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinet A., Decrouy A., Even P. C. Ca(2+)-dependent heat production under basal and near-basal conditions in the mouse soleus muscle. J Physiol. 1992 Sep;455:663–678. doi: 10.1113/jphysiol.1992.sp019321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton G. R., Morgan J. E., Partridge T. A., Sloper J. C. The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol. 1988 Jan-Feb;14(1):53–70. doi: 10.1111/j.1365-2990.1988.tb00866.x. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Decrouy A., Even P. C., Chinet A. Decreased rates of Ca(2+)-dependent heat production in slow- and fast-twitch muscles from the dystrophic (mdx) mouse. Experientia. 1993 Oct 15;49(10):843–849. doi: 10.1007/BF01952595. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B., KAPLAN E. Effects of glucose on fatty acid oxidation by diaphragms from normal and alloxan-diabetic fed and starved rats. Am J Physiol. 1960 Jan;198:39–44. doi: 10.1152/ajplegacy.1960.198.1.39. [DOI] [PubMed] [Google Scholar]
- Ferré P., Leturque A., Burnol A. F., Penicaud L., Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985 May 15;228(1):103–110. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fréminet A., Leclerc L., Poyart C., Huel C., Gentil M. Alanine and succinate accumulation in the perfused rat heart during hypoxia. J Physiol (Paris) 1980;76(2):113–117. [PubMed] [Google Scholar]
- Förster M. E. Citric acid cycle as a "one-step" reaction. J Theor Biol. 1988 Jul 8;133(1):1–11. doi: 10.1016/s0022-5193(88)80020-1. [DOI] [PubMed] [Google Scholar]
- Head S. I. Membrane potential, resting calcium and calcium transients in isolated muscle fibres from normal and dystrophic mice. J Physiol. 1993 Sep;469:11–19. doi: 10.1113/jphysiol.1993.sp019801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOSSOW W. J., CHAIKOFF I. L. Carbohydrate sparing of fatty acid oxidation. I. The relation of fatty acid chain length to the degree of sparing. II. The mechanism by which carbohydrate spares the oxidation of palmitic acid. Arch Biochem Biophys. 1955 Jul;57(1):23–40. doi: 10.1016/0003-9861(55)90173-9. [DOI] [PubMed] [Google Scholar]
- Larner J., Galasko G., Cheng K., DePaoli-Roach A. A., Huang L., Daggy P., Kellogg J. Generation by insulin of a chemical mediator that controls protein phosphorylation and dephosphorylation. Science. 1979 Dec 21;206(4425):1408–1410. doi: 10.1126/science.228395. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Jeanrenaud B., Freychet P. Insulin binding and effects in isolated soleus muscle of lean and obese mice. Am J Physiol. 1978 Apr;234(4):E348–E358. doi: 10.1152/ajpendo.1978.234.4.E348. [DOI] [PubMed] [Google Scholar]
- Letellier T., Malgat M., Mazat J. P. Control of oxidative phosphorylation in rat muscle mitochondria: implications for mitochondrial myopathies. Biochim Biophys Acta. 1993 Feb 8;1141(1):58–64. doi: 10.1016/0005-2728(93)90189-m. [DOI] [PubMed] [Google Scholar]
- Lucas-Heron B., Schmitt N., Ollivier B. Muscular dystrophy: possible role of mitochondrial deficiency in muscle degeneration processes. J Neurol Sci. 1990 Mar;95(3):327–334. doi: 10.1016/0022-510x(90)90078-2. [DOI] [PubMed] [Google Scholar]
- MacLennan P. A., McArdle A., Edwards R. H. Acute effects of phorbol esters on the protein-synthetic rate and carbohydrate metabolism of normal and mdx mouse muscles. Biochem J. 1991 Apr 15;275(Pt 2):477–483. doi: 10.1042/bj2750477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichon-Berthe C., Gautier N., Van Obberghen E., Le Marchand-Brustel Y. Expression of the glucose transporter GLUT4 in the muscular dystrophic mdx mouse. Biochem J. 1993 Apr 1;291(Pt 1):257–261. doi: 10.1042/bj2910257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie M. J., Idström J. P., Mann G. E., Scherstén T., Bylund-Fellenius A. C. A paired-tracer dilution method for characterizing membrane transport in the perfused rat hindlimb. Effects of insulin, feeding and fasting on the kinetics of sugar transport. Biochem J. 1983 Sep 15;214(3):737–743. doi: 10.1042/bj2140737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. M., Atwood H. L., Whittaker J. S., Itakura T., Walker P. M., Mickle D. A., Jeejeebhoy K. N. The effect of fasting and hypocaloric diets on the functional and metabolic characteristics of rat gastrocnemius muscle. Clin Sci (Lond) 1984 Aug;67(2):185–194. doi: 10.1042/cs0670185. [DOI] [PubMed] [Google Scholar]
- Stedman H. H., Sweeney H. L., Shrager J. B., Maguire H. C., Panettieri R. A., Petrof B., Narusawa M., Leferovich J. M., Sladky J. T., Kelly A. M. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991 Aug 8;352(6335):536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Tahiliani A. G., Keene T., Kaplan R. S. Characterization of the inhibitor sensitivity of the coenzyme A transport system in isolated rat heart mitochondria. J Bioenerg Biomembr. 1992 Dec;24(6):635–640. doi: 10.1007/BF00762356. [DOI] [PubMed] [Google Scholar]
- Takagi A., Kojima S., Ida M., Araki M. Increased leakage of calcium ion from the sarcoplasmic reticulum of the mdx mouse. J Neurol Sci. 1992 Jul;110(1-2):160–164. doi: 10.1016/0022-510x(92)90023-e. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Esaki K., Nomura T. Skeletal muscle pathology in X chromosome-linked muscular dystrophy (mdx) mouse. Acta Neuropathol. 1986;69(1-2):91–95. doi: 10.1007/BF00687043. [DOI] [PubMed] [Google Scholar]
- Torres L. F., Duchen L. W. The mutant mdx: inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain. 1987 Apr;110(Pt 2):269–299. doi: 10.1093/brain/110.2.269. [DOI] [PubMed] [Google Scholar]
- Turner P. R., Fong P. Y., Denetclaw W. F., Steinhardt R. A. Increased calcium influx in dystrophic muscle. J Cell Biol. 1991 Dec;115(6):1701–1712. doi: 10.1083/jcb.115.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R., Westwood T., Regen C. M., Steinhardt R. A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988 Oct 20;335(6192):735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- van Breda E., Keizer H. A., Glatz J. F., Geurten P. Use of the intact mouse skeletal-muscle preparation for metabolic studies. Evaluation of the model. Biochem J. 1990 Apr 1;267(1):257–260. doi: 10.1042/bj2670257. [DOI] [PMC free article] [PubMed] [Google Scholar]


