This substudy of the SEQUOIA-HCM (Phase 3 Trial to Evaluate the Efficacy and Safety of Aficamten Compared to Placebo in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy) randomized clinical trial investigates if treatment with aficamten improves exercise response beyond peak oxygen uptake measured by cardiopulmonary exercise testing in obstructive hypertrophic cardiomyopathy.
Key Points
Question
Does aficamten treatment improve exercise response beyond peak oxygen uptake (pVO2) measured by cardiopulmonary exercise testing in obstructive hypertrophic cardiomyopathy (HCM)?
Findings
In the Phase 3 Trial to Evaluate the Efficacy and Safety of Aficamten Compared to Placebo in Adults With Symptomatic Obstructive HCM (SEQUOIA-HCM) randomized clinical trial, 282 patients with symptomatic obstructive HCM received aficamten or placebo for 24 weeks. Aficamten treatment resulted in significant improvement in multiple exercise measures, including a novel composite of exercise performance during peak and submaximal exercise.
Meaning
Aficamten treatment improves several exercise performance measures in patients with obstructive HCM, and improvements in these measures were correlated with other important clinical responses.
Abstract
Importance
Impaired exercise capacity is a cardinal manifestation of obstructive hypertrophic cardiomyopathy (HCM). The Phase 3 Trial to Evaluate the Efficacy and Safety of Aficamten Compared to Placebo in Adults With Symptomatic Obstructive HCM (SEQUOIA-HCM) is a pivotal study characterizing the treatment effect of aficamten, a next-in-class cardiac myosin inhibitor, on a comprehensive set of exercise performance and clinical measures.
Objective
To evaluate the effect of aficamten on exercise performance using cardiopulmonary exercise testing with a novel integrated measure of maximal and submaximal exercise performance and evaluate other exercise measures and clinical correlates.
Design, Setting, and Participants
This was a prespecified analysis from SEQUOIA-HCM, a double-blind, placebo-controlled, randomized clinical trial. Patients were recruited from 101 sites in 14 countries (North America, Europe, Israel, and China). Individuals with symptomatic obstructive HCM with objective exertional intolerance (peak oxygen uptake [pVO2] ≤90% predicted) were included in the analysis. Data were analyzed from January to March 2024.
Interventions
Randomized 1:1 to aficamten (5-20 mg daily) or matching placebo for 24 weeks.
Main Outcomes and Measures
The primary outcome was change from baseline to week 24 in integrated exercise performance, defined as the 2-component z score of pVO2 and ventilatory efficiency throughout exercise (minute ventilation [VE]/carbon dioxide output [VCO2] slope). Response rates for achieving clinically meaningful thresholds for change in pVO2 and correlations with clinical measures of treatment effect (health status, echocardiographic/cardiac biomarkers) were also assessed.
Results
Among 282 randomized patients (mean [SD] age, 59.1 [12.9] years; 115 female [40.8%], 167 male [59.2%]), 263 (93.3%) had core laboratory–validated exercise testing at baseline and week 24. Integrated composite exercise performance improved in the aficamten group (mean [SD] z score, 0.17 [0.51]) from baseline to week 24, whereas the placebo group deteriorated (mean [SD] z score, −0.19 [0.45]), yielding a placebo-corrected improvement of 0.35 (95% CI, 0.25-0.46; P <.001). Further, aficamten treatment demonstrated significant improvements in total workload, circulatory power, exercise duration, heart rate reserve, peak heart rate, ventilatory efficiency, ventilatory power, and anaerobic threshold (all P <.001). In the aficamten group, large improvements (≥3.0 mL/kg per minute) in pVO2 were more common than large reductions (32% and 2%, respectively) compared with placebo (16% and 11%, respectively). Improvements in both components of the primary outcome, pVO2 and VE/VCO2 slope throughout exercise, were significantly correlated with improvements in symptom burden and hemodynamics (all P <.05).
Conclusions and Relevance
This prespecified analysis of the SEQUOIA-HCM randomized clinical trial found that aficamten treatment improved a broad range of exercise performance measures. These findings offer valuable insight into the therapeutic effects of aficamten.
Trial Registration
ClinicalTrials.gov Identifier: NCT05186818
Introduction
A cardinal clinical feature of obstructive hypertrophic cardiomyopathy (HCM) is exercise intolerance. Cardiospecific mechanisms underlying exercise intolerance are thought to arise from the following: (1) dynamic left ventricular outflow tract (LVOT) obstruction, (2) diastolic dysfunction, (3) dynamic mitral regurgitation, and (4) myocardial oxygen supply-demand mismatch resulting in ischemia.1 The evaluation of patients with obstructive HCM during the physiologic stress of exercise with cardiopulmonary exercise testing (CPET) can ascertain the extent to which these cardiospecific limitations impair exercise performance and serve to evaluate potential functional improvements with treatment. CPET also enables an objective and reproducible assessment of all stages of exercise performance. Importantly, peak oxygen uptake (pVO2) and other exercise physiology metrics measured by CPET have already been shown to predict clinical events in obstructive HCM.2
Cardiac myosin inhibitors (CMIs) have been developed as a therapeutic option for patients with obstructive HCM by targeting the underlying etiopathology of the disease. They act by directly reducing excessive actin-myosin crossbridges at the level of the sarcomere and mitigate cardiac hypercontractility. Mavacamten, the first-in-class CMI, has shown efficacy by improving pVO2 and other CPET parameters.3 Aficamten, a next-in-class CMI, was designed with unique physicochemical properties. Aficamten doses can be adjusted to achieve an individualized target dose rapidly (within 6 weeks) as a result of a wide therapeutic window (modest reductions in left ventricular ejection fraction [LVEF] with each dose-level increment) and plasma half-life of 3.4 days. Additionally, minimal drug-drug interactions and rapid reversibility are safety features that allow for precision dosing and relatively infrequent low LVEF excursions less than 50% that can be managed with dose reduction without the need for treatment interruption.1 Aficamten treatment has been demonstrated to relieve obstruction and improve symptoms, cardiac biomarkers, and measures of diastolic function in the phase 2 and open-label extension studies (REDWOOD-HCM4,5 and FOREST-HCM), and was recently shown to improve pVO2, symptoms, health status, and LVOT gradients (LVOT-G) and reduce eligibility for septal reduction therapy in the SEQUOIA-HCM trial.6
In this prespecified analysis of the SEQUOIA-HCM randomized clinical trial, we hypothesized that aficamten would improve a novel measure of integrated exercise performance that combines complementary measures previously independently related to obstructive HCM prognosis and incorporates both submaximal and maximal exercise capacity.7 We further hypothesized that changes in pVO2 would relate to changes in symptoms and cardiac biomarkers, as well as in LVOT-G and other echocardiographic measures.
Methods
Study Oversight
All study participants provided written informed consent before enrollment. The study was conceived, designed, and conducted by an academic steering committee in conjunction with the study sponsor (Supplement 1). All patients provided written informed consent, and the study was carried out in accordance with the provisions of the Declaration of Helsinki and the International Council for Harmonisation Guideline for Good Clinical Practice. An independent data monitoring committee had access to unblinded data for monitoring. Study personnel remained blinded to treatment assignments, dosing, and echocardiogram results through database lock. Results were generated based on a prespecified statistical analysis plan (Supplement 2) that was finalized before database lock. The trial was approved by the regulatory agencies in the participating countries and by the institutional review board or ethics committee at each trial center. This study followed Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Study Design
The rationale for and design of the SEQUOIA-HCM trial have been previously described.1 In brief, this was a phase 3, placebo-controlled, double-blind, multicenter, randomized clinical trial in participants with obstructive HCM. Patients with LVOT-G greater than or equal to 30 mm Hg (resting) and greater than or equal to 50 mm Hg (after Valsalva), New York Heart Association (NYHA) functional class II to III symptoms, baseline pVO2 of 90% or less of predicted, and respiratory exchange ratio (RER) of 1.05 or greater were eligible to participate. Individuals were excluded if they had a history of syncope or sustained ventricular tachyarrhythmia with exercise within 6 months before screening or inability to exercise on a treadmill or cycle. Participants self-identified with the following races and ethnicities: Asian, Black or African American, White, and other, which included American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander; multiracial; or not reported. Race and ethnicity information was included in this study to permit appropriate interpretation of the data and generalize the research.
Randomization
Participants who met screening criteria underwent baseline studies, including history, physical examination, vital signs, echocardiography, CPET, laboratory assessments, and symptom assessments (NYHA functional class, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score [KCCQ-CSS], patient and clinical global impression scales), and they were then randomly assigned 1:1 to receive either aficamten or matching placebo using an interactive Web Response System (Signant Health). Central randomization was stratified by use of β-blockers (yes or no) and CPET exercise modality (treadmill or cycle).
Interventions
Patients receiving aficamten were assigned 5 mg, 10 mg, 15 mg, or 20 mg orally once daily based on site-read echocardiogram-guided titration.1 Aficamten doses were individualized to achieve the lowest effective dose resulting in a Valsalva LVOT-G of less than 30 mm Hg while maintaining an LVEF of 50% or greater over the first 8 weeks of the study starting at 5 mg. Patients, investigators, and the study sponsor were masked to the echocardiogram results and N-terminal pro–brain natriuretic peptide (NT-proBNP) level.
CPET
The CPET core laboratory (Massachusetts General Hospital and Harvard University) certified study sites according to their having demonstrated appropriate and reproducible conduct of qualification CPETs in accordance with the CPET manual of operating procedures, version 1.3 (Supplement 1 and eAppendix in Supplement 3). To achieve within-participant consistency, where possible, CPET was performed on the same equipment, using the same protocol, and was administered by the same staff, at both baseline and week 24. All CPETs were transferred electronically to the core laboratory, where they were interpreted in a blinded fashion. Prespecified criteria for valid CPETs included the absence of equipment malfunction, major CPET protocol deviation, or transient illness/injury unrelated to HCM symptoms that precluded valid CPET completion.
CPET End Points
The primary analysis was change from baseline to week 24 in integrated exercise performance, normalized to a composite of z scores for pVO2 and minute ventilation (VE)/carbon dioxide output (VCO2) slope throughout exercise to capture physiologic responses to maximal and submaximal exercise. The z score was derived by reversing the directionality of the VE/VCO2 slope such that increases in both z score components indicate benefit, and equal weights were used for each component. For each patient, the composite z score was defined as (z1 + z2) / 2, where z1 is the patient’s pVO2 change minus the trial-level mean pVO2 change, divided by the trial-level SD of pVO2 change, and z2 was defined similarly for VE/VCO2 slope, then multiplied by −1, such that positive values of both z1 and z2 represent changes from baseline that are better than average.
Secondary end points included assessments for changes from baseline to week 24 in CPET-derived measures during 2 phases of exercise performance: (1) peak exercise (pVO2, peak workload, peak metabolic equivalents, peak circulatory power [VO2 × systolic blood pressure], exercise duration, peak RER, heart rate reserve, peak heart rate, oxygen pulse at peak exercise, proportionate pulse pressure) and (2) during submaximal exercise (preanaerobic threshold VE/VCO2 slope from the onset of exercise up until and including the first ventilatory anaerobic threshold, derived from the V-slope method, VE/VCO2 slope throughout exercise [from rest to peak exercise], ventilatory power, ventilatory anaerobic threshold, and aerobic efficiency).
Sample Size Calculation
Sample size calculations for the SEQUOIA-HCM trial assumed a between-group difference in change from baseline in pVO2 of 1.5 mL/kg per minute for aficamten vs placebo, a common SD of 3.5 mL/kg per minute, and 10% missing data for the primary end point. A sample size of 270 patients at a randomization ratio of 1:1 (approximately 135 randomized to aficamten and approximately 135 to placebo) was estimated to provide greater than or equal to 90% power to detect a difference of 1.5 mL/kg per minute in pVO2 change from baseline to week 24 with a 2-sided type I error of 0.05.
Statistical Analysis
The full statistical analysis plan for CPET analyses is provided in Supplement 2. Unless otherwise specified, efficacy analyses were performed on the full analysis set, which includes all randomized patients who received 1 or more doses of study drug and had 1 or more postbaseline efficacy assessments. The primary analysis was performed using an analysis of covariance model that included terms of treatment, randomization stratification factors (β-blocker use status and CPET modality), baseline value of the outcome, and baseline body weight as covariates. Missing data were imputed 100 times according to the missing at random (MAR) paradigm. Least squares mean (LSM) treatment difference and SE were combined using Rubin rules to produce an LSM estimate of the treatment difference, its 95% CI, and P value for the test of null hypothesis of no treatment effect. Secondary exercise testing efficacy end points were analyzed using the same methodology as used for the primary end points.
Additional analyses included the following: (1) a sensitivity analysis using an alternate z score with the VE/VCO2 slope preanaerobic threshold (rather than throughout exercise), (2) a responder analysis for the proportional achievement of clinically meaningful thresholds for shifts in pVO2 (small [0 to <1.5 mL/kg per minute], moderate [≥1.5 to <3 mL/kg per minute], or large [≥3.0 mL/kg per minute]) by treatment group and correlations between change in pVO2 and other measures of treatment effect (symptoms, hemodynamics, biomarkers), and (3) an evaluation of how change in the z score and its components relates to change in other SEQUOIA-HCM CPET, symptom-based, echocardiographic, and biomarker end points by correlation and multivariate regression analysis.
Baseline data are presented as number (%), mean (SD), and median (IQR). Two-sided P values <.05 were considered statistically significant. Missing CPET end points at week 24, regardless of type of intercurrent events, were imputed using multiple imputation methodology under the MAR assumption for the primary analysis of the primary estimate because the proportion of patients with week 24 CPET missing was expected to be very low.
Statistical analyses were performed from January to March 2024 by the Brigham and Women’s Hospital Clinical Trials Outcomes Center.
Results
Patient Population
Between February 1, 2022, and May 15, 2023, 282 eligible patients were randomized to aficamten or placebo at 101 sites in 14 countries. Baseline characteristics have been previously published and are shown in Table 1.1,6 The mean (SD) age of participants was 59.1 (12.9) years, 115 (40.8%) were female, and 167 (59.2%) were male. Patients self-identified with the following races and ethnicities: 54 Asian (19.1%), 3 Black or African American (1.1%), 223 White (79.1%), and 2 other (0.7%). Background HCM therapy included 173 participants (61.3%) receiving β-blockers, 81 (28.7%) receiving nondihydropyridine calcium channel blockers, 36 (12.8%) receiving disopyramide, and 41 (14.5%) not taking any HCM medication. Exercise capacity was reduced as evidenced by the baseline mean (SD) pVO2 of 18.5 (4.5) mL/kg per minute, representing a mean (SD) of 56.9% (11.8%) of age- and sex-predicted pVO2,8 and a reduced VE/VCO2 slope throughout exercise mean (SD) of 33.0 (6.1). Other key baseline CPET parameters included workload (mean [SD], 122.5 [40.1] W) and metabolic equivalents (mean [SD], 5.3 [1.3] mL/kg per minute), both of which were impaired.
Table 1. Baseline Characteristicsa.
| Characteristic | Aficamten (n = 142) | Placebo (n = 140) |
|---|---|---|
| Age, y | 59.2 (12.6) | 59.0 (13.3) |
| Sex, No. (%) | ||
| Female | 56 (39.4) | 59 (42.1) |
| Male | 86 (60.6) | 81 (57.9) |
| Race, No. (%) | ||
| Asian | 29 (20.4) | 25 (17.9) |
| Black or African American | 3 (2.1) | 0 |
| White | 108 (76.1) | 115 (82.1) |
| Otherb | 2 (1.4) | 0 |
| Geographic region, No. (%) | ||
| North America | 49 (34.5) | 45 (32.1) |
| China | 24 (16.9) | 22 (15.7) |
| Europe and Israel | 69 (48.6) | 73 (52.1) |
| Medical history, No. (%) | ||
| Hypertension | 75 (52.8) | 70 (50.0) |
| Family history or known gene variant | 47 (33.1) | 44 (31.4) |
| Family history of HCM | 41 (28.9) | 34 (24.3) |
| Pathogenic sarcomere variant | 24 (16.9) | 25 (17.9) |
| Paroxysmal atrial fibrillation | 21 (14.8) | 20 (14.3) |
| Coronary artery disease | 19 (13.4) | 16 (11.4) |
| Diabetes | 14 (9.9) | 9 (6.4) |
| Permanent atrial fibrillation | 2 (1.4) | 1 (0.7) |
| Background HCM therapy, No. (%) | ||
| β-Blocker | 86 (60.6) | 87 (62.1) |
| Calcium channel blocker | 45 (31.7) | 36 (25.7) |
| Disopyramide | 16 (11.3) | 20 (14.3) |
| None | 19 (13.4) | 22 (15.7) |
| Symptoms | ||
| KCCQ-CSS | 76 (18) | 74 (18) |
| NYHA functional class, No. (%) | ||
| II | 108 (76.1) | 106 (75.7) |
| III | 34 (23.9) | 33 (23.6) |
| IV | 0 | 1 (0.7) |
| Cardiac biomarkers | ||
| Median NT-proBNP (IQR), pg/mL | 818 (377-1630) | 692 (335-1795) |
| Median hs-cTnl (IQR), ng/L | 12.9 (7.6-33.6) | 11.5 (7.7-25.0) |
| Echocardiographic parameters | ||
| Valsalva LVOT-G, mm Hg | 83 (32) | 83 (33) |
| Resting LVOT-G, mm Hg | 55 (27) | 55 (32) |
| LVEF, % | 75 (5.5) | 75 (6.3) |
| LAVI, mL/m2 | 40.1 (12.7) | 40.9 (15.1) |
| Maximal wall thickness, cm | 2.1 (0.3) | 2.1 (0.3) |
| Cardiopulmonary exercise test parameters | ||
| Integrated 2-component exercise performance metric | −0.01 (0.82) | 0.02 (0.75) |
| pVO2, mL/kg/min | 18.4 (4.5) | 18.6 (4.6) |
| Workload, W | 120 (40) | 126 (43) |
| Metabolic equivalents, METS | 5.3 (1.3) | 5.3 (1.3) |
| Ventilatory efficiency throughout exercise (VE/VCO2 slope) | 33.2 (6.4) | 32.9 (6.0) |
Abbreviations: HCM, hypertrophic cardiomyopathy; hs-cTnI, high-sensitivity cardiac troponin I; KCCQ-CSS, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVOT-G, left ventricular outflow tract gradient; NT-proBNP, N-terminal pro–brain natriuretic peptide; NYHA, New York Heart Association; pVO2, peak oxygen uptake; VCO2, carbon dioxide output; VE, minute ventilation.
SI conversion factors: To convert hs-cTnI to micrograms per liter, divide by 1000 and multiply by 1; NT-proBNP to nanograms per liter, multiply by 1.
Percentages may not total 100 because of rounding. Value in parenthesis represents the SD unless otherwise specified.
Race was denoted by the patient as part of baseline characteristics. Other ethnic groups included American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander; multiracial; and not reported.
Patient Disposition
At least 1 dose of study medication was received by all randomized patients (eFigure in Supplement 3). At week 24, the number (%) of patients taking each aficamten dose was 5 (3.6), 21 (15.3), 48 (35), and 63 (46) for 5 mg, 10 mg, 15 mg, and 20 mg, respectively. Of those randomized, 263 patients (93.3%) completed both baseline and week 24 CPETs that were deemed physiologically interpretable and valid by the core laboratory. Of the 19 patients (7%; 9 aficamten and 10 placebo) who did not have a core laboratory–interpretable week 24 CPET available for analysis, 6 (2.1%; 3 aficamten and 3 placebo) terminated early from the study before week 24 CPET, and 13 (4.6%; 6 aficamten and 7 placebo) were determined by the core laboratory to have an invalid week 24 CPET (3 did not follow the CPET manual of operations, and 10 were technical failures).
Primary and Secondary End Points
The change from baseline to week 24 in the integrated composite for exercise performance demonstrated improvement in the aficamten group (mean [SD] z score, 0.17 [0.51]) compared with a deterioration in the placebo group (mean [SD] z score, −0.19 [0.45]), yielding a significant placebo-corrected increase (mean [SD] z score, 0.35; 95% CI, 0.25-0.46; P <.001) (Figure 1 and Table 2). Aficamten improved peak exercise performance as measured by the total workload (LSM difference, 12 W; 95% CI, 6-18 W; P <.001), circulatory power (LSM difference, 586 mm Hg × mL/kg/min; 95% CI, 379-793 mm Hg × mL/kg/min; P <.001), exercise duration (LSM difference, 1.0 minute; 95% CI, 0.5-1.4 minutes; P <.001), heart rate reserve (LSM difference, 6 beats per minute; 95% CI, 3-9 beats per minute; P <.001), and peak heart rate (LSM difference, 9 beats per minute; 95% CI, 6-12 beats per minute; P <.001) (Table 2). Aficamten also improved submaximal exercise performance as measured by ventilatory efficiency (both preanaerobic threshold: LSM difference, −1.5; 95% CI, −2.5 to −0.6; P =.002 and throughout exercise: LSM difference, −2.3; 95% CI, −3.2 to −1.4; P <.001), increased ventilatory power (LSM difference, 0.9 mm Hg; 95% CI, 0.6-1.1 mm Hg; P <.001), and increased ventilatory anaerobic threshold (LSM difference, 59 mL per minute; 95% CI, 33-85 mL per minute; P <.001) (Table 2). As anticipated, aficamten treatment was not associated with improvements in oxygen pulse at peak exercise, proportionate pulse pressure at peak or rest, or aerobic efficiency, and there were no between-group differences in peak RER.
Figure 1. Baseline and Week 24 Values and Changes in Integrated Exercise Performance and Its Component Variables.
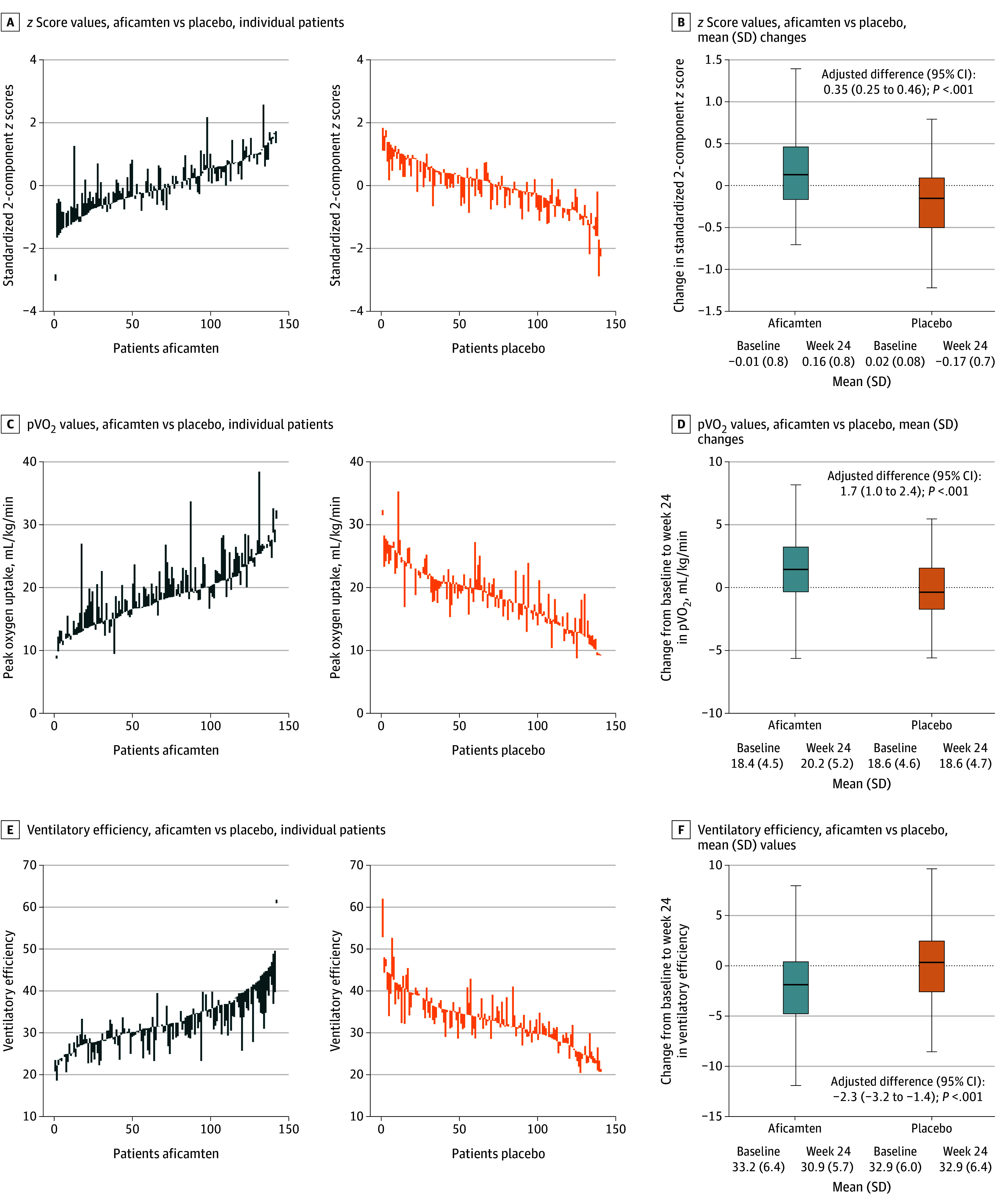
Baseline and week 24 values, connected by vertical lines, are shown for individual patients receiving aficamten (left) and placebo (right) for z score values (A), peak oxygen uptake (pVO2) values (C), and ventilatory efficiency values (E). Integrated exercise performance and its component variables (standardized 2-component z score of pVO2 and VE as measured by minute ventilation [VE]/carbon dioxide output [VCO2] slope throughout all of exercise). The z score was derived by reversing the directionality of VE/VCO2 slope values such that increases in both z score components indicate benefit; equal weights were used for each component. Changes in values (median and IQR) from baseline to week 24 are shown in panels B, D, and F. Box edges indicate the IQRs; the horizontal lines in between the edges indicate the median values. Whiskers extend to the upper and lower adjacent values, and dots represent outside values.
Table 2. Cardiopulmonary Exercise Testing (CPET) Parameters by Treatment Assignment.
| CPET variable | Aficamten, mean (SD) (n = 133) | Placebo, mean (SD) (n = 130) | Adjusted difference (95% CI)b | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 24 | Absolute difference (SD)a | Baseline | Week 24 | Absolute difference (SD)a | |||
| Integrated 2-component z score metricc | −0.01 (0.82) | 0.16 (0.76) | 0.17 (0.51) | 0.02 (0.75) | −0.17 (0.74) | −0.19 (0.45) | 0.35 (0.25 to 0.46) | <.001 |
| pVO2, mL/kg/min | 18.4 (4.5) | 20.2 (5.2) | 1.8 (3.1) | 18.6 (4.6) | 18.6 (4.7) | 0 (2.7) | 1.7 (1.0 to 2.4) | <.001 |
| Peak workload, W | 120 (40) | 134 (50) | 14 (27) | 126 (43) | 127 (44) | 1 (21) | 12 (6 to 18) | <.001 |
| Peak METS, metabolic equivalents | 5.3 (1.3) | 5.8 (1.5) | 0.51 (0.89) | 5.3 (1.3) | 5.3 (1.3) | 0 (0.78) | 0.49 (0.29 to 0.69) | <.001 |
| Peak circulatory power, mm Hg × mL/kg/min | 3013 (924) | 3550 (1140) | 537 (995) | 3160 (1136) | 3074 (1152) | −86 (731) | 586 (379 to 793) | <.001 |
| Exercise duration, min | 11.2 (3.0) | 12.4 (3.9) | 1.2 (2.1) | 11.5 (3.0) | 11.7 (3.2) | 0.1 (1.5) | 1.0 (0.5 to 1.4) | <.001 |
| Peak RER | 1.19 (0.10) | 1.20 (0.11) | 0.01 (0.10) | 1.18 (0.09) | 1.19 (0.10) | 0.01 (0.10) | 0.00 (−0.02 to 0.02) | .84 |
| Heart rate reserve, beats/min | 59 (18) | 66 (22) | 7 (15) | 57 (19) | 59 (20) | 1 (10) | 6 (3 to 9) | <.001 |
| Peak heart rate, beats/min | 128 (20) | 135 (23) | 8 (14) | 128 (23) | 127 (23) | −1 (12) | 9 (6 to 12) | <.001 |
| Oxygen pulse at peak exercise, mL/beat | 0.14 (0.03) | 0.15 (0.03) | 0 (0.02) | 0.15 (0.03) | 0.15 (0.03) | 0 (0.02) | 0.003 (−0.002 to 0.008) | .21 |
| Proportionate pulse pressure, peak exercise | 0.49 (0.11) | 0.51 (0.12) | 0.02 (0.14) | 0.50 (0.10) | 0.50 (0.11) | 0 (0.10) | 0.01 (−0.01 to 0.04) | .29 |
| Proportionate pulse pressure, rest | 0.39 (0.10) | 0.37 (0.09) | −0.02 (0.10) | 0.39 (0.10) | 0.38 (0.10) | −0.01 (0.11) | −0.01 (−0.03 to 0.01) | .33 |
| Ventilatory efficiency pre-VAT, VE/VCO2 slope | 29.2 (5.4) | 27.4 (4.4) | −1.9 (4.7) | 29.1 (4.7) | 28.8 (5.6) | −0.3 (4.2) | −1.5 (−2.5 to −0.6) | .002 |
| Ventilatory efficiency throughout exercise, VE/VCO2 slope | 33.2 (6.4) | 30.9 (5.7) | −2.2 (4.0) | 32.9 (6.0) | 32.9 (6.4) | 0.1 (3.7) | −2.3 (−3.2 to −1.4) | <.001 |
| Ventilatory power, mm Hg | 5.1 (1.5) | 5.9 (1.6) | 0.8 (1.3) | 5.2 (1.6) | 5.1 (1.5) | −0.1 (1.0) | 0.9 (0.6 to 1.1) | <.001 |
| Ventilatory anaerobic threshold, mL/min | 898 (266) | 958 (276) | 60 (107) | 931 (261) | 927 (257) | −3 (108) | 59 (33 to 85) | <.001 |
| Aerobic efficiency, mL/min/W | 8.3 (2.5) | 8.6 (2.5) | 0.3 (1.8) | 8.2 (2.3) | 8.2 (2.4) | 0.1 (1.7) | 0.2 (−0.2 to 0.6) | .22 |
Abbreviations: LSM, least squares mean; METS, metabolic equivalents; pVO2, peak oxygen uptake; RER, respiratory exchange ratio; VAT, ventilatory anaerobic threshold; VCO2, carbon dioxide output; VE, minute ventilation.
The absolute difference corresponds to the change from baseline to week 24.
The adjusted difference corresponds to the LSM treatment difference.
Integrated exercise performance was defined as the 2-component z score of pVO2 and ventilatory efficiency throughout exercise (VE/VCO2 slope). The z score was derived by reversing the directionality of VE/VCO2 slope values such that increases in both z score components indicate benefit; equal weights were used for each component.
In a sensitivity analysis using an alternate z score using the VE/VCO2 slope preanaerobic threshold (rather than throughout exercise), the change from baseline to week 24 in this alternate z score also showed improvement in the aficamten group (mean [SD] z score, 0.15 [0.63]) compared with a deterioration in the placebo group (mean [SD] z score, −0.18 [0.57]), resulting in a significant placebo-corrected increased (mean [SD] z score, 0.32; 95% CI, 0.18-0.45; P < .001).
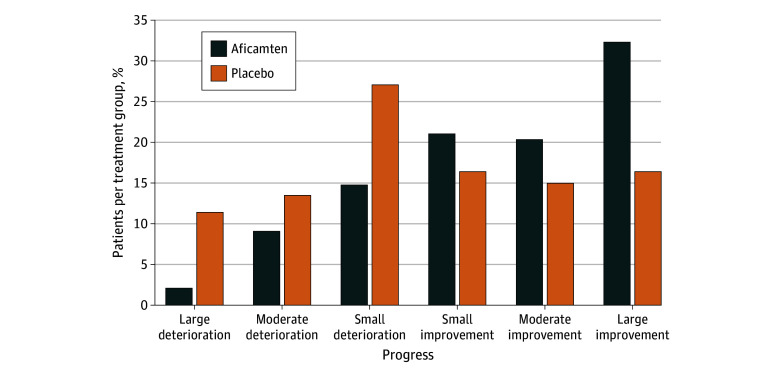
Responder Analysis
Large pVO2 improvements (≥3.0 mL/kg per minute) were more frequent with aficamten treatment compared with placebo (32% vs 16%, respectively), and large pVO2 deteriorations (≤−3.0 mL/kg per minute) were less frequent with aficamten treatment vs placebo (2% vs 11%, respectively) (Figure 2). The odds ratios (ORs) for achieving any improvement (OR, 3.09; 95% CI, 1.88-5.09), moderate to large improvements greater than or equal to 1.5 mL/kg per minute (OR, 2.44; 95% CI, 1.50-3.96), and large improvements greater than or equal to 3.0 mL/kg per minute (OR, 2.44; 95% CI, 1.38-4.29) all favor aficamten treatment and correspond with a number needed to treat of 3.8, 4.7, and 6.3 patients, respectively (eTable 1 in Supplement 3).
Figure 2. Responder Analyses of Categorical Change in Peak Oxygen Uptake (pVO2) With Aficamten vs Placebo at 24 Weeks.
Data represented as proportion of patients experiencing small (0 to <1.5), moderate (≥1.5 to <3), or large (≥3.0) deteriorations or improvements in pVO2 (in milliliters per kilogram per minute) per treatment group.
Clinical Correlations
Cubic spline graphs and univariate correlation analyses revealed significant associations between improvements in pVO2 and improvements in KCCQ-CSS score, NYHA functional class, septal E/e′ (peak E-wave velocity divided by peak e′ velocity, an estimate of left ventricular end-diastolic pressure), resting and Valsalva LVOT-G, NT-proBNP level, and high-sensitivity cardiac troponin I (hs-cTnI) level (Figure 3 and eTable 2 in Supplement 3). Sequential regression multivariate analyses revealed that changes in NT-proBNP level accounted for the greatest amount of variance in change in pVO2, whereas changes in E/e′ and KCCQ-CSS score also significantly added to the observed variance (eTable 3 in Supplement 3). In a univariate analysis, improvements in VE/VCO2 slope throughout exercise were associated with changes in many of the same clinical measures as change in pVO2. However, change in VE/VCO2 slope demonstrated higher correlation with changes in LVOT-G (rest and Valsalva) and left atrial volume index (LAVI) and lower correlation with changes in E/e′ and hs-cTnI level. In the multivariate analysis, changes in NT-proBNP level, LAVI, and mitral regurgitation significantly explained variance in VE/VCO2 slope (eTable 3 in Supplement 3). In addition to distinct correlates for pVO2 and VE/VCO2 slope, only a modest relationship between the change in pVO2 and the change in VE/VCO2 slope (correlation coefficient = −0.23) was observed, highlighting the orthogonal nature of the 2 variables selected for our 2-component z score.
Figure 3. Cubic Spline Graphs: Correlation Between Change in Peak Oxygen Uptake (pVO2) and Clinical Metrics.
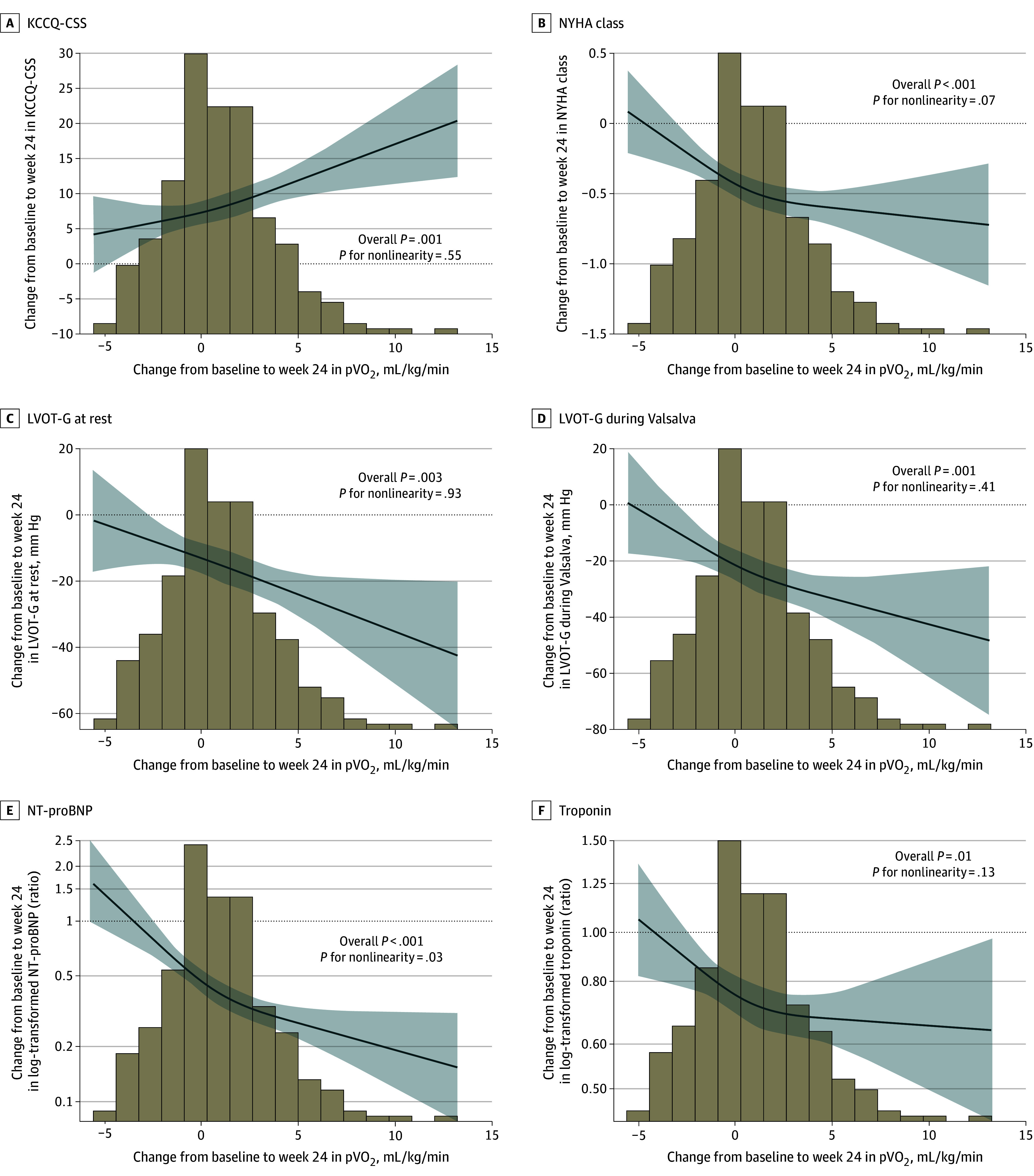
The panels display the change in pVO2 from baseline to 24 weeks for the following: Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score (KCCQ-CSS) (A), New York Heart Association (NYHA) functional class (B), resting left ventricular outflow tract gradient (LVOT-G) (C), Valsalva LVOT-G (D), N-terminal pro–brain natriuretic peptide (NT-proBNP) (E), and high-sensitivity cardiac troponin I (hs-cTnI) (F). Graphs display linear regression with cubic terms. Analyses adjusted for baseline values of both parameters. Solid lines show the association correlate, and shaded areas show the 95% CIs. Histograms show the distribution of change in pVO2.
Discussion
We report a comprehensive prespecified assessment of CPET, in the context of the largest (to our knowledge) placebo-controlled randomized clinical trial in patients with obstructive HCM to date. Given that pVO2 is a metric of maximal exercise capacity that is not necessarily relevant to daily physical activity, we developed a novel metric that integrates both maximal and submaximal exercise performance by CPET (z score of change in pVO2 and VE/VCO2 throughout exercise). We then evaluated whether aficamten treatment would improve this more global metric of exercise performance in this prespecified analysis from the SEQUOIA-HCM trial. This integrated exercise measure was significantly improved with aficamten vs placebo in patients with obstructive HCM, indicating that exercise benefits accrue in a combined fashion during both maximal and submaximal activity reflective of all stages of exertion in everyday life. Further, there is only a modest relationship between changes in pVO2 and VE/VCO2 throughout exercise (correlation coefficient <0.25) but a stronger correlation of both variables with changes in NT-proBNP level. Thus, the measures of pVO2 and VE/VCO2 throughout exercise embedded in the 2-component z score are complementary.
The impact of therapies on morbidity and mortality is difficult to assess in patients with HCM given the low event rate. Thus, defining clinically important outcome measures that serve as effective end points for clinical trials and also meaningfully reflect the treatment’s impact on patient symptoms and function is critical. One approach is to categorize improvements into clinically meaningful increments and evaluate the number needed to treat to achieve those outcomes. In our categorical threshold analyses, patients taking aficamten were twice as likely to achieve a large pVO2 increase (≥3.0 mL/kg per minute) and greater than 5 times less likely to experience worsening than those receiving placebo (Figure 2 and eTable 1 in Supplement 3), with fewer than 4 patients requiring treatment with aficamten to achieve exercise capacity improvement vs placebo. These findings are particularly noteworthy given that improvements in pVO2 of the magnitude and consistency seen in the SEQUOIA-HCM trial are rarely achieved with existing medical therapies for individuals with either HCM or heart failure.1,9
Correlations between observed changes in pVO2 and VE/VCO2 throughout exercise in the SEQUOIA-HCM trial and changes in measures of cardiac structure and function begin to provide mechanistic insights into predominant contributions to exercise limitation in obstructive HCM and their modifiability with aficamten. The observed correlation between changes in LVOT-G with and without Valsalva was expected, although notably higher correlation coefficients were observed between changes in pVO2 and changes in NT-proBNP level as well as E/e′ values. In fact, NT-proBNP level accounted for the highest relative variance explained in change in pVO2 and change in VE/VCO2 throughout exercise in multivariate modeling, suggesting that the drivers of NT-proBNP–level reduction extend beyond simply reducing LVOT-G. Further, these findings support current efforts to understand whether CMI, which has been observed to substantially lower NT-proBNP level in phase 2 studies in nonobstructive HCM, may presage its role in improving functional capacity in nonobstructive HCM also.10 This approach is currently under investigation in the ongoing Phase 3 Trial to Evaluate the Efficacy and Safety of Aficamten Compared to Placebo in Adults With Symptomatic Nonobstructive HCM (ACACIA-HCM), which will leverage the integrated exercise performance end point first described in this study.
CPET metrics that are cardiocentric (VE/VCO2 slope, circulatory power, heart rate reserve, peak heart rate), as well as those that are highly integrated with whole-body physiology (ie, pVO2) (Table 2 and eTable 2 in Supplement 3), improved in the setting of aficamten treatment. We did not observe significant improvement in peak oxygen pulse, the product of stroke volume and peripheral oxygen extraction, likely because peripheral oxygen extraction is far more dynamic than stroke volume in accounting for peak oxygen pulse, and aficamten was not expected to influence peripheral oxygen extraction, which falls in a reciprocal manner in response to increases in cardiac output at peak exercise. Proportional pulse pressure also did not change with aficamten, which may reflect the multitude of inputs to blood pressure and challenges with measurement of diastolic blood pressure at peak exercise (which proportionate pulse pressure largely depends on).
By expanding the range of CPET parameters evaluated, we gain a more holistic understanding of the physiologic responses to exercise in patients with obstructive HCM. Within this context, pVO2 is primarily influenced by cardiac output, whereby failure to increase cardiac output relative to increased peripheral oxygen extraction has been observed in the setting of obstructive HCM during exercise.11,12,13 In contrast, for other conditions (eg, heart failure with preserved EF), the determinants of pVO2 frequently extend beyond cardiac output to include factors such as peripheral abnormalities (ie, impaired peripheral oxygen extraction by muscles), making improvement in exercise capacity harder to achieve with cardiospecific pharmacologic interventions.14,15 Finally, the observation that aficamten improved both heart rate reserve and peak heart rate independent of β-blockade is particularly interesting. It suggests a mechanism that may account for the absence of interaction with β-blocker use for the primary end point of change in pVO2 in the SEQUOIA-HCM trial, and it stands in contrast to the β-blocker interaction observed with mavacamten in the Clinical Study to Evaluate Mavacamten (MYK-461) in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy (EXPLORER-HCM).16
We further showed that CPET is a reproducible tool for use in clinical trials, performed at over 100 sites in 14 countries. These data also strengthen justification for CPET as a clinical tool to explain exertional intolerance in patients with obstructive HCM, assess response to treatment, and provide data for potential prognostic extrapolation from other observational studies.7,17 CPET enables simultaneous assessment of multiple pathophysiologic abnormalities characteristic of HCM, including early anaerobic threshold, ventilatory inefficiency, chronotropic incompetence, and reduced blood pressure augmentation. Collectively, improvement in these findings supports the effect that aficamten has on both submaximal and maximal exercise performance, spanning the array of experiences and activities that are important to patients with symptomatic obstructive HCM.
Limitations
This study has limitations. First, patients with prior septal reduction therapy and those with nonobstructive HCM were excluded from the study, as were those unable to exercise, thereby limiting the generalizability of our study population to all patients with HCM. Second, CPET assessments were conducted at only 2 time points (baseline and week 24), and the study duration of 24 weeks may not inform what happens to exercise response patterns relative to placebo over shorter or longer exposure periods. Third, 13 patients (4.6%) were not included in this analysis due to CPET technical issues or deviations from the CPET manual of operations.
Conclusions
Our comprehensive prespecified analysis of CPET metrics in the SEQUOIA-HCM randomized clinical trial, including a novel integrated exercise performance metric, showed improved exercise response patterns in patients with obstructive HCM who were treated with aficamten. Improvements in exercise performance correlated with improvements in cardiac structure and function extending beyond reduction in LVOT-G and also with change in symptom burden. These findings offer valuable mechanistic and clinical insights into the beneficial therapeutic effects of aficamten in patients with obstructive HCM.
CPET Manual of Operating Procedures.
Statistical Analysis Plan.
eAppendix. CPET Operating Procedures
eFigure. CONSORT Diagram: Recruitment, Randomization, and Follow-Up in the SEQUOIA-HCM Trial
eTable 1. Responder Analyses of Categorical Change in pVO2 With Aficamten vs Placebo at 24 Weeks
eTable 2. Univariate Correlations Between Change in Components of Integrated Exercise Response z Score and Changes in Other Measures
eTable 3. Multivariate Regression Model to Evaluate Independent Degree of Variance Explained in Change in pVO2 and VE/VCO2 Slope From Baseline to Week 24 by Clinical Variables
Nonauthor Collaborators. SEQUOIA-HCM Investigators.
Data Sharing Statement.
References
- 1.Coats CJ, Maron MS, Abraham TP, et al. ; SEQUOIA-HCM Investigators . Exercise capacity in patients with obstructive hypertrophic cardiomyopathy: SEQUOIA-HCM baseline characteristics and study design. JACC Heart Fail. 2024;12(1):199-215. doi: 10.1016/j.jchf.2023.10.004 [DOI] [PubMed] [Google Scholar]
- 2.Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy—executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142(25):e533-e557. doi: 10.1161/CIR.0000000000000938 [DOI] [PubMed] [Google Scholar]
- 3.Wheeler MT, Olivotto I, Elliott PM, et al. Effects of mavacamten on measures of cardiopulmonary exercise testing beyond peak oxygen consumption: a secondary analysis of the EXPLORER-HCM randomized trial. JAMA Cardiol. 2023;8(3):240-247. doi: 10.1001/jamacardio.2022.5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maron MS, Masri A, Choudhury L, et al. ; REDWOOD-HCM Steering Committee and Investigators . Phase 2 study of aficamten in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2023;81(1):34-45. doi: 10.1016/j.jacc.2022.10.020 [DOI] [PubMed] [Google Scholar]
- 5.Owens AT, Masri A, Abraham TP, et al. ; REDWOOD-HCM INVESTIGATORS . Aficamten for drug-refractory severe obstructive hypertrophic cardiomyopathy in patients receiving disopyramide: REDWOOD-HCM cohort 3. J Card Fail. 2023;29(11):1576-1582. doi: 10.1016/j.cardfail.2023.07.003 [DOI] [PubMed] [Google Scholar]
- 6.Maron MS, Masri A, Nassif ME, et al. ; SEQUOIA-HCM Investigators . Aficamten for symptomatic obstructive hypertrophic cardiomyopathy. N Engl J Med. 2024;390(20):1849-1861. doi: 10.1056/NEJMoa2401424 [DOI] [PubMed] [Google Scholar]
- 7.Coats CJ, Rantell K, Bartnik A, et al. Cardiopulmonary exercise testing and prognosis in hypertrophic cardiomyopathy. Circ Heart Fail. 2015;8(6):1022-1031. doi: 10.1161/CIRCHEARTFAILURE.114.002248 [DOI] [PubMed] [Google Scholar]
- 8.Fletcher GF, Balady G, Froelicher VF, Hartley LH, Haskell WL, Pollock ML; Writing Group . Exercise standards: a statement for health care professionals from the American Heart Association. Circulation. 1995;91(2):580-615. doi: 10.1161/01.CIR.91.2.580 [DOI] [PubMed] [Google Scholar]
- 9.Lewis GD, Docherty KF, Voors AA, et al. Developments in exercise capacity assessment in heart failure clinical trials and the rationale for the design of METEORIC-HF. Circ Heart Fail. 2022;15(5):e008970. doi: 10.1161/CIRCHEARTFAILURE.121.008970 [DOI] [PubMed] [Google Scholar]
- 10.Masri A, Sherrid MV, Abraham TP, et al. ; REDWOOD-HCM Investigators . Efficacy and safety of aficamten in symptomatic nonobstructive hypertrophic cardiomyopathy: results from the REDWOOD-HCM trial, cohort 4. J Card Fail. Published online March 15, 2024. doi: 10.1016/j.cardfail.2024.02.020 [DOI] [PubMed] [Google Scholar]
- 11.Chomsky DB, Lang CC, Rayos GH, et al. Hemodynamic exercise testing—a valuable tool in the selection of cardiac transplantation candidates. Circulation. 1996;94(12):3176-3183. doi: 10.1161/01.CIR.94.12.3176 [DOI] [PubMed] [Google Scholar]
- 12.Critoph CH, Patel V, Mist B, Elliott PM. Cardiac output response and peripheral oxygen extraction during exercise among symptomatic hypertrophic cardiomyopathy patients with and without left ventricular outflow tract obstruction. Heart. 2014;100(8):639-646. doi: 10.1136/heartjnl-2013-304914 [DOI] [PubMed] [Google Scholar]
- 13.MacNamara JP, Dias KA, Hearon CM Jr, et al. Limits to submaximal and maximal exercise in patients with hypertrophic cardiomyopathy. J Appl Physiol (1985). 2022;133(4):787-797. doi: 10.1152/japplphysiol.00566.2021 [DOI] [PubMed] [Google Scholar]
- 14.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58(3):265-274. doi: 10.1016/j.jacc.2011.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8(2):286-294. doi: 10.1161/CIRCHEARTFAILURE.114.001825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler MT, Jacoby D, Elliott PM, et al. Effect of β-blocker therapy on the response to mavacamten in patients with symptomatic obstructive hypertrophic cardiomyopathy. Eur J Heart Fail. 2023;25(2):260-270. doi: 10.1002/ejhf.2737 [DOI] [PubMed] [Google Scholar]
- 17.Masri A, Pierson LM, Smedira NG, et al. Predictors of long-term outcomes in patients with hypertrophic cardiomyopathy undergoing cardiopulmonary stress testing and echocardiography. Am Heart J. 2015;169(5):684-692.e1. doi: 10.1016/j.ahj.2015.02.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CPET Manual of Operating Procedures.
Statistical Analysis Plan.
eAppendix. CPET Operating Procedures
eFigure. CONSORT Diagram: Recruitment, Randomization, and Follow-Up in the SEQUOIA-HCM Trial
eTable 1. Responder Analyses of Categorical Change in pVO2 With Aficamten vs Placebo at 24 Weeks
eTable 2. Univariate Correlations Between Change in Components of Integrated Exercise Response z Score and Changes in Other Measures
eTable 3. Multivariate Regression Model to Evaluate Independent Degree of Variance Explained in Change in pVO2 and VE/VCO2 Slope From Baseline to Week 24 by Clinical Variables
Nonauthor Collaborators. SEQUOIA-HCM Investigators.
Data Sharing Statement.