Abstract
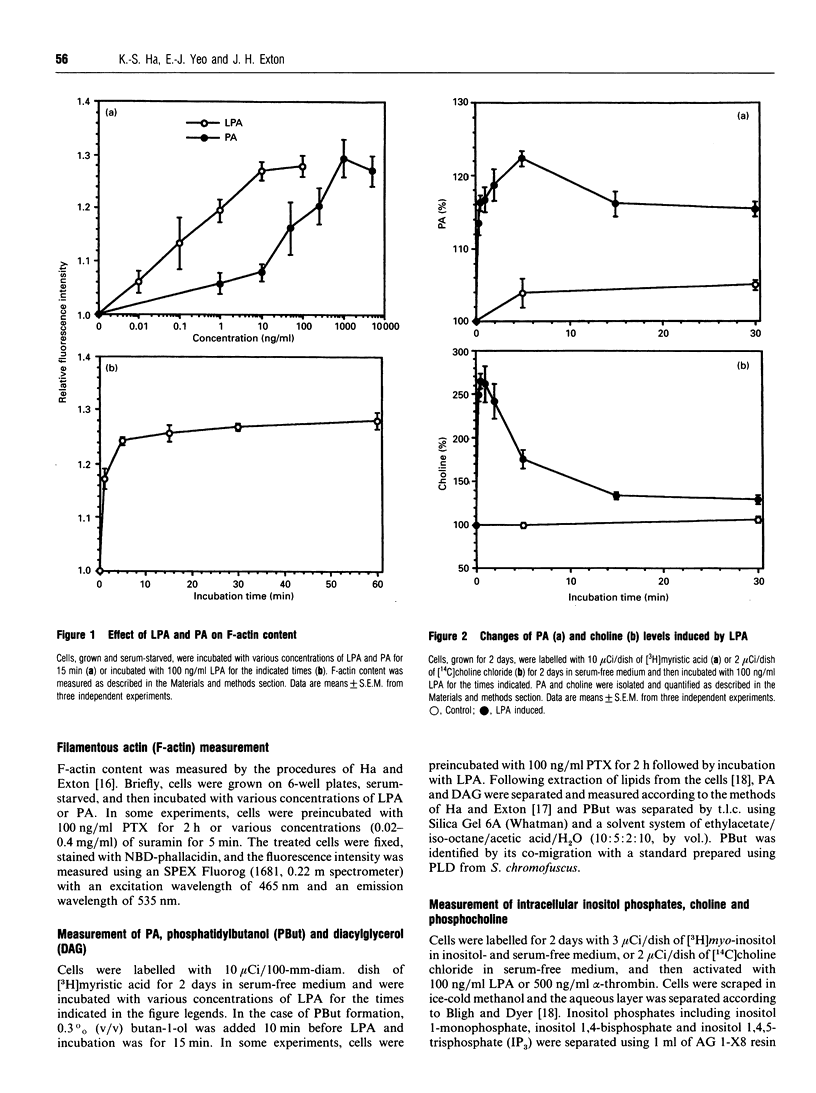
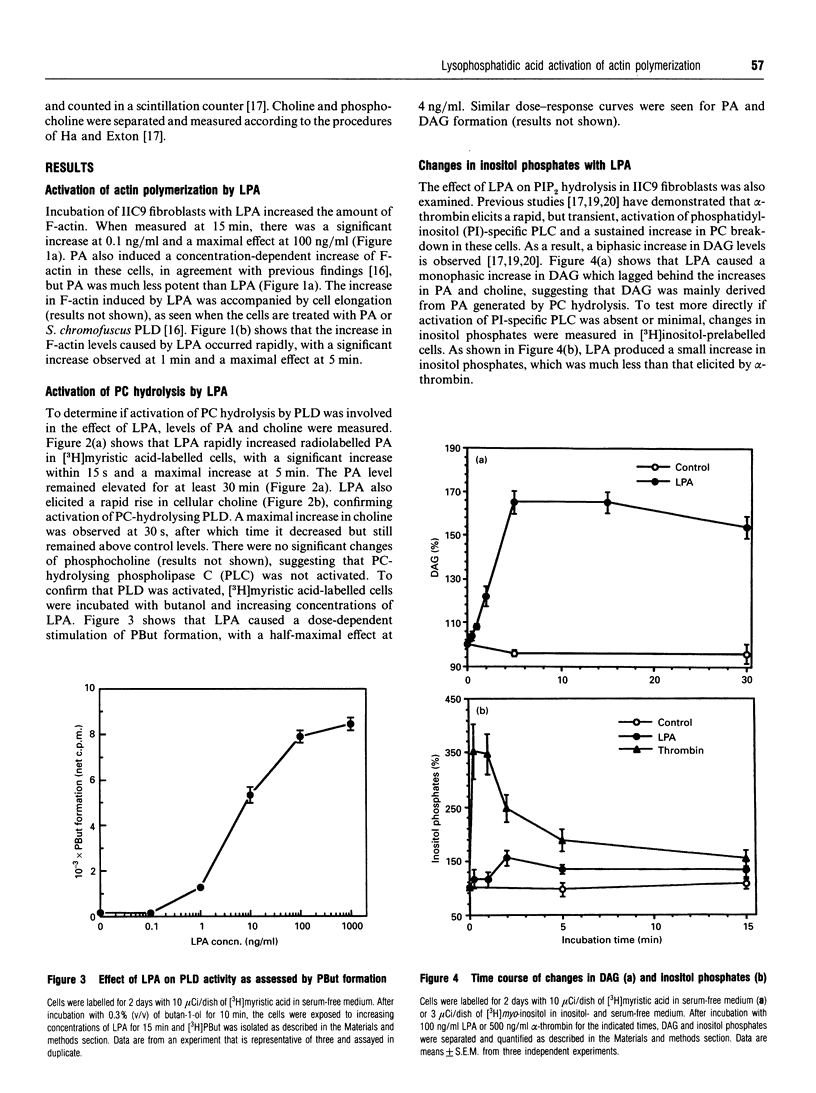
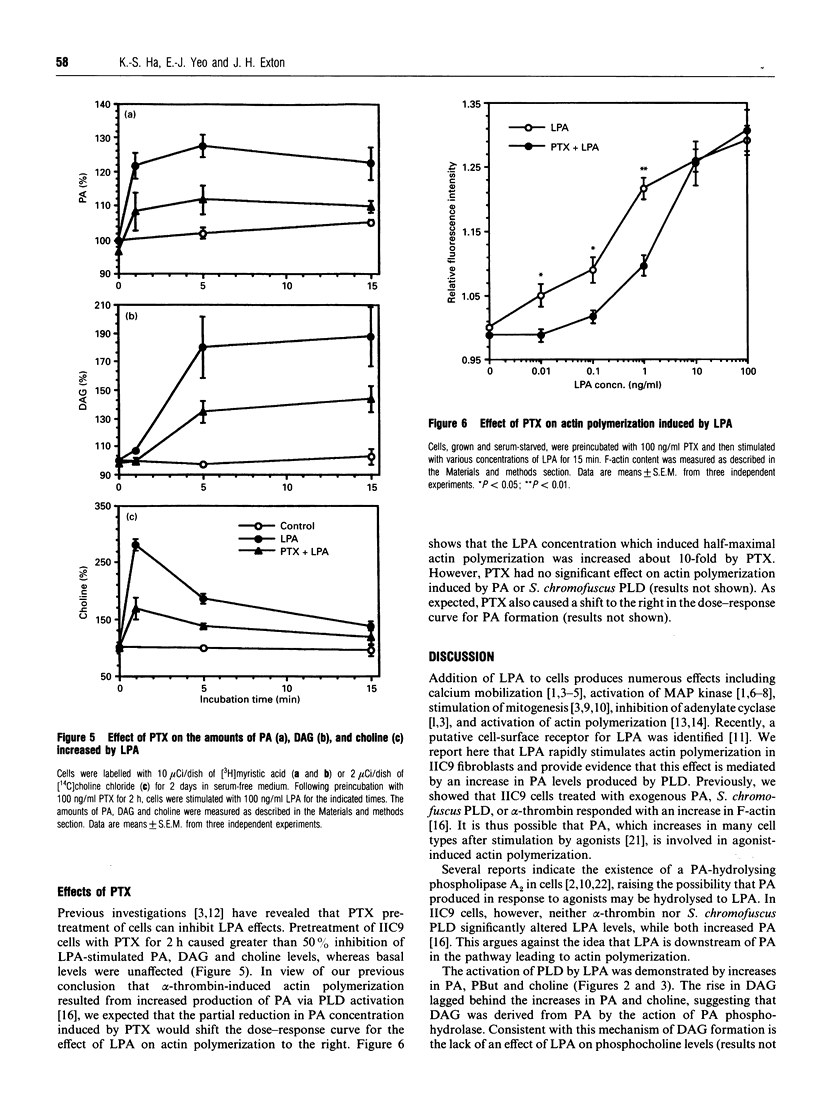
Incubation of IIC9 fibroblasts with lysophosphatidic acid (LPA) induced an increase in the amount of filamentous actin (F-actin), which was concentration-dependent with a maximal effect at 100 ng/ml. Phosphatidic acid (PA) also produced a concentration-dependent increase of F-actin, but it was less potent than LPA. The LPA-induced increase in F-actin was rapid and sustained for at least 60 min. LPA rapidly increased the levels of PA and choline, with maximal increases at 5 min and 30 s respectively. LPA also caused a monophasic increase in diacylglycerol (DAG) which lagged behind the increases in PA and choline. LPA stimulated phosphatidylbutanol formation in the presence of butanol and produced a small increase in inositol phosphates that was much less than that induced by alpha-thrombin. Pretreatment of cells with pertussis toxin (PTX) caused greater than 50% inhibition of the LPA-stimulated increases in PA, DAG and choline. PTX increased the LPA concentration required to induce half-maximal actin polymerization by about 10-fold. PTX caused a similar shift in the dose-response curve for LPA-induced PA formation. These results suggest that LPA induces an increase in PA by activating a phosphatidylcholine-hydrolysing phospholipase D via a PTX-sensitive G-protein and that the increase in PA is involved in the activation of actin polymerization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cook S. J., Rubinfeld B., Albert I., McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993 Sep;12(9):3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Lu M. L., Lo S. H., Lin S., Butler J. A., Druker B. J., Roberts T. M., An Q., Chen L. B. Presence of an SH2 domain in the actin-binding protein tensin. Science. 1991 May 3;252(5006):712–715. doi: 10.1126/science.1708917. [DOI] [PubMed] [Google Scholar]
- Eichholtz T., Jalink K., Fahrenfort I., Moolenaar W. H. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993 May 1;291(Pt 3):677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Fukami K., Takenawa T. Phosphatidic acid that accumulates in platelet-derived growth factor-stimulated Balb/c 3T3 cells is a potential mitogenic signal. J Biol Chem. 1992 Jun 5;267(16):10988–10993. [PubMed] [Google Scholar]
- Gerrard J. M., Robinson P. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochim Biophys Acta. 1989 Feb 20;1001(3):282–285. doi: 10.1016/0005-2760(89)90112-4. [DOI] [PubMed] [Google Scholar]
- Ha K. S., Exton J. H. Activation of actin polymerization by phosphatidic acid derived from phosphatidylcholine in IIC9 fibroblasts. J Cell Biol. 1993 Dec;123(6 Pt 2):1789–1796. doi: 10.1083/jcb.123.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha K. S., Exton J. H. Differential translocation of protein kinase C isozymes by thrombin and platelet-derived growth factor. A possible function for phosphatidylcholine-derived diacylglycerol. J Biol Chem. 1993 May 15;268(14):10534–10539. [PubMed] [Google Scholar]
- Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L. R., Marshall C. J. Lysophosphatidic acid stimulates mitogen-activated protein kinase activation via a G-protein-coupled pathway requiring p21ras and p74raf-1. J Biol Chem. 1993 Oct 5;268(28):20717–20720. [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Moolenaar W. H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2(+)-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J Biol Chem. 1990 Jul 25;265(21):12232–12239. [PubMed] [Google Scholar]
- Kumagai N., Morii N., Fujisawa K., Yoshimasa T., Nakao K., Narumiya S. Lysophosphatidic acid induces tyrosine phosphorylation and activation of MAP-kinase and focal adhesion kinase in cultured Swiss 3T3 cells. FEBS Lett. 1993 Aug 30;329(3):273–276. doi: 10.1016/0014-5793(93)80236-n. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., van der Bend R. L., van Corven E. J., Jalink K., Eichholtz T., van Blitterswijk W. J. Lysophosphatidic acid: a novel phospholipid with hormone- and growth factor-like activities. Cold Spring Harb Symp Quant Biol. 1992;57:163–167. doi: 10.1101/sqb.1992.057.01.021. [DOI] [PubMed] [Google Scholar]
- Pessin M. S., Raben D. M. Molecular species analysis of 1,2-diglycerides stimulated by alpha-thrombin in cultured fibroblasts. J Biol Chem. 1989 May 25;264(15):8729–8738. [PubMed] [Google Scholar]
- Plevin R., MacNulty E. E., Palmer S., Wakelam M. J. Differences in the regulation of endothelin-1- and lysophosphatidic-acid-stimulated Ins(1,4,5)P3 formation in rat-1 fibroblasts. Biochem J. 1991 Dec 15;280(Pt 3):609–615. doi: 10.1042/bj2800609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. Distinct patterns of actin organization regulated by the small GTP-binding proteins Rac and Rho. Cold Spring Harb Symp Quant Biol. 1992;57:661–671. doi: 10.1101/sqb.1992.057.01.072. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Shiono S., Kawamoto K., Yoshida N., Kondo T., Inagami T. Neurotransmitter release from lysophosphatidic acid stimulated PC12 cells: involvement of lysophosphatidic acid receptors. Biochem Biophys Res Commun. 1993 Jun 15;193(2):667–673. doi: 10.1006/bbrc.1993.1676. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Tsai M. H., Hall A., Stacey D. W. Inhibition by phospholipids of the interaction between R-ras, rho, and their GTPase-activating proteins. Mol Cell Biol. 1989 Nov;9(11):5260–5264. doi: 10.1128/mcb.9.11.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. M., Rangan L. A., Shin H. S., Raben D. M. Kinetic analysis of 1,2-diacylglycerol mass levels in cultured fibroblasts. Comparison of stimulation by alpha-thrombin and epidermal growth factor. J Biol Chem. 1988 Jul 5;263(19):9374–9380. [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J., Turner C. E., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation of the focal adhesion-associated protein paxillin in Swiss 3T3 cells. J Biol Chem. 1993 Oct 15;268(29):22060–22065. [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., van Rijswijk A., Jalink K., van der Bend R. L., van Blitterswijk W. J., Moolenaar W. H. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bend R. L., Brunner J., Jalink K., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. Identification of a putative membrane receptor for the bioactive phospholipid, lysophosphatidic acid. EMBO J. 1992 Jul;11(7):2495–2501. doi: 10.1002/j.1460-2075.1992.tb05314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bend R. L., de Widt J., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. The biologically active phospholipid, lysophosphatidic acid, induces phosphatidylcholine breakdown in fibroblasts via activation of phospholipase D. Comparison with the response to endothelin. Biochem J. 1992 Jul 1;285(Pt 1):235–240. doi: 10.1042/bj2850235. [DOI] [PMC free article] [PubMed] [Google Scholar]


