Abstract
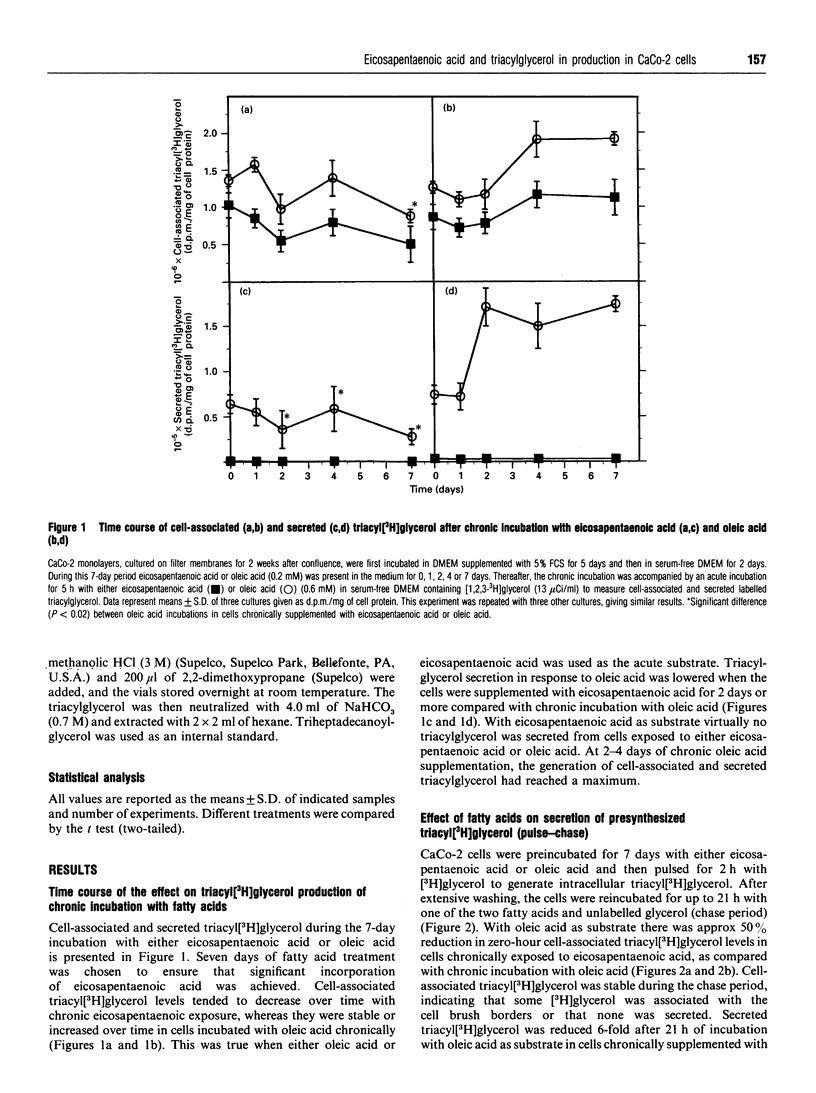
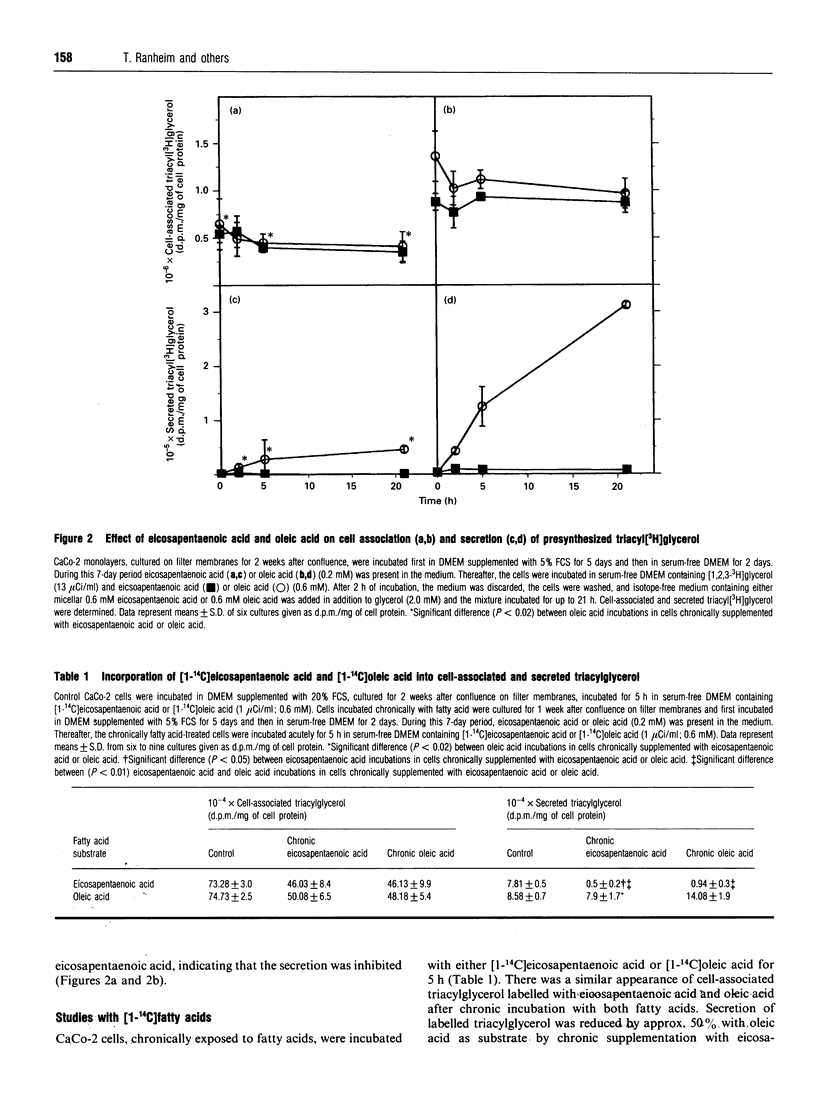
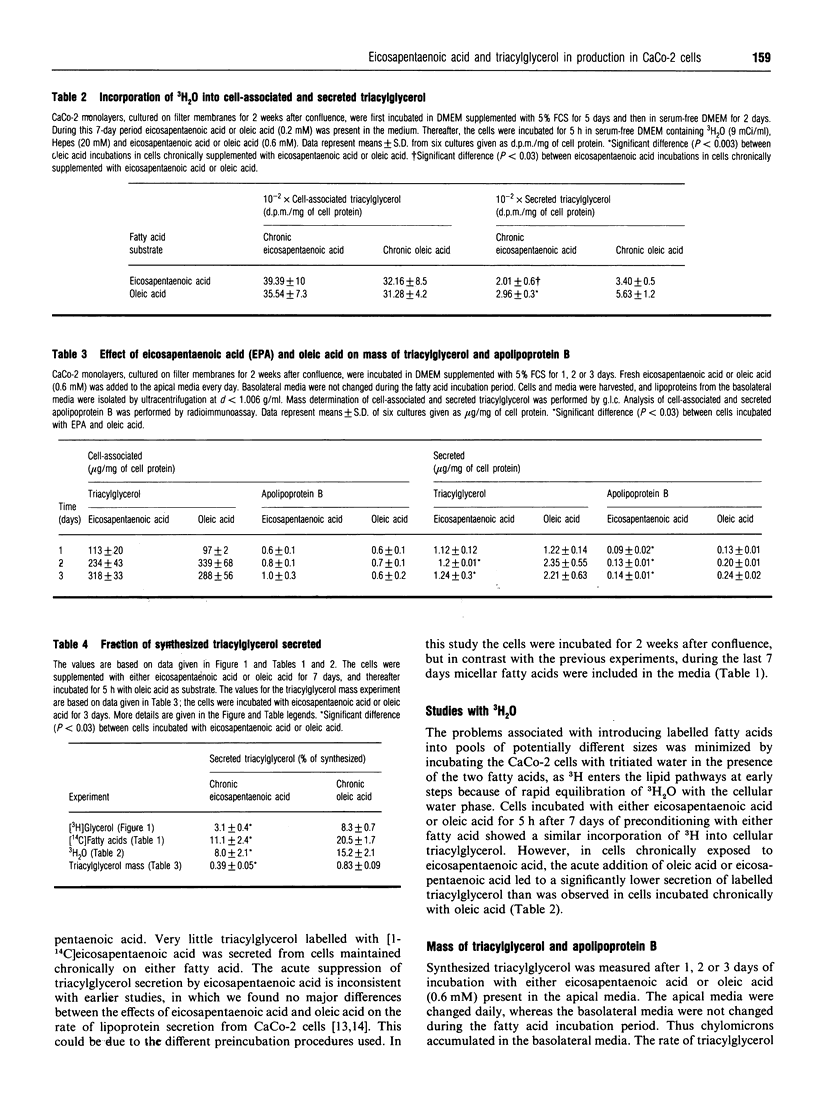
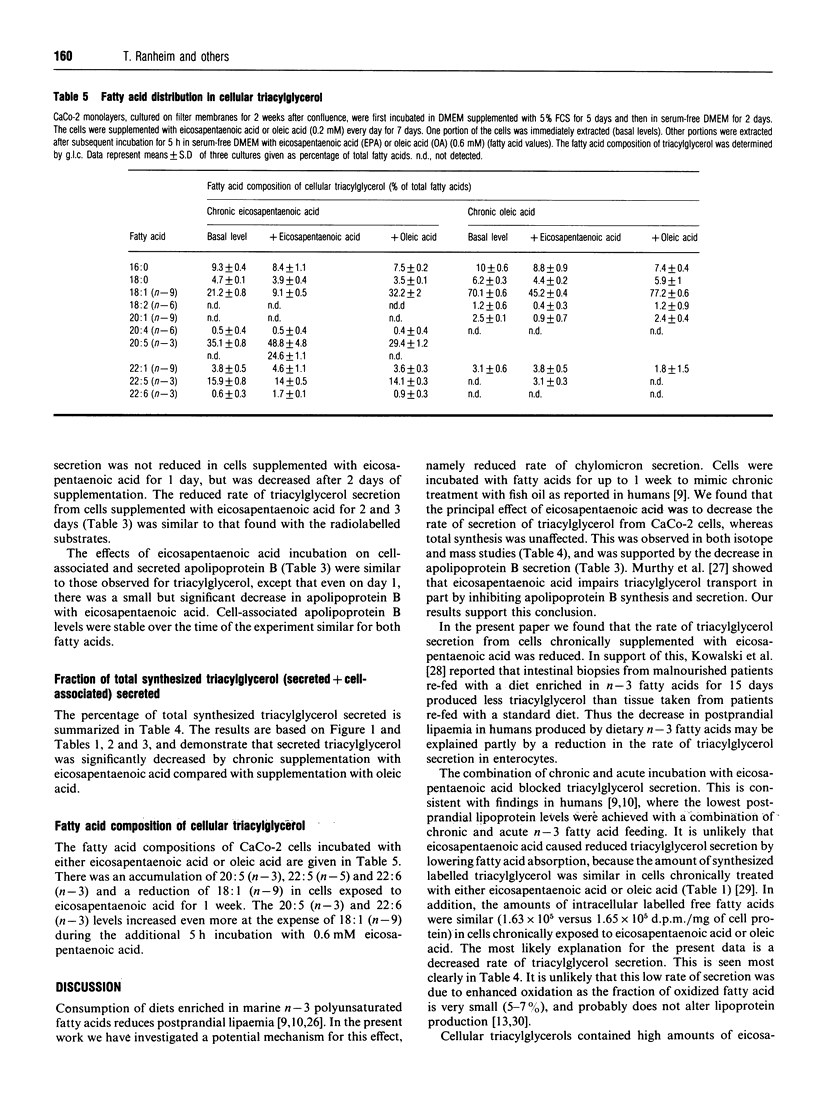
CaCo-2 monolayers, cultured for 1 week after reaching confluence, were incubated with micellar solutions of fatty acids for up to 7 days. These conditioned cells were incubated acutely (5 h) with eicosapentaenoic acid and oleic acid, and the levels of cell-associated and secreted triacylglycerol were determined. With acute addition of oleic acid, both cell-associated and secreted triacylglycerol were decreased in cells chronically exposed to eicosapentaenoic acid. This effect was observed after as little as 2 days of chronic incubation with eicosapentaenoic acid. A further decrease was found when these cells were incubated acutely with eicosapentaenoic acid, regardless of which radioisotopes were used to label precursors in the incubation media. The secretion of both labelled and total triacylglycerol and apolipoprotein B was reduced approximately 50% in cells incubated chronically with eicosapentaenoic acid. The amounts of triacylglycerol and apolipoprotein B within the cells were not decreased by chronic exposure to eicosapentaenoic acid. Our data indicate that CaCo-2 cells chronically incubated with eicosapentaenoic acid secrete significantly less triacylglycerol than cells incubated chronically with oleic acid. When eicosapentaenoic acid was also included acutely, triacylglycerol secretion was reduced even more. We conclude that chronic exposure of eicosapentaenoic acid to this intestinal cell type reduces the rate of chylomicron secretion and may help explain the decreased postprandial lipaemia observed in humans taking fish oil supplements.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang H. O., Dyerberg J., Hjøorne N. The composition of food consumed by Greenland Eskimos. Acta Med Scand. 1976;200(1-2):69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- Bang H. O., Dyerberg J., Sinclair H. M. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980 Dec;33(12):2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- Brown A. J., Roberts D. C. Moderate fish oil intake improves lipemic response to a standard fat meal. A study in 25 healthy men. Arterioscler Thromb. 1991 May-Jun;11(3):457–466. doi: 10.1161/01.atv.11.3.457. [DOI] [PubMed] [Google Scholar]
- Dashti N., Smith E. A., Alaupovic P. Increased production of apolipoprotein B and its lipoproteins by oleic acid in Caco-2 cells. J Lipid Res. 1990 Jan;31(1):113–123. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Field F. J., Fujiwara D., Born E., Chappell D. A., Mathur S. N. Regulation of LDL receptor expression by luminal sterol flux in CaCo-2 cells. Arterioscler Thromb. 1993 May;13(5):729–737. doi: 10.1161/01.atv.13.5.729. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. S., Connor W. E., Alam N., Illingworth D. R. Reduction of postprandial triglyceridemia in humans by dietary n-3 fatty acids. J Lipid Res. 1988 Nov;29(11):1451–1460. [PubMed] [Google Scholar]
- Harris W. S., Connor W. E. The effects of salmon oil upon plasma lipids, lipoproteins, and triglyceride clearance. Trans Assoc Am Physicians. 1980;93:148–155. [PubMed] [Google Scholar]
- Harris W. S. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J Lipid Res. 1989 Jun;30(6):785–807. [PubMed] [Google Scholar]
- Herzberg G. R., Chernenko G. A., Barrowman J. A., Kean K. T., Keough K. M. Intestinal absorption of fish oil in rats previously adapted to diets containing fish oil or corn oil. Biochim Biophys Acta. 1992 Mar 4;1124(2):190–194. doi: 10.1016/0005-2760(92)90097-f. [DOI] [PubMed] [Google Scholar]
- Klevay L. M. The Lifestyle Heart Trial. Nutr Rev. 1992 Jan;50(1):29–29. doi: 10.1111/j.1753-4887.1992.tb02463.x. [DOI] [PubMed] [Google Scholar]
- Kowalski S., Charles F., Nano J. L., Fournel S., Hébuterne X., Rampal P. Lipid metabolism by the intestinal mucosa in malnourished subjects following enteral nutrition supplemented with omega3 fatty acids. Clin Nutr. 1993 Jun;12(3):174–181. doi: 10.1016/0261-5614(93)90077-h. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leaf A., Weber P. C. Cardiovascular effects of n-3 fatty acids. N Engl J Med. 1988 Mar 3;318(9):549–557. doi: 10.1056/NEJM198803033180905. [DOI] [PubMed] [Google Scholar]
- Levin M. S., Talkad V. D., Gordon J. I., Stenson W. F. Trafficking of exogenous fatty acids within Caco-2 cells. J Lipid Res. 1992 Jan;33(1):9–19. [PubMed] [Google Scholar]
- Lindsey S., Pronczuk A., Hayes K. C. Low density lipoprotein from humans supplemented with n-3 fatty acids depresses both LDL receptor activity and LDLr mRNA abundance in HepG2 cells. J Lipid Res. 1992 May;33(5):647–658. [PubMed] [Google Scholar]
- Mallordy A., Besnard P., Carlier H. Research of an in vitro model to study the expression of fatty acid-binding proteins in the small intestine. Mol Cell Biochem. 1993 Jun 9;123(1-2):85–92. doi: 10.1007/BF01076478. [DOI] [PubMed] [Google Scholar]
- Murthy S., Albright E., Mathur S. N., Davidson N. O., Field F. J. Apolipoprotein B mRNA abundance is decreased by eicosapentaenoic acid in CaCo-2 cells. Effect on the synthesis and secretion of apolipoprotein B. Arterioscler Thromb. 1992 Jun;12(6):691–700. doi: 10.1161/01.atv.12.6.691. [DOI] [PubMed] [Google Scholar]
- Murthy S., Albright E., Mathur S. N., Field F. J. Effect of eicosapentaenoic acid on triacylglycerol transport in CaCo-2 cells. Biochim Biophys Acta. 1990 Jul 16;1045(2):147–155. doi: 10.1016/0005-2760(90)90144-m. [DOI] [PubMed] [Google Scholar]
- Phillipson B. E., Rothrock D. W., Connor W. E., Harris W. S., Illingworth D. R. Reduction of plasma lipids, lipoproteins, and apoproteins by dietary fish oils in patients with hypertriglyceridemia. N Engl J Med. 1985 May 9;312(19):1210–1216. doi: 10.1056/NEJM198505093121902. [DOI] [PubMed] [Google Scholar]
- Ranheim T., Gedde-Dahl A., Rustan A. C., Drevon C. A. Fatty acid uptake and metabolism in CaCo-2 cells: eicosapentaenoic acid (20:5(n-3)) and oleic acid (18:1(n-9)) presented in association with micelles or albumin. Biochim Biophys Acta. 1994 Jun 2;1212(3):295–304. doi: 10.1016/0005-2760(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Ranheim T., Gedde-Dahl A., Rustan A. C., Drevon C. A. Influence of eicosapentaenoic acid (20:5, n-3) on secretion of lipoproteins in CaCo-2 cells. J Lipid Res. 1992 Sep;33(9):1281–1293. [PubMed] [Google Scholar]
- Simopoulos A. P. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991 Sep;54(3):438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Traber M. G., Kayden H. J., Rindler M. J. Polarized secretion of newly synthesized lipoproteins by the Caco-2 human intestinal cell line. J Lipid Res. 1987 Nov;28(11):1350–1363. [PubMed] [Google Scholar]
- Trotter P. J., Storch J. Fatty acid uptake and metabolism in a human intestinal cell line (Caco-2): comparison of apical and basolateral incubation. J Lipid Res. 1991 Feb;32(2):293–304. [PubMed] [Google Scholar]
- Weintraub M. S., Zechner R., Brown A., Eisenberg S., Breslow J. L. Dietary polyunsaturated fats of the W-6 and W-3 series reduce postprandial lipoprotein levels. Chronic and acute effects of fat saturation on postprandial lipoprotein metabolism. J Clin Invest. 1988 Dec;82(6):1884–1893. doi: 10.1172/JCI113806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schacky C., Fischer S., Weber P. C. Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J Clin Invest. 1985 Oct;76(4):1626–1631. doi: 10.1172/JCI112147. [DOI] [PMC free article] [PubMed] [Google Scholar]


