Abstract
While induced pluripotent stem cells hold promise as a potential source of osteoblasts for skeletal regeneration, the induction of pluripotency followed by directed differentiation into osteoblasts is time-consuming and low yield. In contrast, direct lineage reprogramming without an intervening stem/progenitor cell stage would be a more efficient approach to generate osteoblasts. We screened combinations of osteogenic transcription factors and identified four factors, Runx2, Osx, Dlx5, and ATF4, that rapidly and efficiently reprogram mouse fibroblasts derived from 2.3 kb type I collagen promoter-driven green fluorescent protein (Col2.3GFP) transgenic mice into induced osteoblast cells (iOBs). iOBs exhibit osteoblast morphology, form mineralized nodules, and express Col2.3GFP and gene markers of osteoblast differentiation. The global transcriptome profiles validated that iOBs resemble primary osteoblasts. Genome-wide DNA methylation analysis demonstrates that within differentially methylated loci, the methylation status of iOBs more closely resembles primary osteoblasts than mouse fibroblasts. We further demonstrate that Col2.3GFP+ iOBs have transcriptome profiles similar to GFP+ cells harvested from Col2.3GFP mouse bone chips. Functionally, Col2.3GFP+ iOBs form mineralized bone structures after subcutaneous implantation in immunodeficient mice and contribute to bone healing in a tibia bone fracture model. These findings provide an approach to derive and study osteoblasts for skeletal regeneration.
Keywords: Reprogramming, fibroblast, osteoblast, bone
Introduction
Bone exhibits robust intrinsic capacity for regeneration; however, large bony defects caused by trauma, congenital malformation, or tumor may require exogenous bone graft material for repair or regeneration (1). Osteoblasts are bone-forming cells that secrete extracellular matrix that is subsequently mineralized to provide mechanical support and scaffolding for muscle attachment (2). Cells of the osteoblast lineage are also important for the support of bone marrow hematopoiesis and immune cell development (3–8). Therefore, the ability to efficiently and reliably generate patient-specific osteoblasts would have great clinical implications for the treatment of bone defects and diseases (9).
Osteoblasts are derived from bone marrow mesenchymal stem cells (MSCs) (10–12). MSCs harvested from bone marrow stromal cells, as well as pluripotent stem cells including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have been used as sources of osteoblasts, and demonstrate osteogenic potential in vitro and/or in vivo (1,9,13–19). However, mesenchymal stromal cells gradually lose their multipotency and are limited by large donor variation (1,20,21), while ESCs and iPSCs are limited by low osteogenic differentiation efficiency and the risk of teratoma formation (22). Another approach, the direct conversion (or reprogramming) of adult somatic cells to an alternative cell lineage, has shown potential for clinical regeneration. Functional neurons, hematopoietic progenitors, hepatocytes, cardiomyocytes, and other tissues have been generated directly from mouse or human fibroblasts, which are easily accessible from patients (23–27). Forced expression of Runx2, a master regulator of osteoblast differentiation, in non-osteoblastic fibroblast cells and osteoblast-like cells can increase osteogenic gene expression and mineralized nodule deposition (28–31). Recently, it was reported that forced expression of four transcription factors, Runt-related transcription factor 2 (Runx2), Osterix (Osx), Octamer-binding transcription factor 3/4 (Oct4) and L-Myc, can directly convert human fibroblasts to functional osteoblasts as assayed by gene expression and mineralization (32,33). Other combinations including Oct6, Oct9, or N-myc can also convert human fibroblasts to osteoblasts (34). However, osteoblast gene expression and mineralization in vitro are not sufficient assays of osteoblast characterization as even the presence of only a small fraction of osteoblast lineage cells can yield positive results. Moreover, these assays do not correlate with in vivo bone formation, a more robust determination of osteoblast function (15). We have demonstrated that mouse embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) derived from Col2.3GFP transgenic mice, in which GFP expression marks osteoblasts (35), can be successfully differentiated to Col2.3GFP+ osteoblast cells (22). We confirmed that Col2.3GFP+ cells are enriched for osteoblast populations. However, directed differentiation of pluripotent stem cells into osteoblasts is limited by low yields (22).
In this study we report that mouse fibroblasts derived from Col2.3GFP transgenic mice can be rapidly and reliably reprogrammed into Col2.3GFP+ induced osteoblast (iOB) cells. We identified a combination of four osteoblast-specific transcription factors, Runx2, Osx, Distal-less homeobox 5 (Dlx5), and Activating Transcription Factor 4 (ATF4), that could efficiently generate induced osteoblasts (iOBs). We demonstrate that Col2.3GFP+ iOBs exhibit osteoblast-like morphology and produce mineralized bone nodules. Genome-wide gene expression profiles and methylation patterns demonstrate that iOBs resemble primary osteoblasts, and Col2.3GFP+ iOBs have characteristic gene expression profile similar to the Col2.3GFP+ osteoblast population derived from mouse bone chips. Furthermore, osteoblast function was confirmed in in vivo ectopic bone formation and orthopedic bone healing models.
Results
Screening for osteoblast-inducing factors
In order to screen for combinations of transcription factors that could convert fibroblasts to functional iOBs, we developed a system in which the efficiency of osteoblast induction could be quantitated by reporter-based fluorescence-activated cell sorting (FACS). We harvested tail tip fibroblasts from Col2.3GFP transgenic mice, in which GFP is expressed specifically in osteoblasts (35), and confirmed the absence of GFP expression in fibroblasts to exclude the contamination of primary osteoblast lineage cells. Based on literature review we selected five candidate transcription factors, Runx2, Dlx5, Ostrix, ATF4 and Msx2 (10,36–39). Runx2 is a master regulator and critical for osteoblast differentiation (40,41) and heterodimerizes with transcriptional co-activator core binding factor ß (Cbfß), which has been shown to be required for the function of Runx2 in bone development and osteoblast differentiation (38,39,42,43). Therefore, we also selected Cbfß as a candidate reprogramming factor.
Col2.3GFP mouse tail tip fibroblasts were transduced with lentivirus expressing all six candidate factors (Figure 1A). As early as 8–10 days after transduction, osteoblast-like clusters that expressed Col2.3GFP could be observed (Figure 1B), suggesting the induction of Col2.3GFP+ osteoblast cells. Next, we sought to narrow down the factors that most efficiently drive direct reprogramming into osteoblasts by screening for calcium deposition and mineralization using Alizarin Red S and/or Von Kossa staining. As expected, Runx2 is required for direct reprogramming as no Alizarin Red S staining was visible in the absence of Runx2 (Figure S1). In contrast, expression of Msx2 abolished Von Kossa staining, consistent with reports that Msx2 expression may inhibit osteoblast differentiation (38,44) (Figure S2A). Surprisingly, we found that expression of Cbfß also decreased Von Kossa staining (Figure S2B). Therefore, we excluded Msx2 and Cbfß from further reprogramming experiments.
Figure 1.

Screening for osteoblast inducing factors. (A) Schematic representation of the strategy to test osteoblast inducing factors. (B) 8 days after transduction of lentivirus containing six candidate transcription factors, live cell GFP image demonstrates Col2.3GFP expression. Scale bars: 1000 μm. Lentivirus containing pLV-tet empty vector was used as mock control. (C) 25 days after transduction of lentivirus containing various combination transcription factors, representative Alizarin Red S and Von kossa staining images in reprogrammed cells. (D) 25 days after transduction, flow cytometry analysis Col2.3GFP+ percentage in reprogrammed cells. All data represent mean ± SD (n=6; One-way ANOVA; *, P=0.006; +, P=0.005; ^, P=0.026; #, P<0.001; relative to mock). (E) 25 days after transduction, qRT-PCR analysis with osteogenic specific markers in reprogrammed cells. All data represent mean ± SD (For Col1a1 expression: n=5 for mock, Runx2, Runx2+Dlx5, or Runx2+Dlx5+Osx+ATF4 group; n=4 for Runx2+Dlx5+Osx group; n=3 for Runx2+ATF5, Runx2+Osx, or Runx2+Osx+ATF5, Runx2+Dlx5+ATF4 group; For Bglap expression: n=3; For Ibsp expression: n=5 for mock, Runx2, Runx2+Dlx5, Runx2+Dlx5+Osx, or Runx2+Dlx5+Osx+ATF4 group; n=3 for Runx2+ATF5, Runx2+Osx, or Runx2+Osx+ATF5, Runx2+Dlx5+ATF4 group; One-way ANOVA; **, P=0.006; +, P=0.012; *, P=0.046; #, P<0.001; ^, P=0.011; relative to mock). See also Figure S1–S4.
Various combinations of Runx2, Dlx5, Osx and ATF4 expression all efficiently induced calcium deposition by Alizarin Red S staining, with mineralization confirmed by Von Kossa staining in these reprogrammed cells (Figure 1C). We have previously published that Col2.3GFP expression is a robust cell-autonomous assay that enriches for osteoblast populations derived from multiple sources including pluripotent stem cells (22). We therefore used flow cytometry to quantitate the frequency of Col2.3GFP+ cells, and found that expression of all four remaining factors, Runx2-Dlx5-Osx-ATF4 (RDOA), gave the highest reprogramming efficiency (Figure 1D). Osteoblast-related gene expression showed that compared to the mock-transduced control group, all groups show increased mRNA levels for the osteoblast markers Col1a1, osteocalcin (Bglap) and bone sialoprotein (Ibsp) (Figure 1E), with the highest expression of Bglap and Ibsp seen in RDOA-transduced cells. In fibroblasts transduced with RDOA factors, compact cell clusters, cuboidal and nodular osteoblast-like cells expressing Col2.3GFP were visible (Figure 2A, b–d and f–h), while the mock transduced control group did not exhibit any Col2.3GFP+ signal under osteogenic conditions, further excluding the possibility of preosteoblast lineage contamination (Figure 2A, a and e). Immunostaining revealed that Col2.3GFP-expressing nodules also expressed osteocalcin, a marker of mature osteoblasts (Figure 2B).
Figure 2.

Runx2-Dlx5-Osx-ATF4 rapidly and stably induce osteoblast reprogramming. (A) Runx2-Dlx5-Osx-ATF4 induced iOBs exhibit osteoblast cell morphology. After 25 days of direct reprogramming, iOBs exhibit compact cell clusters (b), cuboidal (c) and nodular (d) osteoblast-like cell morphology and GFP expression (f-h) (live GFP images). No cell clusters or GFP+ cells are visible in the mock transduction group (a and e). (B) Immunofluorescence staining for GFP and the mature osteoblast marker osteocalcin. Nuclei were stained with DAPI. Scale bars: 1000 μm. (C) Flow cytometry analysis shows that the percentage of Col2.3GFP+ cells increased over time course during reprogramming (left panel). All data represent mean ± SD (n=3; One-way ANOVA; *, P=0.018; #, P<0.001; relative to Day 5). At day 25 of reprogramming, RDOA induced around 3.46 % Col2.3GFP+ iOB cells (right panel). (D) qRT-PCR analysis shows the expression of osteogenic specific markers increased over time course during reprogramming. All data represent mean ± SD (n=3; Multiple t test; For Col1a1: #, P <0.001; *, P = 0.019; + P = 0.011; For Bglap: ++, P = 0.009; **, P = 0.007; *, P = 0.040; +, P = 0.038; ^, P = 0.045; For Ibsp: *, P = 0.011; +, P = 0.006; #, P <0.001; relative to Fibroblast). (E) qRT-PCR analysis shows that expression of endogenous reprogramming factors increased over time course during reprogramming. All data represent mean ± SD (n=3; Multiple t test; For Runx2 (endogenous): *, P = 0.028; +, P = 0.012; ^, P = 0.046; **, P = 0.001; For Sp7 (endogenous): #, P <0.001; **, P = 0.001; *, P = 0.012; +, P = 0.003; ^, P = 0.004; For Dlx5 (endogenous): #, P <0.001; *, P = 0.020; +, P = 0.039; ^, P = 0.042; **, P = 0.015; ++, P = 0.044; relative to Fibroblast). See also Figure S5 - S7.
Next, we examined whether RDOA factors can reprogram mouse fibroblasts in the absence of ascorbic acid. Surprisingly, we found four factors induced a few Col2.3GFP+ cells in the absence of any osteogenic reagent (Figure S3A), although the morphology of the cell cluster expressing Col2.3GFP+ does not exhibit characteristic cuboidal and/ or nodular morphology of osteoblast cells, and the Col2.3GFP+ frequency is low (Figure S3B). Interestingly, RDOA alone also induced osteogenic marker gene expression with comparable levels as in the presence of ascorbic acid with the exception of lower Ibsp expression (Figure S3C). These results suggest that RDOA alone is capable of initiating the osteogenic gene expression program but requires ascorbic acid to enhance osteoblast cell maturation.
It has been reported that human fibroblasts can be directly reprogrammed to osteoblasts with the expression of four transcription factors, Runx2-Osx-Oct4-L-myc (RXOL) (32). We therefore compared the efficiency of RDOA and RXOL in direct reprogramming of murine osteoblasts. We found that RXOL was unable to induce detectable Col2.3GFP expression in murine osteoblasts (Figure S4A). Alizarin Red and Von Kossa staining were also significantly decreased in RXOL- compared to RDOA-treated cells (Figure S4B). However, we did detect significant induction of Bglap and Ibsp mRNA levels in RXOL- and RDOA-treated cells (Figure S4C), suggesting that while both RXOL and RDOA can induce expression of osteoblast genes, RDOA factors are more efficient in reprogramming mouse fibroblasts to mature osteoblasts than RXOL.
Runx2-Dlx5-Osx-ATF4 rapidly and stably induces osteoblast reprogramming
We next examined the kinetics of direct reprogramming. RDOA transduction progressively increased the frequency of Col2.3GFP+ osteoblasts over 25 days in culture (Figure 2C). Significant alkaline phosphatase activity was present by day 5 (Figure S5, left panel), and robust calcium deposition and mineralization could be detected by day 15 and increased over time (Figure S5, middle and right panels). Expression of osteoblast genes Bglap and Ibsp also progressively increased with differentiation (Figure 2D). By day 20 of reprogramming, RDOA-induced Col1a1, Bglap, and Ibsp levels were comparable to those in osteoblasts differentiated from primary calvarial cells (calvarial-OBs) (Figure 2D).
Using primers targeting the untranslated region (UTR) and specific for endogenous mRNA levels, we found that endogenous Runx2, Dlx5, and Osx levels increased over time in iOBs to a similar level as in calvarial-OBs (Figure 2E). We did not examine endogenous ATF4 since it is not regulated at the transcriptional level (45). In contrast, total mRNA (including endogenous and exogenous) levels of the four factors were initially strongly upregulated and then gradually decreased over time and decreased to approximately baseline levels if doxycycline was withdrawn at day 8, with no difference in calcium deposition and mineralization (Figure S6A–E), suggesting that continuous lentiviral expression of the exogenous transgenes is not required for maintenance of the iOB cell state. In order to examine exogenous expression of the four factors, we designed primers spanning the sequence on the transfected plasmid through the coding region to detect only exogenous Runx2, Dlx5, Sp7/Osx and ATF4. As shown in Figure S7, compared to mock transfection, all four exogenous transcription factors increased at the beginning of reprogramming and decreased with the time. By day 20 of reprogramming, all four exogenous transcription factors decreased to the level comparable with the mock control. These data suggested that transient expression of the four transcription factors is sufficient to induce osteoblast reprogramming.
We also assessed whether fibroblasts were converted to osteoblasts by passing through an intermediate pluripotency stage. We examined expression of two pluripotency markers, Pou5f1/Oct4 and Nanog, throughout the direct reprograming process. We found no significant upregulation of either Pou5f1/Oct 4 and Nanog and their mRNA levels were negligible compared with the levels in mouse induced pluripotent cells (miPSCs) (Figure S6F). These results demonstrated that iOBs were directly reprogrammed without an intervening stem or progenitor cell state.
Induced osteoblast transcriptomes resemble primary osteoblast transcriptomes
Next, we performed genome wide RNA-sequencing (RNA-seq) on fibroblasts, calvarial-OBs, and iOBs. As expected, osteoblast-related genes Bglap2, Bglap, Bglap3, Ibsp, Alpl, Col1a1 and Col1a2 were significantly upregulated in iOB cells compared to fibroblast cells (Figure 3A). Genome-wide gene expression changes of iOBs versus fibroblasts are shown in Figure 3B. A total of 3222 genes were found to be significantly upregulated while 2427 genes were significantly downregulated. The osteoblast marker Bglap2 was the most significantly upregulated gene based on the lowest adjusted p value (FDR=1.06−197) (Figure 3B). Gene Ontology Analysis demonstrated that significantly upregulated genes were highly enriched in osteoblast differentiation, bone mineralization, and osteogenesis annotation terms (Figure 3C). Upregulated genes were also enriched in other categories related to bone development and maturation processes including extracellular matrix, calcium, cell adhesion, cell junction and collagen annotation terms. Significantly downregulated genes were enriched in cell cycle, cell division, chromatin binding, methylation, chromatin regulator and covalent chromatin modification annotation terms, suggesting that fundamental cellular biological processes and epigenetic status (e.g., methylation) change during direct reprogramming (Figure 3D). Of note, downregulated genes were also enriched in extracellular matrix and osteoblast differentiation categories, suggesting that these genes might be involved in negative regulation of these two biological processes.
Figure 3.

iOB transcriptomes resemble osteoblast transcriptomes. Genome wide RNA-seq on fibroblasts, calvarial-OBs, and iOBs was performed in triplicate for each group. (A) Osteoblast and bone related gene expression levels. Log2-fold change of expression level and −log10 (P value) in iOBs versus fibroblasts are shown on the x axis. (B) A plot of gene expression changes in iOBs versus fibroblasts. The log2-fold change for gene expression level is plotted on the y-axis and the average of the counts normalized by size factor is shown on the x-axis. Each gene is represented with a dot. Differentially expressed genes with an adjusted p (FDR) value below 0.05 are shown in red. Compared to the fibroblast population, 3222 genes were significantly upregulated, and 2427 genes were downregulated in iOB cells. Bglap2 was the most significantly upregulated gene based on the lowest adjusted p value (FDR=1.06E-197). (C) and (D) Gene Ontology Analysis of biological process for significantly upregulated (C) and downregulated (D) genes in iOBs versus fibroblasts. −log10 (P value) of gene enriched terms were shown. (E) Heatmap of sample-to-sample distances using rlog-transformed values of RNA-seq data. For each sample, the distance calculation is based on the contribution of rlog from all the genes. (F) PCA plot using the rlog-transformed values of RNA-seq data. Each color represents a sample type. Each sample has three replicates. (G) Heatmap of relative rlog-transformed values of total genes across samples. Each row represents a gene. Each column represents a cell type. (H) Heatmap of relative rlog-transformed values of differentially expressed genes in iOBs versus fibroblast across samples. Each row represents a gene. Each column represents a cell type. (E-H) Fib: Fibroblast; Cal: Calvarial. (I) Gene set enrichment analysis (GSEA) show osteogenic genes are enriched in iOBs. Normalized enrichment score, NES:1.57, FDR q=0 and p=0.
We compared the global gene expression profiles by RNA-seq analysis of fibroblasts, iOBs and primary calvarial cell-derived osteoblasts (calvarial-OBs). The heatmap of sample-to-sample distance demonstrated that iOBs and calvarial-OBs were clustered together and separated from fibroblasts (Figure 3E). The global expression patterns were further analyzed by principal component analysis (PCA). The first principal component (PC1), which captured 74% of variation, revealed that iOBs cluster more closely to calvarial-OBs than fibroblasts (Figure 3F).
A hierarchical heatmap of genome-wide gene expression pattern confirmed that iOBs clustered together with calvarial-OBs and separate from fibroblasts (Figure 3G). A heatmap depicting differentially expressed genes between iOBs and fibroblasts revealed that, compared to whole genome-wide gene expression pattern, the expression trends of these genes are more similar in iOBs and calvarial-OBs, demonstrating that the transcriptional program characteristic of fibroblasts was globally reprogrammed toward that of osteoblast lineage (Figure 3H). To further determine whether these cells underwent osteoblast conversion, we carried out gene set enrichment analysis (GSEA) using a mouse osteogenesis gene set including 82 osteogenic differentiation-related genes. The GSEA analysis revealed that iOBs significantly expressed many osteogenic genes (normalized enrichment score, NES:1.57, FDR q=0 and p=0) (Figure 3I), confirming that fibroblasts underwent global gene expression change to an osteogenic fate.
Induced osteoblast cell GFP+ population resembles Col2.3GFP mouse bone chip GFP+ osteoblasts
We have previously shown that among osteoblasts differentiated from Col2.3GFP mouse embryonic cells, sorted Col2.3GFP+ cells are a more enriched osteoblast population (22). We therefore sorted Col2.3GFP+ cells from iOBs (iOB GFP+) and carried out genome-wide gene expression profiling. There was a total of 3877 significantly increased genes and 3547 significantly decreased genes in iOB GFP+ population compared to fibroblasts (data not shown). Within the top 10 upregulated genes, 7 genes were related to osteogenesis, bone formation or extracellular matrix (Figure 4A), including the known osteoblast markers Bglap2, Bglap and Ibsp and osteocyte marker Dmp1. Col13a1 has an important role in bone modeling (46) and Col11a2 is extracellular matrix protein that is for critical skeletal morphogenesis and proper mineralization of bone (47). Panx3 belongs to the innexin family, structural components of gap junctions that are critical for communication between osteoblasts (48). The 3877 upregulated genes are significantly enriched in extracellular matrix, calcium, ossification, osteogenesis, bone mineralization and osteoblast differentiation annotation terms (Figure 4B). Gene Ontology Analysis for significantly downregulated genes showed similar categories of enrichment as total iOB cells (Figure 4C).
Figure 4.
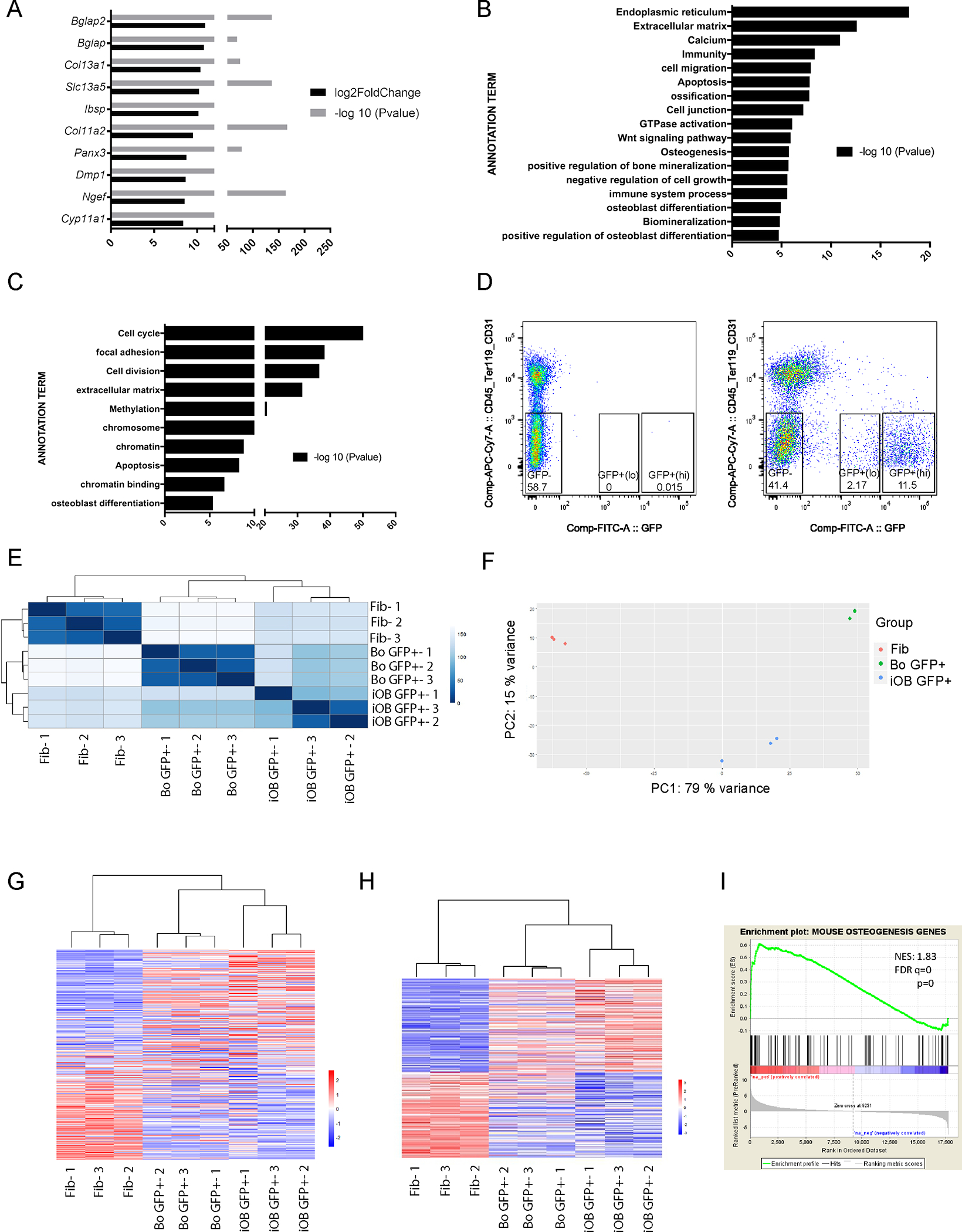
iOB Col2.3GFP+ transcriptomes resemble mouse bone chip Col2.3GFP+ population transcriptomes. Genome wide RNA-seq was performed in triplicate on each group. (A) Top 10 significantly upregulated genes in iOB GFP+ population versus fibroblast cells. (B) and (C) Gene Ontology Analysis of biological process for significantly upregulated (B) and downregulated (C) genes in iOB GFP+ population versus fibroblast cells. (D) FACS sorting of CD45−/Ter119−/CD31− /Col2.3GFP+(lo), CD45−/Ter119−/CD31− /Col2.3GFP+(hi), and CD45−/Ter119−/CD31− /GFP− cells from mouse bone chip culture. Three biological replicates were processed. Mouse bone chip culture from WT mice were used as sorting gate negative control. (E) Heatmap of sample-to-sample distances using rlog-transformed values of RNA-seq data. For each sample, the distance calculation is based on the contribution of rlog from all the genes. (F) PCA plot using the rlog-transformed values of RNA-seq data. Each color represents a sample type. Each sample has three replicates. (G) Heatmap of relative rlog-transformed values of total genes across samples. Each row represents a gene. Each column represents a cell type. (H) Heatmap of relative rlog-transformed values of differentially expressed genes in iOB GFP+ population versus fibroblast cells across samples. Each row represents a gene. Each column represents a cell type. (E-H) Fib: Fibroblast; Bo: Bone chip. (I) Gene set enrichment analysis (GSEA) show osteogenic genes are enriched in iOB GFP+ population. Normalized enrichment score, NES:1.83, FDR q=0 and p=0.
We have shown that Col2.3GFP+(lo) and GFP+(hi) populations sorted from mice long bone osteoblasts are comprised of immature and mature osteoblasts, respectively (22). We therefore compared the transcriptomes of iOB GFP+ population with bone chip-derived osteoblast Col2.3GFP+(hi) population (bone chip GFP+; Figure 4D). Sample-to-sample distance heatmap revealed that iOB GFP+ clustered together with bone chip GFP+ cells and separately from fibroblast cells (Figure 4E). Second, principal component analysis confirmed that iOB GFP+ are much closer to bone chip GFP+ cells in the first principal component (PC1), which captured 79% of variation (Figure 4F). Third, iOB GFP+ also closely grouped together with bone chip GFP+ cells in the hierarchical whole genome gene clustering (Figure 4G). As for differentially expressed genes in iOB GFP+ compared to fibroblasts, the gene expression patterns are much more similar between iOB GFP+ and bone chip GFP+ cells (Figure 4H). Finally, GSEA analysis demonstrated that iOB GFP+ population also significantly expressed many osteogenic genes (NES:1.83, FDR q=0 and p=0) (Figure 4I). The higher normalized enrichment score of iOB GFP+ population than total iOBs (NES:1.83 vs NES:1.57) suggests that iOB GFP+ cells are a more enriched osteoblast population. Taken together, these data confirmed osteoblast conversion from fibroblasts and demonstrated that iOB GFP+ cells are a more enriched osteoblast population with similarity to Col2.3GFP+(hi) mature osteoblasts from mice bone.
DNA methylation status changes during direct reprogramming
Chromatin regulation and methylation annotation terms were enriched among downregulated genes during direct reprogramming (Figure 3D and 4C), indicating that DNA methylation might play an important regulatory role in gene expression changes. We therefore harvested genomic DNA from fibroblasts, iOBs and calvarial-OBs and investigated the DNA methylation status by Methyl-CpG binding domain protein – sequencing (MBD-seq). We first examined the methylation status of osteogenic markers (e.g., Bglap, Ibsp) and calculated the relative methylation score (rms) for each gene. CpG-island methylation, particularly when located in the promoter or first exon regions, has been reported to lead to transcriptional silencing (49). We identified rms peaks in the Bglap2 locus and in the Bglap3 promoter region (500 bp upstream of the transcription start site) in fibroblasts, but rms peaks were absent in iOBs and calvarial-OBs (Figure 5A). At the locus for Ibsp, another osteoblast marker, while rms peaks in the last exon region were present in fibroblasts, iOBs and calvarial-OBs, other rms peaks in the Ibsp promoter, first and fifth intron regions were present only in fibroblasts. These data suggest that DNA demethylation at Bglap and Ibsp loci may contribute the dramatic induction of these genes during direct reprogramming. Based on relative methylation score, we calculated and identified totally 9743 differential methylation regions (DMRs) between iOBs and fibroblasts. The heatmap of these 9743 DMRs across iOBs, calvarial-OBs and fibroblast demonstrated that calvarial-OBs had an intermediate methylation level in most of the DMRs and that iOBs clustered more closely with calvarial-OBs (Figure 5B), although there is substantial heterogeneity. Next, we examined whether the methylation status changes affected gene expression. We identified 1197 genes associated with DMRs and found that 393 genes (49.5%) overlapped with differentially expressed genes (Figure 5C). Therefore, epigenetic methylation status changes might be one of the mechanisms of regulating gene expression changes during direct reprogramming of fibroblasts into iOBs.
Figure 5.
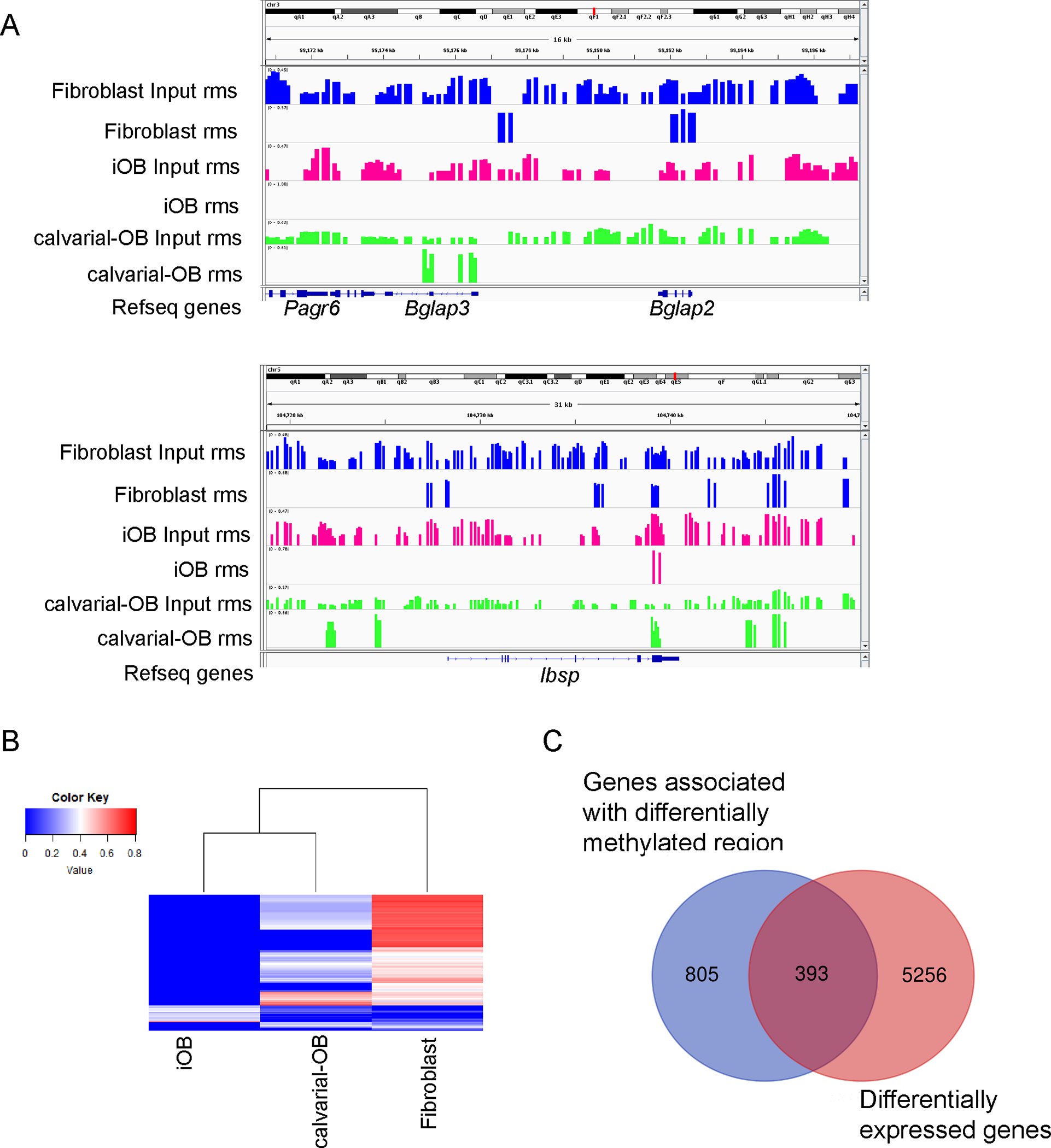
iOB methylation status changes. (A) Decreased methylation peak in osteogenic marker Bglap3, Bglap2 and Ibsp locus. Visualization of regions was done by Integrative Genomics Viewer (IGV). Included tracks are rms (relative methylation score) for different groups. (B) Heatmap of mean rms values of differentially methylated regions (DMRs) in iOB versus fibroblast across samples. Each row represents a differentially methylated region. Each column represents a cell type. (C) The number of genes associated with differentially methylated regions (DMRs) is respected to the number of differentially expressed genes in iOB versus fibroblast.
Induced osteoblast cells form mineralized bone in vivo
To test whether iOBs can form mineralized bone in vivo, we implanted iOB and Matrigel mixtures subcutaneously into immunodeficient mice. Acellular Matrigel and fibroblast Matrigel mixtures were also implanted as controls. By week 4, two of four iOB implants displayed significant mineralized tissue formation by X-ray and micro-CT scanning, whereas only one in four fibroblast implants displayed minimal mineralized tissue formation (Figure 6A). Acellular Matrigel did not exhibit any mineralization in microCT scanning (data not shown). Histological analysis revealed that mineralized nodules formed by implanted iOBs stained positively with Alizarin Red S and Von Kossa, and colocalized with live Col2.3GFP+ signal (Figure 6B), suggesting that the mineral nodule structures were donor iOB cells in origin. Immunostaining for osteocalcin within the mineral nodule confirmed the presence of mature osteoblasts. In contrast, no Alizarin Red S, Von Kossa, live GFP and osteocalcin signaling were detected in fibroblast implants (Figure 6B). By 12 weeks iOB implants displayed extensive microCT signals and prominent ossicles (Figure 6C). Enhanced deposition of cortical bone area was revealed by H&E and Masson Aniline Blue staining, with osteocyte-like cells embedded in cortical bony structures. Collectively, these results suggest that iOBs demonstrate mineralization and bone-forming capacity in vivo.
Figure 6.

iOBs implanted in vivo form mineralized bone structure. Acellular Matrigel, fibroblast Matrigel mixtures and iOB Matrigel mixtures were subcutaneously implanted into immunodeficient mice (n = 4 per group). (A) Fibroblasts and iOBs that had been cultured in osteogenic medium in vitro for 20 days were mixed with equal volume of Matrigel and then subcutaneously implanted into the back of 7-week-old nude mice. Four weeks later, representative microCT x-ray and 3D images of explanted cell lumps show significant mineralized structure in iOB implant and limited radiodense region in fibroblast implant. (B) Serial cryosections were made in iOB and fibroblast of four- week implants. Mineralized structures were visualized using Alizerin Red S and Von Kossa staining. The nucleus of implanted fibroblasts and iOBs were stained with DAPI. Live GFP positive signal showed Col2.3GFP+ expression. Matrix protein osteocalcin was stained by immunofluorescence. (C) Representative microCT x-ray and 3D images of 12-week explanted ossicles from iOB implants. Serial sections were made from paraffin-embedded iOB implant samples. Cortical bony like structures were shown in H&E and Masson Aniline Blue staining.
Induced osteoblast cells can contribute to bone fracture healing
Next, we examined the biological activity of iOBs in an orthotopic model. A 1 mm diameter pin hole defect was created in the tibiae of immunodeficient mice. We transplanted acellular Matrigel, fibroblast or iOB Matrigel mixtures at the bone defect sites and estimated bone fracture healing by X-ray, micro-computed tomography (microCT) and histological analysis. Some pin holes were used as empty control without any transfection. Entire defect sites of tibiae were analyzed by microCT after day 1, week 1, 2, 3 and 4 of transplantation. By week 4, x-ray and microCT images revealed that greater healing in iOB-transplanted tibiae compared to fibroblast-transplanted tibiae, acellular Matrigel and empty control (Figure 7A and B). Bone volume increased significantly in iOB-transplanted tibiae, whereas trabecular spacing was significantly decreased (Figure 7C). Since pin hole formation also resulted in some secondary fractures, we cannot distinguish whether iOBs may have contributed to intramembranous and/or endochondral healing in this model. Histological analysis revealed callus formation at the bone defect site (Figure 7D) and the new bone formation (Figure 7D, k and l) in iOB transplanted tibiae. Col2.3GFP+ iOBs were localized on the surface of the callus (Figure 7D, c and i), especially along with the new thickened bone formation (Figure 7D, m and o), suggesting that iOBs actively contributed to bone fracture healing.
Figure 7.

iOBs contribute to bone fracture healing. Representative microCT 3D (A) and x-ray (B) images of bone fracture sites of tibiae after 1 day and 4-week of surgery and transplantation. (C) MicroCT analysis of Bone Volume/Total Volume (BV/TV), trabecular number (Tb.N) and trabecular spacing (Tb.Sp) absolute increase at week 4 over day 1. All data represent mean ± SD (n=3 for empty control; n=5 for Acellular Matrigel/Matrigel only; n=6 for Matrigel + Fibroblast; n=8 for Matrigel + iOB; Multiple t test). (D) Representative histology of serial cryosections made in Week 4 iOB- transplants. Hematoxylin and eosin staining showed overall morphology of bone callus at fracture site (d and j). New formed bony structures were stained with Von Kossa and Masson Aniline Blue staining (e, f, k, and l, red dot line area). The nucleus was stained with DAPI (b and h). Live GFP positive signal showed Col2.3GFP+ expression on the surface of the callus (c and i) and colocalized along with the new thickened bone (m and o, red dot line area).
Discussion
An unlimited source of bone-forming osteoblasts has significant clinical implications for skeletal regeneration. Because cell differentiation and reprogramming protocols invariably yield a heterogeneous population of cells, we sought to identify candidate osteoblast reprogramming factors using Col2.3GFP as a marker of osteoblasts. Assays for osteoblast differentiation that rely on osteoblast gene expression and mineralization can yield positive results with only a small percentage of osteoblasts, and do not accurately predict in vivo bone formation (15). We have demonstrated that the Col2.3GFP reporter provides a reliable means to identify and harvest an enriched population of osteoblasts (22). Col2.3GFP expression can be used to quantitate osteoblasts, making it possible to rigorously screen combinations of transcription factors that favor osteoblast induction and differentiation. In this study, we demonstrated that the combination of four transcription factors, Runx2, Dlx5, Osx, and ATF4, can more rapidly and efficiently reprogram mouse fibroblasts to Col2.3GFP+ induced osteoblasts (iOBs). Our previous studies have shown that directed differentiation of mouse pluripotent cells into Col2.3GFP+ osteoblasts has low yield (around 0.2%) and requires a lengthy process (45 days) (22). In contrast, here we report that fibroblasts can be reprogrammed with a yield up to 4% Col2.3GFP+ iOBs within 25 days, a significant improvement in efficiency.
A number of studies have identified transcription factors involved in osteoblast differentiation (10,19,37–39), from which we selected candidate reprogramming factors. Among these Runx2 is a master regulator for osteoblast differentiation (40,41), and our data confirm that Runx2 alone is indispensable and sufficient to reprogram fibroblasts into iOBs. Cbfß is an important co-regulator of Runx2 and is required for the function of Runx2 in bone development, likely by enhancing Runx2 interactions with DNA (38,39). Intriguingly, we found that forced expression of Cbfß reduced the direct programming efficiency. Overexpression of Runx2 has been shown to inhibit osteoblast maturation (50). Therefore, a high level of Runx2/Cbfß heterodimers may be detrimental to osteoblast reprogramming.
Dlx5 is a homeobox-containing transcription factor involved osteoblast differentiation and bone development (38,44,51–53). Dlx5 has been reported to stimulate Runx2 expression by binding to Runx2 promoter and play an essential role in the osteoblast-specific activation of the enhancer of Runx2 (54,55). Consistent with these reports, we find that Dlx5 with Runx2 synergistically increases the reprogramming efficiency. On the contrary, forced expression of Msx2, another homeobox-containing transcription factor, inhibited direct reprogramming. While Msx and Dlx families share similarities in sequence as well as expression patterns during vertebrate development (44), Msx proteins function as transcriptional repressors, while Dlx proteins are transcriptional activators. Msx and Dlx appear to have the antagonistic activities with respect to proliferation and differentiation of osteogenic precursors as well as regulating Osteocalcin promotor (44,56). Therefore, overexpression of Msx2 may either attenuate Dlx5 transcription activator activity or direct repress direct reprogramming.
Osterix is a transcription factor that is essential in late osteogenesis (57). During osteoblast differentiation program, mesenchymal progenitors are initially committed to Runx2 positive osteoblast precursors and then transition to Runx2/Osx double positive osteoblast progenitors. Osx is required for differentiation of preosteoblasts into fully mature and functional osteoblasts (39). However, forced overexpression Osx together with Runx2 attenuated the reprogramming efficiency. Similarly, forced expression of ATF4 with Runx2 also impaired osteoblast reprogramming. ATF4 has an important role in the more mature osteoblast lineage cells by regulating osteoblast-derived osteocalcin and receptor activator of nuclear factor-кB ligand (RANKL) and promotes efficient amino acid import to ensure proper protein synthesis by osteoblast (36,58,59). Our data indicate that early Osx or ATF4 overexpression may suppress osteoblast reprogramming, and the effect of inhibition may also explain why RDOA does not increase the efficiency much greater than Runx2 alone. It will be interesting to examine whether expression Osx and ATF4 at later stages of direct reprogramming would instead further boost efficiency. Recently, Dlx5 has been shown to bind the Sp7/Osx zinc finger domain and recruit Sp7/Osx osteoblast enhancers (60). These studies including our data suggest that Dlx5 and Runx2 might synergistically stimulate direct reprogramming to form preosteoblasts and facilitate osteoblast maturation cooperating with Osx and ATF4. Further dissection of reprogramming mechanisms and optimization of the reprogramming process is required to increase reprogramming efficiency.
In addition to transcription factors, growth factors and cell signaling pathways including TGFβ, BMP, Wnt and cAMP/PKA have also been implicated in osteoblast direct reprogramming (61–63). Since virus-mediated gene delivery has clinical limitations, small molecules that efficiently induce Col2.3GFP expression and drive osteoblast reprogramming would be a promising alternative. Although the induced Col2.3GFP population is enriched in the osteoblast lineage, the population is still heterogenous. Thus, further studies of Col2.3GFP in combination with other cell markers will help to further optimize the reprogramming protocol, and to determine whether in vitro direct reprogramming recapitulates physiological osteoblast differentiation.
In this study, we demonstrated that iOBs can ectopically form mineralized nodules expressing Col2.3GFP in vivo, suggesting that iOBs can contribute to bone formation and remodeling in vivo. We further demonstrated that iOBs facilitated bone fracture healing after transplanted into immunodeficient mice at bone defect sites, highlighting the potential of iOBs for bone regeneration. Given the occurrence of secondary fractures during pinhole formation, we cannot distinguish the contribution of iOBs to endochondral versus intramembranous bone formation in this model. Future studies will more carefully examine the capacity of iOBs to form endochondral as opposed to intramembranous bone. Importantly, no tumor formation was observed upon transplantation of iOBs, in contrast to teratoma formation upon in vivo differentiation of iPSCs at mouse cranial defect sites (data not shown). Another advantage of direct reprogramming is that the timing is much more rapid than reprograming to pluripotent stem cells followed by osteogenic differentiation. These studies highlight the potential feasibility of direct reprogramming of patient-specific human fibroblast cells for cell based skeletal regeneration.
Methods
Molecular cloning and lentivirus production
Mouse pCMV6-Etry clone containing Runx2, Cbfb, Dlx5, Sp7/Osx, Atf4, Msx2 and L-myc cDNA were purchased from ORIGENE (OriGene Technologies, Inc. MD). The cDNAs of candidate genes were amplified by RT-PCR with specific primers and replaced the Oct4 cDNA in the doxycycline-inducible lentiviral vectors pLV-tetO-Oct4 (Addgene, Cat.No. 19766). pLV-tetO empty vector was used as mock transfection control. Lentiviral vector expressing rtTA (reverse tetracycline-controlled transactivator) was used to generate virus containing rtTA and co-transduced with lentivirus expressing reprogramming factors. Lentiviral vectors along with the packaging vectors psPAX2 and pMAD2.G (Addgene; Cat.No. 16620 and 16619, respectively) were transfected in 293T cells using Lipofectamine 2000 regent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Viral supernatants were collected after 48 hours and concentrated by using Centrifugal Filter Units (Merck Millipore Ltd, IRELAND) according to the manufacturer’s instruction to produce virus stocks with titers of 1 × 108 to 1 × 109 infectious units per milliliter.
Tissue culture and direct reprogramming
Col2.3GFP fibroblasts were derived from Col2.3GFP transgenic mice (35). Genotyping was performed on tail genomic DNA to confirm the presence of the Col2.3GFP transgene. Tail tips were snipped, minced, trypsinized, and plated to derive fibroblast cells (22). Col2.3GFP fibroblasts were expanded for two passages and frozen for future use. For direct reprogramming, Col2.3GFP fibroblast cells were seeded on gelatin-coated 6 well plates (1 × 105 cells/well) in fibroblast growing medium (DMEM medium (Invitrogen, Carlsbad, CA) supplemented with 10 % fetal bovine serum (FBS, Gemini Bio Products, CA)). The following day, the medium was changed to fibroblast growing medium containing virus and 8 ng/ml polybrene (day 1). After 24 hours (day 2), the viruses were washed out and cells recovered in fresh fibroblast growing medium. On day 3, the cells were trypsinized and split (1:2) to new gelatin-coated dishes. On day 5, the fibroblast growing medium was replaced by osteogenic medium ((αMEM (Invitrogen, Carlsbad, CA), 10 % FBS, 50 μg/mL ascorbic acid (Sigma, St. Louis, MO)) supplemented with 2 μg/mL doxycycline. For mineralization assays, 10 mM β-glycerol phosphate (Sigma, St. Louis, MO) and 100 nM dexamethasone (Sigma, St. Louis, MO) were added to the osteogenic medium. Osteogenic medium was changed every three days. More reagent details are provided in Supplemental Table S1.
Calvarial osteoblasts were harvested by serial collagenase digestion as previously described (64), differentiated in osteogenic medium, and harvested after three weeks (22). Bone chips were harvested from Col2.3GFP transgenic mice and subjected to osteogenic differentiation as previously described (65).
Flow Cytometry
To examine Col2.3GFP expression by flow cytometry, cells were harvested with 0.25% trypsin, filtered through a 70 μm cell strainer, washed once in Staining Buffer (BD Biosciences, San Diego, CA) and suspended in PBS. The cells were analyzed by a flow cytometer (FACSCAN, BD Bioscience) in the Stanford Shared FACS Facility. Col2.3GFP+ cells were sorted by fluorescence-activated cell sorting (FACS) using a flow cytometer (Verdi, BD Bioscience), fitted with an 85 μM nozzle. For sorting of Col2.3GFP+ from bone chip differentiation, cells were stained with biotin anti-mouse CD45 antibody, biotin anti-mouse Ter-119 antibody and biotin anti-mouse CD31 antibody (cat NO: 103104, 116204, and 102404; BioLegend, San Diego, CA). After washing, cells were stained with Streptavidin APC/Cy7 (cat NO: 405208; BioLegend) and Propidium iodide (PI). The CD45−/Ter119−/CD31− and GFP positive or negative cells were sorted by FACS using a flow cytometer (Verdi, BD Bioscience) fitted with an 85 μM nozzle. Data were analyzed using the FlowJo software (Tree Star, Inc., Ashland, Oregon). More antibody details are provided in Supplemental Table S2.
Immunofluorescence and Fluorescence Microscopy
Cells were fixed on cover slips with 4% paraformaldehyde for 15 min, permeabilized with 1% TritonX-100/PBS for 30 min, then blocked with PBS-BT (1× PBS, 3% BSA, and 0.1% Triton X-100) for 30 min, all at room temperature. For cryosection, the slides were warmed at room temperature for 30 min followed by PBS-BT blocking. Cover slips with cells or cryosection slides with tissue samples were then incubated in primary and secondary antibodies diluted in PBS-BT. Nuclei were counterstained with DAPI using the Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA). GFP antibody (A-11120) was purchased from Thermo Fisher Scientific (Waltham, MA). Osteocalcin (bs-4917R) antibody was purchased from Bioss Antibodies Inc. (Woburn, MA). Images and live cell images were acquired with an EVOS FL Cell Imaging System (Live technologies, South San Francisco, CA).
Von Kossa and Alizarin Red S staining
Cells were rinsed twice with PBS and fixed in 10 % formaldehyde for 10 minutes, then washed with distilled water (DDW). Cells were incubated in 5% silver nitrate solution under UV light for 30 minutes, washed with DDW, rinsed with 5% sodium thiosulfate for 5 min to remove unreacted silver, washed again with DDW and stored in PBS. For Alizarin Red S staining cells were fixed and washed, stained with 2% Alizarin Red S solution for 20 minutes, then washed with DDW to remove unincorporated excess dye.
Quantitative reverse transcription PCR (RT-qPCR) and RNA-sequencing
RNA was extracted by using PureLink® RNA Mini Kit (Invitrogen, Carlsbad, CA). RNA concentration and purity were measured by NanoDrop (Thermo Scientific, Wilmington, DE) and then used for reverse transcription using iScript Kit (Bio-Rad, Hercules, CA). Quantitative RT-PCR (qRT-PCR) was performed following standard methods. Primer sequences are provided in Supplementary Table S3. Relative mRNA level was normalized to of the levels of β-Actin. PCR was performed in triplicate for each sample, and 3 independent experiments were carried out.
For RNA-sequencing (RNA-seq), the integrity of extracted RNA was assayed by on-chip electrophoresis (Agilent Bioanalyzer) and only samples with a high RNA integrity (RIN) value were used for RNA-seq. Poly-A mRNA was purified using Dynabeads® mRNA Purification Kit (Invitrogen) according to the manufacturer’s instructions. RNA sequencing libraries were prepared using the NEBNext® Ultra™ II DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA) according to the manufacturer’s instructions. Library quality was verified using the Agilent High Sensitivity DNA Kit on Agilent’s 2100 Bioanalyzer. For each library, average 420 bp fragments were sequenced using paired end reads (2 × 100 bp) on the Illumina HiSeq 2500 platform (Stanford Personalized Medicine Sequencing Core), with an average of 30 million reads per sample. Paired end sequencing reads (100bp) were generated and aligned to mouse reference sequence NCBI Build 37/mm9 with the STAR (v2.4.2a) algorithm (66). Normalization of RefSeq annotated genes, expression level and differential expression analysis were performed using the Bioconductor package DESeq2 in R (Version 3.2.2) (67). Genes with False Discovery Rate (FDR) <0.05 were defined as differentially expressed genes. Gene ontology analysis was performed using DAVID (david.abcc.ncifcrf.gov). For Gene Set Enrichment Analysis (GSEA, Broad Institute), the gene expression profiles were ranked based on log2foldchange of iOB or iOB Col2.3GFP+ vs fibroblast and used as metrics. Gene set associated with osteogenesis were obtained from www.qiagen.com (68). All transcriptome raw data are publicly available in GEO (accession number GSE139907).
MBD (Methyl-CpG binding domain protein)-sequencing
Genomic DNA was extracted by NucleoSpin Tissu kit (MACHEREY-NAGEL Inc., Germany). DNA concentration was measured by NanoDrop. Genomic DNA was sonicated to generate 100–350 bp fragments. 100ng of fragmented DNA were subjected to MethylCollector Ultra (MBD2b and MBD3L1) (Active Motif 55005, Carlsbad, California, USA) kit according to the manufacturer’s instructions. Briefly, sheared DNA fragments were incubated with His-tagged MBD2b/MBD3L1 protein complexes. Methylated protein-DNA complexes were then captured by nickel-coated magnetic beads. Enriched DNA fragments and input DNA were purified by using a MinElute Reaction Cleanup kit (Qiagen 28204, Germantown, Maryland, USA). Purified DNA were used for generating MBD- sequencing libraries using the NEBNext® Ultra™ II DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA) according to the manufacturer’s instructions. Library quality was verified using the Agilent High Sensitivity DNA Kit on Agilent’s 2100 Bioanalyzer. For each library, average 400 bp fragments were sequenced using paired end reads (2 × 100 bp) on the Illumina HiSeq 2500 platform (Stanford Personalized Medicine Sequencing Core). Paired-end reads were mapped to mouse reference sequence NCBI Build 37/mm9 using BOWTIE 2 (69). Genome wide methylation signal and differential methylation regions were calculated by using Bioconductor package MEDIPS under R environment (70). All methylation data are publicly available at in GEO (accession number GSE139907).
Mice
7-week-old male CD-1 Nude Mice Crl:CD1-Foxn1nu purchased from Charles River (Wilmington, MA) were housed in Innovive recyclable individually ventilated cages in a designated pathogen-free area facility and fed irradiated mouse chow and autoclaved water. The Veterinary Service Center (VSC) at Stanford University provides laboratory animal care and is administered by the Department of Comparative Medicine. The laboratory animal care program at Stanford University is fully accredited by the Association for Accreditation and Assessment of Laboratory Animal Care (AAALAC). All animal surgeries were approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC). More mice details are provided in Supplemental Table S4.
Subcutaneous implantation
After anesthesia using inhaled isoflurane, fibroblasts and iOBs that had been cultured in osteogenic medium in vitro for 20 days were mixed with equal volume of Matrigel and then subcutaneously implanted into the back of 7-week-old male CD-1 Nude Mice Crl:CD1-Foxn1nu. Each implantation was conducted by injection of 1×10^6 cell and Matrigel mixture using 25G needles. Acellular Matrigel was also injected. Small nodules were explanted after 2, 3, 4,8 12 weeks of implantation after mice euthanasia by carbon dioxide inhalation. The mineralized structure in nodules were analyzed by X-ray, microCT and histology.
Pin hole tibia bone fracture
After anesthesia using inhaled isoflurane, a small incision was made slightly below the knee of 7-week old male CD-1 Nude Mice Crl:CD1-Foxn1nu. A full thickness flap was created on the anterior surface of the tibia and a small pin hole was created in the tibial plateau using a dental drill. The pin hole had outer diameter of 1.0 mm. In order to avoid transplanted cells being expelled by hematoma, the wound was closed temporary for 3 hours using 6.0 nylon sutures. After 3 hours, the wound was reopened, and the pin hole was clear of hematoma. Fibroblasts or iOBs that had been cultured in osteogenic medium in vitro for 20 days were mixed with equal volume of Matrigel and 1×10^6 cells were transplanted by using 25 G needles. Empty control or Acellular Matrigel were also included. The wound was closed again by surgical sutures. Bone microtomography was monitored at day 1, week1, 2, 3, and 4. Day 1 data were used as base line for calculation of bone volume, trabecular number and trabecular spacing absolute increase over day 1. At week 2 and week 4, some of mice were euthanized and tibiae were explanted for histological analysis.
Micro-computed tomography and Histological Analysis
Mineralization of subcutaneous implants and tibiae with pin hole defect were monitored by micro-computed tomography (microCT) using the vivaCT 40 Preclinical MicroCT Scanner (SCANCO Medical, AG). For small nodules formed after subcutaneous implantation, scanning was conducted with X-Ray setting at Energy/intensity: 70 kVp,114 μA, 8w; with CT-Scan setting at high resolution, 21.5 mm FOV (field of view)/Diameter, 10. 5 μm Voxelsize and 618 slices. The region of interest was selected to cover the lumps completely. For pin hole tibia bone fracture, scanning was conducted with X-Ray setting at Energy/intensity: 55 kVp,145 μA, 8w; with CT-Scan at high resolution, 38.9 mm FOV (field of view)/Diameter, 19.0 μm Voxelsize and 211 slices. The pin hole area was selected as center of the region of interest. For bone parameter analysis, VOI (view of interest) was selected as Dimension X:300; Y:300; Z:190 to cover the pin hole area completely. 3D images were completed by accompany software. X-ray images were analyzed by Inveon™ Research Workplace 4.2 (IRW 4.2, Siemens Healthcare GmbH, Germany) software.
For histological analysis, tissue samples were harvested and fixed for 24 hoursin 10% neutral-buffered formalin followed by 24 hours 30 % sucrose incubation. Tissue samples were embedded in embedding medium SCEM (Section-lab, Japan) for cryosection. 8-μm serial section was made on a cryofilm (Section-lab, Japan) using tape-stabilized sectioning method for histology analysis. Some tissue samples were decalcified in 25 % formic acid for 2 weeks and subjected to standard paraffin-embedding process. Paraffin-embedded tissue samples were sectioned at 8-μm. Standard Hematoxylin and eosin (H&E) staining and Aniline Blue staining according to Masson’s trichrome method (Newcomer Supply, Inc., Middleton, WI) were processed on sectioned slides from frozen or paraffin-embedded tissue samples. Images were acquired by an EVOS FL Cell Imaging System (Live technologies, South San Francisco, CA).
Statistical Analysis
Statistical analysis was performed using Microsoft Excel and GraphPad Prism 6 (GraphPad Software, San Diego, CA). Quantitative data are presented as mean ± SD. Statistical significance was assessed by One-way ANOVA with Tukey’s multiple comparison test or Multiple t test followed by correction for multiple comparisons using the Holm-Sidak method.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health grant DP2OD008466 to JYW. We thank Drs. Tzu-hua Lin, Jukka Pajarinen, Stuart B Goodman, Bogi Heinrich Meno Conrad, Fan Yang, Fiorella Carla Grandi and Nidhi Bhutani (Department of Orthopaedics, Stanford University) for advice on methods. We also like to thank Dr. Frezghi Habte (Stanford Small Animal Imaging Facility) for assistance with microCT.
Footnotes
Competing interests
The authors declare no competing interests.
Data availability
The raw data required to reproduce these findings are available in the Open Data section.
References
- 1.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. May 31 2011;9:66. Epub 2011/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long F Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. Dec 22 2011;13(1):27–38. Epub 2011/12/23. [DOI] [PubMed] [Google Scholar]
- 3.Panaroni C, Tzeng YS, Saeed H, Wu JY. Mesenchymal progenitors and the osteoblast lineage in bone marrow hematopoietic niches. Curr Osteoporos Rep. Mar 2014;12(1):22–32. Epub 2014/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panaroni C, Wu JY. Interactions between B lymphocytes and the osteoblast lineage in bone marrow. Calcif Tissue Int. Sep 2013;93(3):261–8. Epub 2013/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. Dec 23 2011;12(1):49–60. Epub 2011/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng YH, Chitteti BR, Streicher DA, Morgan JA, Rodriguez-Rodriguez S, Carlesso N, et al. Impact of maturational status on the ability of osteoblasts to enhance the hematopoietic function of stem and progenitor cells. J Bone Miner Res. May 2011;26(5):1111–21. Epub 2011/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askmyr M, Sims NA, Martin TJ, Purton LE. What is the true nature of the osteoblastic hematopoietic stem cell niche? Trends Endocrinol Metab. Aug 2009;20(6):303–9. Epub 2009/07/15. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. May 1 2007;109(9):3706–12. Epub 2007/01/18. [DOI] [PubMed] [Google Scholar]
- 9.Wu JY. Pluripotent Stem Cells and Skeletal Regeneration--Promise and Potential. Curr Osteoporos Rep. Oct 2015;13(5):342–50. Epub 2015/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. May 15 2003;423(6937):349–55. Epub 2003/05/16. [DOI] [PubMed] [Google Scholar]
- 11.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–92. Epub 2001/05/22. [DOI] [PubMed] [Google Scholar]
- 12.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. Apr 02 1999;284(5411):143–7. Epub 1999/04/02. [DOI] [PubMed] [Google Scholar]
- 13.Both SK, van Apeldoorn AA, Jukes JM, Englund MC, Hyllner J, van Blitterswijk CA, et al. Differential bone-forming capacity of osteogenic cells from either embryonic stem cells or bone marrow-derived mesenchymal stem cells. J Tissue Eng Regen Med. Mar 2011;5(3):180–90. Epub 2010/08/19. [DOI] [PubMed] [Google Scholar]
- 14.Lou X. Induced Pluripotent Stem Cells as a new Strategy for Osteogenesis and Bone Regeneration. Stem Cell Rev. May 29 2015. Epub 2015/05/30. [DOI] [PubMed] [Google Scholar]
- 15.Phillips MD, Kuznetsov SA, Cherman N, Park K, Chen KG, McClendon BN, et al. Directed differentiation of human induced pluripotent stem cells toward bone and cartilage: in vitro versus in vivo assays. Stem Cells Transl Med. Jul 2014;3(7):867–78. Epub 2014/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi B, Hyun JS, Montoro DT, Lo DD, Chan CK, Hu S, et al. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc Natl Acad Sci U S A. Dec 11 2012;109(50):20379–84. Epub 2012/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilousova G, Jun du H, King KB, De Langhe S, Chick WS, Torchia EC, et al. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells. Feb 2011;29(2):206–16. Epub 2011/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Bronson S, Niyibizi C. Derivation of murine induced pluripotent stem cells (iPS) and assessment of their differentiation toward osteogenic lineage. J Cell Biochem. Mar 01 2010;109(4):643–52. Epub 2009/12/30. [DOI] [PubMed] [Google Scholar]
- 19.Duplomb L, Dagouassat M, Jourdon P, Heymann D. Concise review: embryonic stem cells: a new tool to study osteoblast and osteoclast differentiation. Stem Cells. Mar 2007;25(3):544–52. Epub 2006/11/11. [DOI] [PubMed] [Google Scholar]
- 20.Siddappa R, Licht R, van Blitterswijk C, de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. Aug 2007;25(8):1029–41. Epub 2007/05/01. [DOI] [PubMed] [Google Scholar]
- 21.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med. Feb 2007;4(2):e9. Epub 2007/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu H, Kimura T, Swami S, Wu JY. Pluripotent stem cells as a source of osteoblasts for bone tissue regeneration. Biomaterials. Mar 2019;196:31–45. Epub 2018/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, et al. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. May 2013;31(5):434–9. Epub 2013/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. May 11 2011;475(7356):386–9. Epub 2011/05/13. [DOI] [PubMed] [Google Scholar]
- 25.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. Feb 25 2010;463(7284):1035–41. Epub 2010/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. Nov 25 2010;468(7323):521–6. Epub 2010/11/09. [DOI] [PubMed] [Google Scholar]
- 27.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. Aug 06 2010;142(3):375–86. Epub 2010/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. May 30 1997;89(5):747–54. Epub 1997/05/30. [DOI] [PubMed] [Google Scholar]
- 29.Phillips JE, Guldberg RE, Garcia AJ. Dermal fibroblasts genetically modified to express Runx2/Cbfa1 as a mineralizing cell source for bone tissue engineering. Tissue Eng. Aug 2007;13(8):2029–40. Epub 2007/05/23. [DOI] [PubMed] [Google Scholar]
- 30.Yang S, Wei D, Wang D, Phimphilai M, Krebsbach PH, Franceschi RT. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J Bone Miner Res. Apr 2003;18(4):705–15. Epub 2003/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byers BA, Pavlath GK, Murphy TJ, Karsenty G, Garcia AJ. Cell-type-dependent up-regulation of in vitro mineralization after overexpression of the osteoblast-specific transcription factor Runx2/Cbfal. J Bone Miner Res. Nov 2002;17(11):1931–44. Epub 2002/11/05. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto K, Kishida T, Sato Y, Nishioka K, Ejima A, Fujiwara H, et al. Direct conversion of human fibroblasts into functional osteoblasts by defined factors. Proc Natl Acad Sci U S A. May 12 2015;112(19):6152–7. Epub 2015/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto K, Sato Y, Honjo K, Ichioka H, Oseko F, Sowa Y, et al. Generation of Directly Converted Human Osteoblasts That Are Free of Exogenous Gene and Xenogenic Protein. J Cell Biochem. Nov 2016;117(11):2538–45. Epub 2016/03/19. [DOI] [PubMed] [Google Scholar]
- 34.Mizoshiri N, Kishida T, Yamamoto K, Shirai T, Terauchi R, Tsuchida S, et al. Transduction of Oct6 or Oct9 gene concomitant with Myc family gene induced osteoblast-like phenotypic conversion in normal human fibroblasts. Biochem Biophys Res Commun. Nov 27 2015;467(4):1110–6. Epub 2015/10/27. [DOI] [PubMed] [Google Scholar]
- 35.Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. Jan 2002;17(1):15–25. Epub 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 36.Long F Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. Jan 2012;13(1):27–38. Epub 2011/12/23. [DOI] [PubMed] [Google Scholar]
- 37.Illich DJ, Demir N, Stojkovic M, Scheer M, Rothamel D, Neugebauer J, et al. Concise review: induced pluripotent stem cells and lineage reprogramming: prospects for bone regeneration. Stem Cells. Apr 2011;29(4):555–63. Epub 2011/02/11. [DOI] [PubMed] [Google Scholar]
- 38.Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. Dec 1 2006;99(5):1233–9. Epub 2006/06/24. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. Aug 2003;19(8):458–66. Epub 2003/08/07. [DOI] [PubMed] [Google Scholar]
- 40.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. May 30 1997;89(5):765–71. Epub 1997/05/30. [DOI] [PubMed] [Google Scholar]
- 41.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. May 30 1997;89(5):755–64. Epub 1997/05/30. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Ma J, Zhu G, Jules J, Wu M, McConnell M, et al. Cbfbeta deletion in mice recapitulates cleidocranial dysplasia and reveals multiple functions of Cbfbeta required for skeletal development. Proc Natl Acad Sci U S A. Jun 10 2014;111(23):8482–7. Epub 2014/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida CA, Furuichi T, Fujita T, Fukuyama R, Kanatani N, Kobayashi S, et al. Core-binding factor beta interacts with Runx2 and is required for skeletal development. Nat Genet. Dec 2002;32(4):633–8. Epub 2002/11/16. [DOI] [PubMed] [Google Scholar]
- 44.Bendall AJ, Abate-Shen C. Roles for Msx and Dlx homeoproteins in vertebrate development. Gene. Apr 18 2000;247(1–2):17–31. Epub 2000/04/25. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Karsenty G. ATF4, the osteoblast accumulation of which is determined post-translationally, can induce osteoblast-specific gene expression in non-osteoblastic cells. J Biol Chem. Nov 5 2004;279(45):47109–14. Epub 2004/09/21. [DOI] [PubMed] [Google Scholar]
- 46.Ylonen R, Kyronlahti T, Sund M, Ilves M, Lehenkari P, Tuukkanen J, et al. Type XIII collagen strongly affects bone formation in transgenic mice. J Bone Miner Res. Aug 2005;20(8):1381–93. Epub 2005/07/12. [DOI] [PubMed] [Google Scholar]
- 47.Vikkula M, Mariman EC, Lui VC, Zhidkova NI, Tiller GE, Goldring MB, et al. Autosomal dominant and recessive osteochondrodysplasias associated with the COL11A2 locus. Cell. Feb 10 1995;80(3):431–7. Epub 1995/02/10. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro F. Variable conformation of GAP junctions linking bone cells: a transmission electron microscopic study of linear, stacked linear, curvilinear, oval, and annular junctions. Calcif Tissue Int. Oct 1997;61(4):285–93. Epub 1997/10/06. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. Jun 2008;9(6):465–76. Epub 2008/05/09. [DOI] [PubMed] [Google Scholar]
- 50.Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, et al. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol. Oct 1 2001;155(1):157–66. Epub 2001/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tadic T, Dodig M, Erceg I, Marijanovic I, Mina M, Kalajzic Z, et al. Overexpression of Dlx5 in chicken calvarial cells accelerates osteoblastic differentiation. J Bone Miner Res. Jun 2002;17(6):1008–14. Epub 2002/06/11. [DOI] [PubMed] [Google Scholar]
- 52.Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, Mantero S, et al. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. Sep 1999;126(17):3795–809. Epub 1999/08/06. [DOI] [PubMed] [Google Scholar]
- 53.Miyama K, Yamada G, Yamamoto TS, Takagi C, Miyado K, Sakai M, et al. A BMP-inducible gene, dlx5, regulates osteoblast differentiation and mesoderm induction. Dev Biol. Apr 1 1999;208(1):123–33. Epub 1999/03/17. [DOI] [PubMed] [Google Scholar]
- 54.Lee MH, Kim YJ, Yoon WJ, Kim JI, Kim BG, Hwang YS, et al. Dlx5 specifically regulates Runx2 type II expression by binding to homeodomain-response elements in the Runx2 distal promoter. J Biol Chem. Oct 21 2005;280(42):35579–87. Epub 2005/08/24. [DOI] [PubMed] [Google Scholar]
- 55.Kawane T, Komori H, Liu W, Moriishi T, Miyazaki T, Mori M, et al. Dlx5 and mef2 regulate a novel runx2 enhancer for osteoblast-specific expression. J Bone Miner Res. Sep 2014;29(9):1960–9. Epub 2014/04/03. [DOI] [PubMed] [Google Scholar]
- 56.Newberry EP, Latifi T, Towler DA. Reciprocal regulation of osteocalcin transcription by the homeodomain proteins Msx2 and Dlx5. Biochemistry. Nov 17 1998;37(46):16360–8. Epub 1998/11/18. [DOI] [PubMed] [Google Scholar]
- 57.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. Jan 11 2002;108(1):17–29. Epub 2002/01/17. [DOI] [PubMed] [Google Scholar]
- 58.Xiao G, Jiang D, Ge C, Zhao Z, Lai Y, Boules H, et al. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J Biol Chem. Sep 2 2005;280(35):30689–96. Epub 2005/07/08. [DOI] [PubMed] [Google Scholar]
- 59.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. Apr 30 2004;117(3):387–98. Epub 2004/04/28. [DOI] [PubMed] [Google Scholar]
- 60.Hojo H, Ohba S, He X, Lai LP, McMahon AP. Sp7/Osterix Is Restricted to Bone-Forming Vertebrates where It Acts as a Dlx Co-factor in Osteoblast Specification. Dev Cell. May 9 2016;37(3):238–53. Epub 2016/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto K, Kishida T, Nakai K, Sato Y, Kotani SI, Nishizawa Y, et al. Direct phenotypic conversion of human fibroblasts into functional osteoblasts triggered by a blockade of the transforming growth factor-beta signal. Sci Rep. May 31 2018;8(1):8463. Epub 2018/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yanjiao Li YW, Yu Juehua, Ma Zhaoxia, Bai Qiong, Wu Xingfei, Bao Pengfei, Li Lirong, Ma Daiping, Liu Jinxue, Liu Change, Chen Fangyun, Hu Min. Direct conversion of human fibroblasts into osteoblasts and osteocytes with small molecules and a single factor, Runx2. BioRxiv. 2017. Epub Apri 14 2017. [Google Scholar]
- 63.Micha D, Voermans E, Eekhoff MEW, van Essen HW, Zandieh-Doulabi B, Netelenbos C, et al. Inhibition of TGFbeta signaling decreases osteogenic differentiation of fibrodysplasia ossificans progressiva fibroblasts in a novel in vitro model of the disease. Bone. Mar 2016;84:169–80. Epub 2016/01/16. [DOI] [PubMed] [Google Scholar]
- 64.Yang D, Guo J, Divieti P, Shioda T, Bringhurst FR. CBP/p300-interacting protein CITED1 modulates parathyroid hormone regulation of osteoblastic differentiation. Endocrinology. Apr 2008;149(4):1728–35. Epub 2008/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panaroni C, Fulzele K, Saini V, Chubb R, Pajevic PD, Wu JY. PTH Signaling in Osteoprogenitors Is Essential for B-Lymphocyte Differentiation and Mobilization. J Bone Miner Res. Dec 2015;30(12):2273–86. Epub 2015/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. Jan 01 2013;29(1):15–21. Epub 2012/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. Epub 2014/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Decker M, Martinez-Morentin L, Wang G, Lee Y, Liu Q, Leslie J, et al. Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat Cell Biol. Jun 2017;19(6):677–88. Epub 2017/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. Mar 4 2012;9(4):357–9. Epub 2012/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lienhard M, Grimm C, Morkel M, Herwig R, Chavez L. MEDIPS: genome-wide differential coverage analysis of sequencing data derived from DNA enrichment experiments. Bioinformatics. Jan 15 2014;30(2):284–6. Epub 2013/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data required to reproduce these findings are available in the Open Data section.


