Abstract
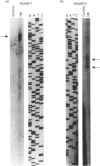
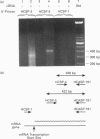
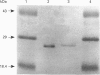
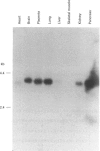
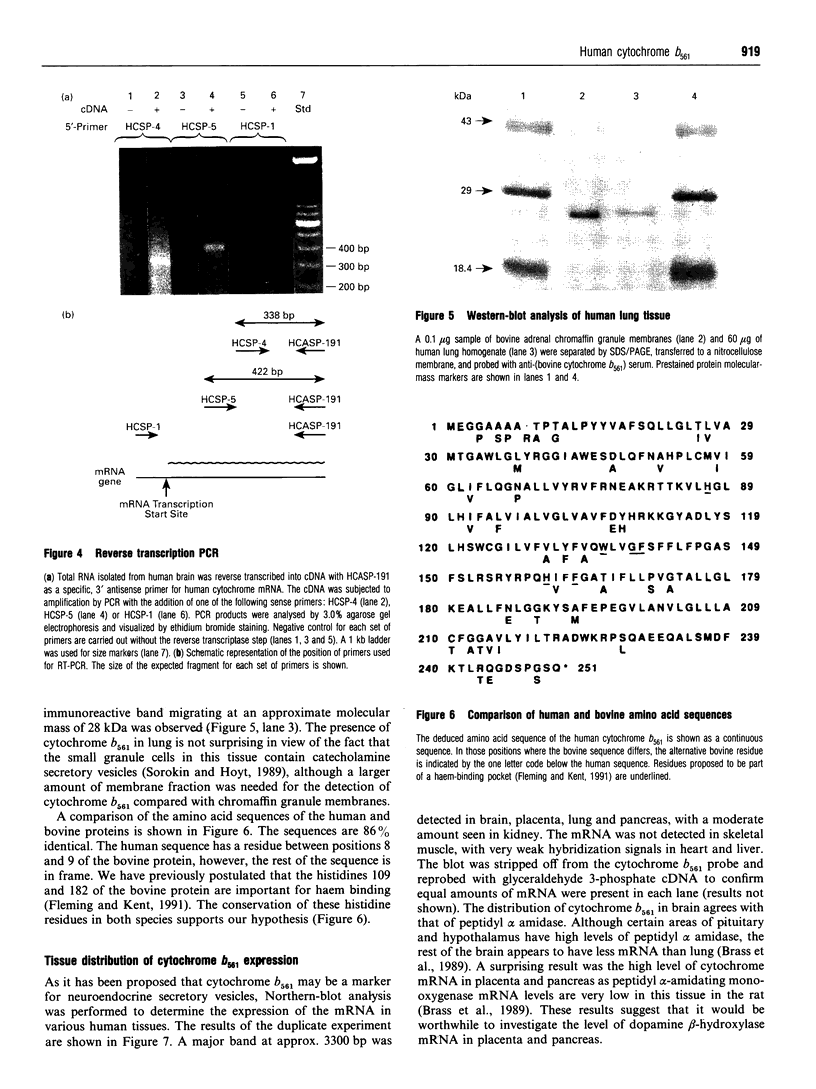
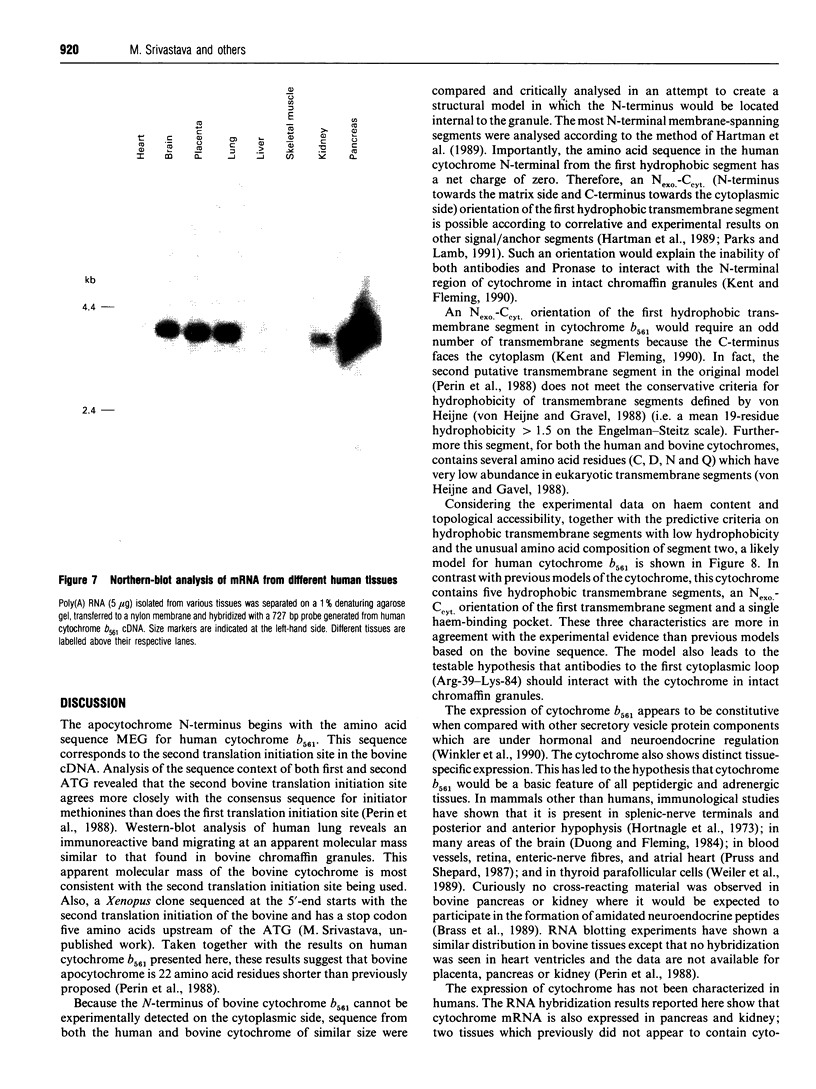
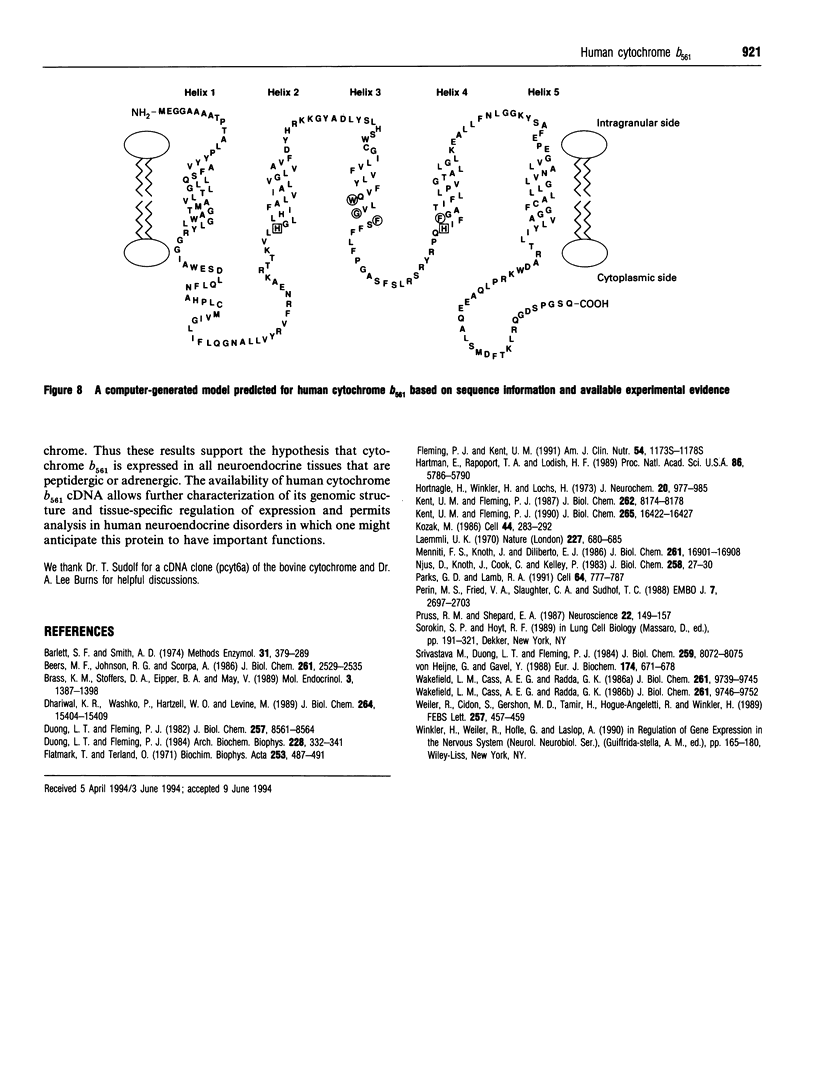
Cytochrome b561 is a major transmembrane protein of catecholamine and neuropeptide secretory vesicles. In this report, we describe the cloning and properties of a full-length cDNA encoding human neuroendocrine cytochrome b561 from a human caudate cDNA library and a human peripheral blood genomic library. The human cDNA contains two major transcription start sites and only one translation start site that codes for an apocytochrome b561, which is 22 amino acid residues smaller than the previously deduced amino acid sequence from bovine cDNA. This smaller version of cytochrome b561 may contain only five transmembrane segments rather than the previously proposed six segments. The new model is in agreement with our previous results on transmembrane topology of the gene product. Northern-blot analysis shows an expanded tissue distribution of cytochrome mRNA expression where previous immunological assays were negative. These results support the hypothesis that cytochrome b561 is a marker for peptidergic and adrenergic tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett S. F., Smith A. D. Adrenal chromaffin granules: isolation and disassembly. Methods Enzymol. 1974;31:379–389. doi: 10.1016/0076-6879(74)31042-7. [DOI] [PubMed] [Google Scholar]
- Beers M. F., Johnson R. G., Scarpa A. Evidence for an ascorbate shuttle for the transfer of reducing equivalents across chromaffin granule membranes. J Biol Chem. 1986 Feb 25;261(6):2529–2535. [PubMed] [Google Scholar]
- Braas K. M., Stoffers D. A., Eipper B. A., May V. Tissue specific expression of rat peptidylglycine alpha-amidating monooxygenase activity and mRNA. Mol Endocrinol. 1989 Sep;3(9):1387–1398. doi: 10.1210/mend-3-9-1387. [DOI] [PubMed] [Google Scholar]
- Dhariwal K. R., Washko P., Hartzell W. O., Levine M. Ascorbic acid within chromaffin granules. In situ kinetics of norepinephrine biosynthesis. J Biol Chem. 1989 Sep 15;264(26):15404–15409. [PubMed] [Google Scholar]
- Duong L. T., Fleming P. J. Isolation and properties of cytochrome b561 from bovine adrenal chromaffin granules. J Biol Chem. 1982 Aug 10;257(15):8561–8564. [PubMed] [Google Scholar]
- Duong L. T., Fleming P. J. The asymmetric orientation of cytochrome b561 in bovine chromaffin granule membranes. Arch Biochem Biophys. 1984 Jan;228(1):332–341. doi: 10.1016/0003-9861(84)90074-2. [DOI] [PubMed] [Google Scholar]
- Flatmark T., Terland O. Cytochrome b 561 of the bovine adrenal chromaffin granules. A high potential b-type cytochrome. Biochim Biophys Acta. 1971 Dec 7;253(2):487–491. doi: 10.1016/0005-2728(71)90052-1. [DOI] [PubMed] [Google Scholar]
- Fleming P. J., Kent U. M. Cytochrome b561, ascorbic acid, and transmembrane electron transfer. Am J Clin Nutr. 1991 Dec;54(6 Suppl):1173S–1178S. doi: 10.1093/ajcn/54.6.1173s. [DOI] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörtnagl H., Winkler H., Lochs H. Immunological studies on a membrane protein (chromomembrin B) of catecholamine-storing vesicles. J Neurochem. 1973 Apr;20(4):977–985. doi: 10.1111/j.1471-4159.1973.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Kent U. M., Fleming P. J. Cytochrome b561 is fatty acylated and oriented in the chromaffin granule membrane with its carboxyl terminus cytoplasmically exposed. J Biol Chem. 1990 Sep 25;265(27):16422–16427. [PubMed] [Google Scholar]
- Kent U. M., Fleming P. J. Purified cytochrome b561 catalyzes transmembrane electron transfer for dopamine beta-hydroxylase and peptidyl glycine alpha-amidating monooxygenase activities in reconstituted systems. J Biol Chem. 1987 Jun 15;262(17):8174–8178. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Menniti F. S., Knoth J., Diliberto E. J., Jr Role of ascorbic acid in dopamine beta-hydroxylation. The endogenous enzyme cofactor and putative electron donor for cofactor regeneration. J Biol Chem. 1986 Dec 25;261(36):16901–16908. [PubMed] [Google Scholar]
- Njus D., Knoth J., Cook C., Kelly P. M. Electron transfer across the chromaffin granule membrane. J Biol Chem. 1983 Jan 10;258(1):27–30. [PubMed] [Google Scholar]
- Parks G. D., Lamb R. A. Topology of eukaryotic type II membrane proteins: importance of N-terminal positively charged residues flanking the hydrophobic domain. Cell. 1991 Feb 22;64(4):777–787. doi: 10.1016/0092-8674(91)90507-u. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Slaughter C. A., Südhof T. C. The structure of cytochrome b561, a secretory vesicle-specific electron transport protein. EMBO J. 1988 Sep;7(9):2697–2703. doi: 10.1002/j.1460-2075.1988.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss R. M., Shepard E. A. Cytochrome b561 can be detected in many neuroendocrine tissues using a specific monoclonal antibody. Neuroscience. 1987 Jul;22(1):149–157. doi: 10.1016/0306-4522(87)90205-3. [DOI] [PubMed] [Google Scholar]
- Srivastava M., Duong L. T., Fleming P. J. Cytochrome b561 catalyzes transmembrane electron transfer. J Biol Chem. 1984 Jul 10;259(13):8072–8075. [PubMed] [Google Scholar]
- Wakefield L. M., Cass A. E., Radda G. K. Electron transfer across the chromaffin granule membrane. Use of EPR to demonstrate reduction of intravesicular ascorbate radical by the extravesicular mitochondrial NADH:ascorbate radical oxidoreductase. J Biol Chem. 1986 Jul 25;261(21):9746–9752. [PubMed] [Google Scholar]
- Wakefield L. M., Cass A. E., Radda G. K. Functional coupling between enzymes of the chromaffin granule membrane. J Biol Chem. 1986 Jul 25;261(21):9739–9745. [PubMed] [Google Scholar]
- Weiler R., Cidon S., Gershon M. D., Tamir H., Hogue-Angeletti R., Winkler H. Adrenal chromaffin granules and secretory granules from thyroid parafollicular cells have several common antigens. FEBS Lett. 1989 Nov 6;257(2):457–459. doi: 10.1016/0014-5793(89)81595-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988 Jul 1;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]