Abstract
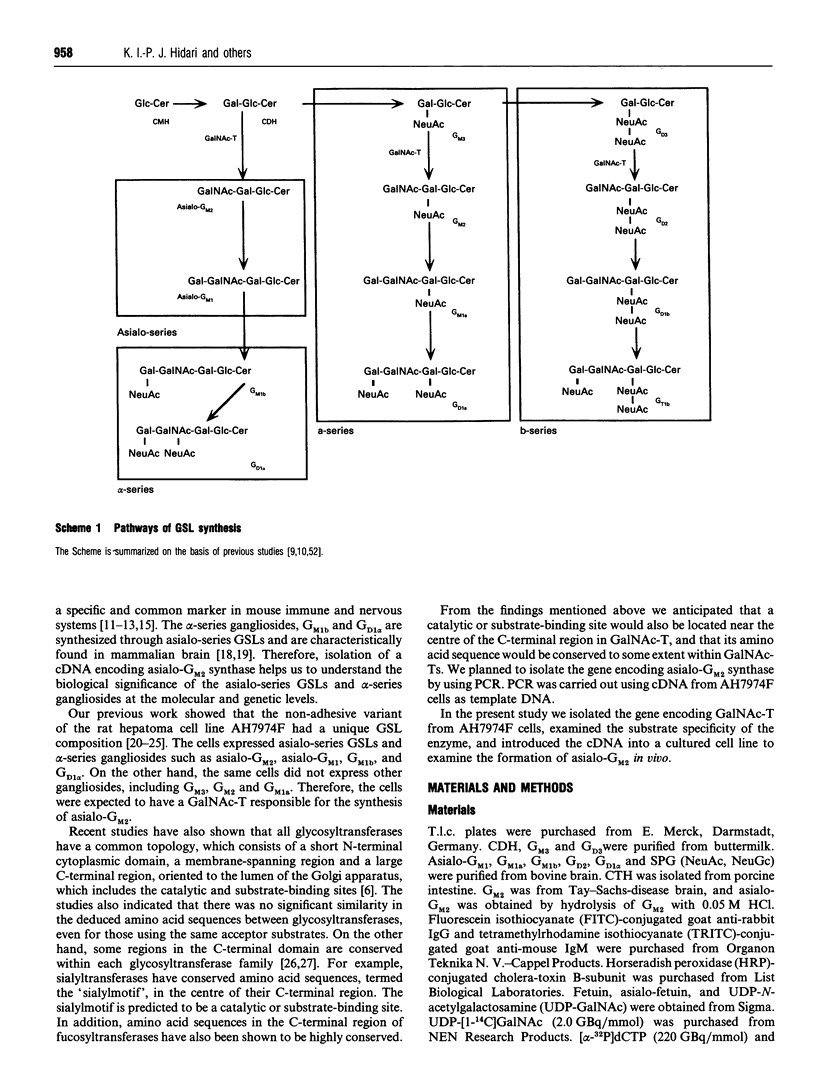
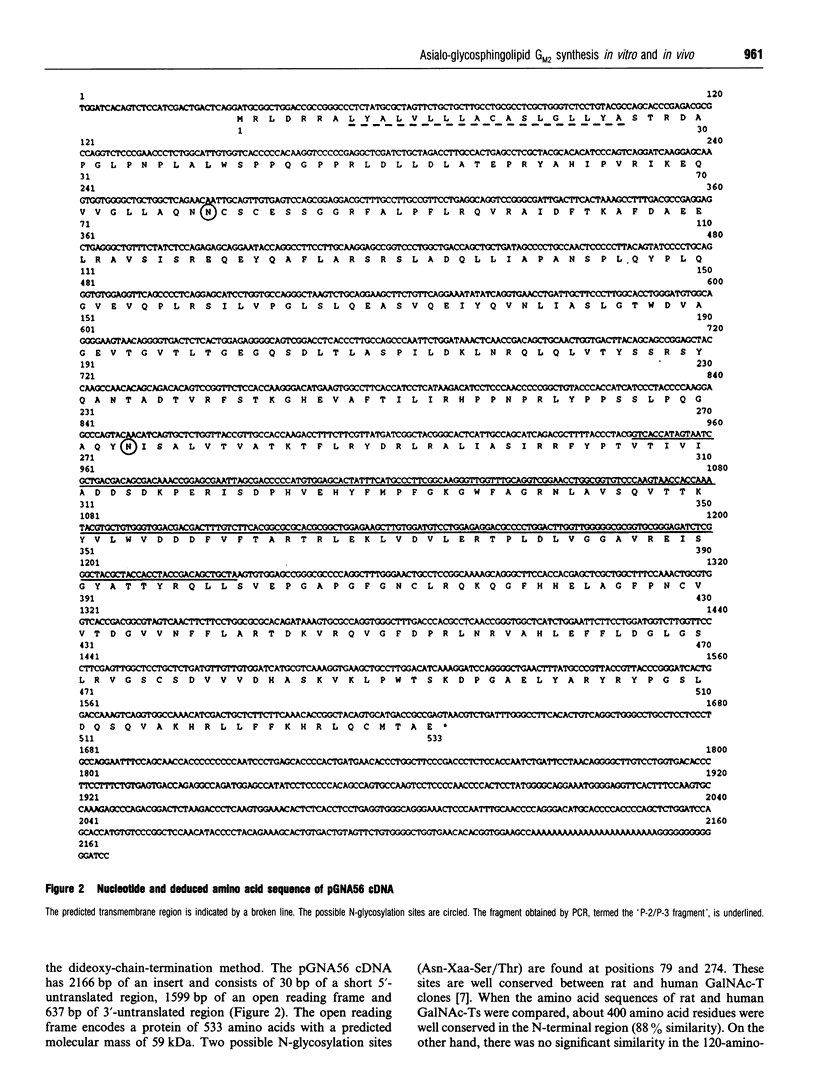
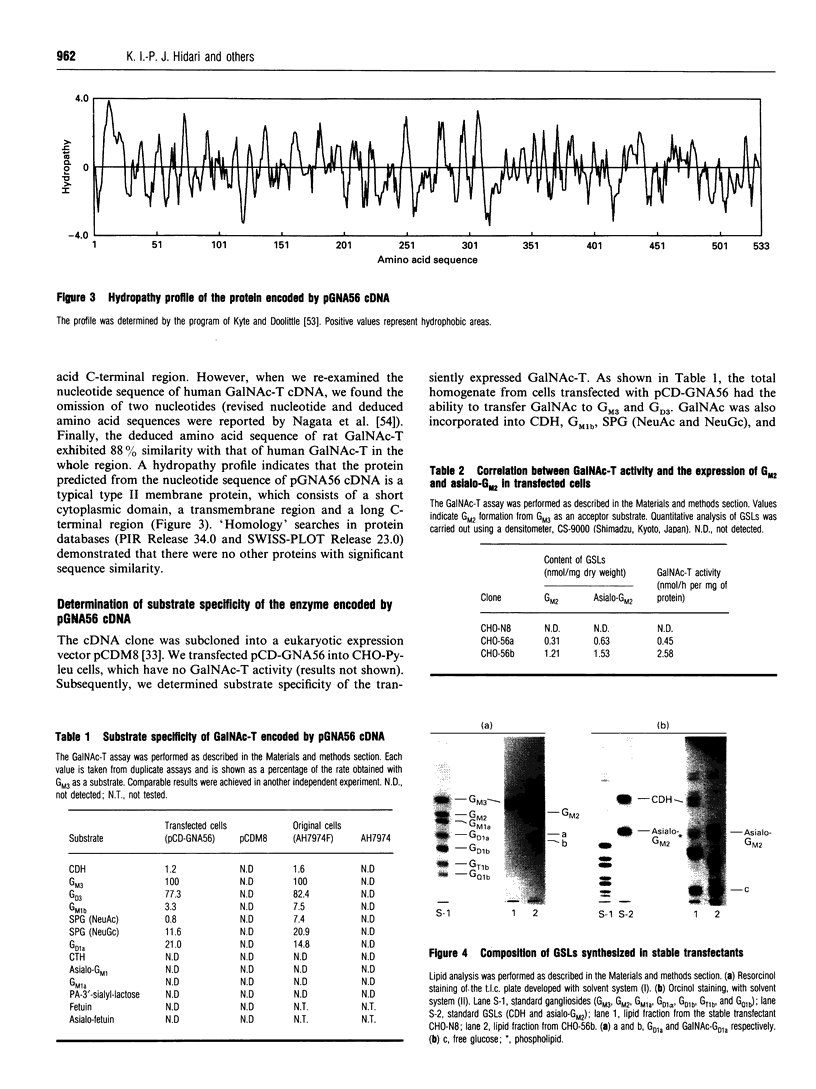
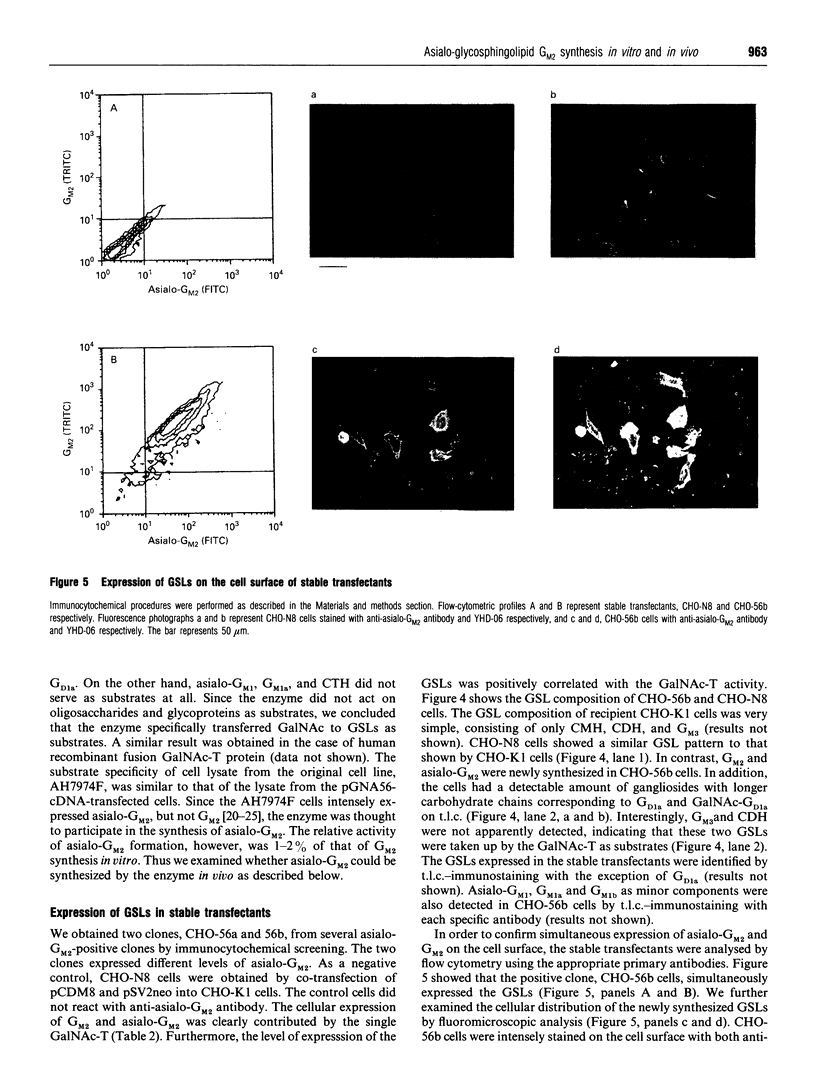
We have cloned a cDNA encoding beta 1-4N-acetylgalactosaminyltransferase (EC 2.4.1.92) (GalNAc-T) from rat ascites hepatoma of the free-cell type AH7974F. The cell line only expressed asialo-series glycosphingolipids (GSLs) including asialo-GM2 [Taki, T., Hirabayashi, Y., Ishiwata, Y., Matsumoto, M., and Kojima, K. (1979) Biochim. Biophys. Acta 572, 113-120]. The cDNA, pGNA56, was isolated by screening AH7974F cDNA library in lambda gt10 with a probe. The probe was obtained from AH7974F cDNA by PCR using primers with the nucleotide sequence of the human GalNAc-T cDNA. The amino acid sequence deduced from the nucleotide sequence of pGNA56 exhibited 88% similarity to the human GalNAc-T sequence. The enzyme was a typical type II membrane protein, which consisted of a short N-terminal residue, a transmembrane region, and a long C-terminal residue, including the catalytic domain. The substrate specificity of rat GalNAc-T was determined using homogenates from cells into which the cDNA clone was transfected. The enzyme catalysed not only the formation of GM2 and GD2 from GM3 and GD3 respectively, but also asialo-GM2 from CDH. It also acted on GSL substrates, including GM1b, sialylparagloboside and GD1 alpha. On the other hand, the enzyme did not transfer GalNAc to soluble substrates such as glycoproteins and oligosaccharide. The GSL compositional and immunocytochemical analyses of stable transfectants obtained by transfection of the cDNA showed simultaneous expression of asialo-GM2 and GM2 on the plasma membrane. Therefore, we concluded that the formation of asialo-GM2, GM2 and GD2 was catalysed by the single GalNAc-T. Northern-blot hybridization showed that the GalNAc-T mRNA was strongly expressed in rat brain, testis, and spleen. The gene was also expressed in rat normal liver to a lesser extent. We found the GSLs in asialo- and alpha-pathways such as asialo-GM1 and GD1 alpha in the rat tissues by using a sensitive t.l.c.-immunostaining method. These observations also supported our conclusion that the single GalNAc-T synthesizes asialo-GM2, GM2 and GD2 in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bierhuizen M. F., Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal beta 1-3-GalNAc-R (GlcNAc to GalNAc) beta 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D., Piller F., Gillard B., Marcus D., Cartron J. P. Identification of a novel ganglioside on erythrocytes with blood group Cad specificity. J Biol Chem. 1985 Jul 5;260(13):7813–7816. [PubMed] [Google Scholar]
- Dennert G., Yogeeswaran G., Yamagata S. Cloned cell lines with natural killer activity. Specificity, function, and cell surface markers. J Exp Med. 1981 Mar 1;153(3):545–556. doi: 10.1084/jem.153.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi T., Hanai N., Yamaguchi K., Oshima M. Localization of UDP-GalNAc:NeuAc alpha 2,3Gal-R beta 1,4(GalNAc to Gal)N-acetylgalactosaminyltransferase in human stomach. Enzymatic synthesis of a fundic gland-specific ganglioside and GM2. J Biol Chem. 1991 Dec 15;266(35):24038–24043. [PubMed] [Google Scholar]
- Dohi T., Nishikawa A., Ishizuka I., Totani M., Yamaguchi K., Nakagawa K., Saitoh O., Ohshiba S., Oshima M. Substrate specificity and distribution of UDP-GalNAc:sialylparagloboside N-acetylgalactosaminyltransferase in the human stomach. Biochem J. 1992 Nov 15;288(Pt 1):161–165. doi: 10.1042/bj2880161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Higashi H., Fukui Y., Ueda S., Kato S., Hirabayashi Y., Matsumoto M., Naiki M. Sensitive enzyme-immunostaining and densitometric determination on thin-layer chromatography of N-glycolylneuraminic acid-containing glycosphingolipids, Hanganutziu-Deicher antigens. J Biochem. 1984 May;95(5):1517–1520. doi: 10.1093/oxfordjournals.jbchem.a134760. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y., Hyogo A., Nakao T., Tsuchiya K., Suzuki Y., Matsumoto M., Kon K., Ando S. Isolation and characterization of extremely minor gangliosides, GM1b and GD1 alpha, in adult bovine brains as developmentally regulated antigens. J Biol Chem. 1990 May 15;265(14):8144–8151. [PubMed] [Google Scholar]
- Hirabayashi Y., Nakao T., Matsumoto M., Obata K., Ando S. Improved method for large-scale purification of brain gangliosides by Q-sepharose column chromatography. Immunochemical detection of C-series polysialogangliosides in adult bovine brains. J Chromatogr. 1988 Jul 22;445(2):377–384. doi: 10.1016/s0021-9673(01)84550-7. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y., Taki T., Matsumoto M., Kojima K. Comparative study on glycolipid composition between two cell types of rat ascites hepatoma cells. Biochim Biophys Acta. 1978 Apr 28;529(1):96–105. [PubMed] [Google Scholar]
- Hirabayashi Y., Taki T., Matsumoto M. Tumor ganglioside - natural occurrence of GM1b. FEBS Lett. 1979 Apr 15;100(2):253–257. doi: 10.1016/0014-5793(79)80345-2. [DOI] [PubMed] [Google Scholar]
- Iwamori M., Shimomura J., Tsuyuhara S., Nagai Y. Gangliosides of various rat tissues: distribution of ganglio-N-tetraose-containing gangliosides and tissue-characteristic composition of gangliosides. J Biochem. 1984 Mar;95(3):761–770. doi: 10.1093/oxfordjournals.jbchem.a134667. [DOI] [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Koizumi K., Ito Y., Kojima K., Fujii T. Isolation and characterization of the plasma membranes from rat ascites hepatomas and from normal rat livers, including newborn, regenerating, and adult livers. J Biochem. 1976 Apr;79(4):739–748. doi: 10.1093/oxfordjournals.jbchem.a131126. [DOI] [PubMed] [Google Scholar]
- Kojima N., Hakomori S. Cell adhesion, spreading, and motility of GM3-expressing cells based on glycolipid-glycolipid interaction. J Biol Chem. 1991 Sep 15;266(26):17552–17558. [PubMed] [Google Scholar]
- Kojima N., Hakomori S. Specific interaction between gangliotriaosylceramide (Gg3) and sialosyllactosylceramide (GM3) as a basis for specific cellular recognition between lymphoma and melanoma cells. J Biol Chem. 1989 Dec 5;264(34):20159–20162. [PubMed] [Google Scholar]
- Kusunoki S., Tsuji S., Nagai Y. Ganglio-N-tetraosylceramide (asialo GM1), an antigen common to the brain and immune system: its localization in myelin. Brain Res. 1985 May 13;334(1):117–124. doi: 10.1016/0006-8993(85)90573-6. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Malagolini N., Dall'Olio F., Di Stefano G., Minni F., Marrano D., Serafini-Cessi F. Expression of UDP-GalNAc:NeuAc alpha 2,3Gal beta-R beta 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989 Dec 1;49(23):6466–6470. [PubMed] [Google Scholar]
- Matsumoto M., Taki T., Kojima K. Study on sialic acid-containing glycolipid in rat ascites hepatoma cells. Jpn J Exp Med. 1976 Apr;46(2):135–138. [PubMed] [Google Scholar]
- Nagata Y., Yamashiro S., Yodoi J., Lloyd K. O., Shiku H., Furukawa K. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J Biol Chem. 1992 Jun 15;267(17):12082–12089. [PubMed] [Google Scholar]
- Nagata Y., Yamashiro S., Yodoi J., Lloyd K. O., Shiku H., Furukawa K. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J Biol Chem. 1994 Mar 4;269(9):7045–7045. [PubMed] [Google Scholar]
- Okada Y., Matsuura H., Hakomori S. Inhibition of tumor cell growth by aggregation of a tumor-associated glycolipid antigen: a close functional association between gangliotriaosylceramide and transferrin receptor in mouse lymphoma L-5178Y. Cancer Res. 1985 Jun;45(6):2793–2801. [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Piller F., Blanchard D., Huet M., Cartron J. P. Identification of a alpha-NeuAc-(2----3)-beta-D-galactopyranosyl N-acetyl-beta-D-galactosaminyltransferase in human kidney. Carbohydr Res. 1986 Jun 1;149(1):171–184. doi: 10.1016/s0008-6215(00)90376-8. [DOI] [PubMed] [Google Scholar]
- Pohlentz G., Klein D., Schmitz D., Schwarzmann G., Peter-Katalinić J., Sandhoff K. Biosynthesis of gangliosides from asialogangliosides in rat liver Golgi vesicles. Biol Chem Hoppe Seyler. 1988 Jan;369(1):55–63. doi: 10.1515/bchm3.1988.369.1.55. [DOI] [PubMed] [Google Scholar]
- Pohlentz G., Klein D., Schwarzmann G., Schmitz D., Sandhoff K. Both GA2, GM2, and GD2 synthases and GM1b, GD1a, and GT1b synthases are single enzymes in Golgi vesicles from rat liver. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7044–7048. doi: 10.1073/pnas.85.19.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokukawa C., Kushi Y., Ueno K., Handa S. Structural study on gangliosides from rat liver and erythrocytes. J Biochem. 1982 Nov;92(5):1481–1488. doi: 10.1093/oxfordjournals.jbchem.a134072. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanai Y., Yamasaki M., Nagai Y. Monoclonal antibody directed to a Hanganutziu-Deicher active ganglioside, GM2 (NeuGc). Biochim Biophys Acta. 1988 Feb 19;958(3):368–374. doi: 10.1016/0005-2760(88)90222-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Senn H. J., Cooper C., Warnke P. C., Wagner M., Decker K. Ganglioside biosynthesis in rat liver. Characterization of UDP-N-acetylgalactosamine -- GM3 acetylgalactosaminyltransferase. Eur J Biochem. 1981 Nov;120(1):59–67. doi: 10.1111/j.1432-1033.1981.tb05670.x. [DOI] [PubMed] [Google Scholar]
- Smith P. L., Baenziger J. U. A pituitary N-acetylgalactosamine transferase that specifically recognizes glycoprotein hormones. Science. 1988 Nov 11;242(4880):930–933. doi: 10.1126/science.2460923. [DOI] [PubMed] [Google Scholar]
- Smith P. L., Baenziger J. U. Molecular basis of recognition by the glycoprotein hormone-specific N-acetylgalactosamine-transferase. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):329–333. doi: 10.1073/pnas.89.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. L., Baenziger J. U. Recognition by the glycoprotein hormone-specific N-acetylgalactosaminetransferase is independent of hormone native conformation. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7275–7279. doi: 10.1073/pnas.87.18.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman D. J., Milman G. Short-term, high-efficiency expression of transfected DNA. Mol Cell Biol. 1984 Aug;4(8):1641–1643. doi: 10.1128/mcb.4.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya A., Hosomi O., Kogure T. Identification and characterization of UDP-GalNAc: NeuAc alpha 2-3Gal beta 1-4Glc(NAc) beta 1-4(GalNAc to Gal)N-acetylgalactosaminyltransferase in human blood plasma. J Biochem. 1987 Jan;101(1):251–259. doi: 10.1093/oxfordjournals.jbchem.a121898. [DOI] [PubMed] [Google Scholar]
- Taki T., Hirabayashi Y., Ishikawa H., Ando S., Kon K., Tanaka Y., Matsumoto M. A ganglioside of rat ascites hepatoma AH 7974F cells. Occurrence of a novel disialoganglioside (GD1 alpha) with a unique N-acetylneuraminosyl (alpha 2-6)-N-acetylgalactosamine structure. J Biol Chem. 1986 Mar 5;261(7):3075–3078. [PubMed] [Google Scholar]
- Taki T., Hirabayashi Y., Ishiwata Y., Matsumoto M., Kojima K. Biosynthesis of different gangliosides in two types of rat ascites hepatoma cells with different degrees of cell adhesiveness. Biochim Biophys Acta. 1979 Jan 29;572(1):113–120. [PubMed] [Google Scholar]
- Taki T., Hirabayashi Y., Matsumoto M., Kojima K. Enzymic synthesis of a new type of fucose-containing glycolipid with fucosyltransferase of rat ascites hepatoma cell, AH 7974F. Biochim Biophys Acta. 1979 Jan 29;572(1):105–112. [PubMed] [Google Scholar]
- Taki T., Hirabayashi Y., Suzuki Y., Matsumoto M., Kojima K. Comparative study of glycolipid compositions of plasma membranes among two types of rat ascites hepatoma and normal rat liver. J Biochem. 1978 May;83(5):1517–1520. doi: 10.1093/oxfordjournals.jbchem.a132062. [DOI] [PubMed] [Google Scholar]
- Ueno K., Kushi Y., Rokukawa C., Handa S. Distribution of gangliosides in parenchymal and non-parenchymal cells of rat liver. Biochem Biophys Res Commun. 1982 Mar 30;105(2):681–687. doi: 10.1016/0006-291x(82)91488-7. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D. X., Livingston B. D., Medzihradszky K. F., Kelm S., Burlingame A. L., Paulson J. C. Primary structure of Gal beta 1,3(4)GlcNAc alpha 2,3-sialyltransferase determined by mass spectrometry sequence analysis and molecular cloning. Evidence for a protein motif in the sialyltransferase gene family. J Biol Chem. 1992 Oct 15;267(29):21011–21019. [PubMed] [Google Scholar]
- Weston B. W., Nair R. P., Larsen R. D., Lowe J. B. Isolation of a novel human alpha (1,3)fucosyltransferase gene and molecular comparison to the human Lewis blood group alpha (1,3/1,4)fucosyltransferase gene. Syntenic, homologous, nonallelic genes encoding enzymes with distinct acceptor substrate specificities. J Biol Chem. 1992 Feb 25;267(6):4152–4160. [PubMed] [Google Scholar]
- Yanagisawa K., Taniguchi N., Makita A. Purification and properties of GM2 synthase, UDP-N-acetylgalactosamine: GM3 beta-N-acetylgalactosaminyltransferase from rat liver. Biochim Biophys Acta. 1987 Jun 23;919(3):213–220. doi: 10.1016/0005-2760(87)90260-8. [DOI] [PubMed] [Google Scholar]
- Young W. W., Jr, Hakomori S. I., Durdik J. M., Henney C. S. Identification of ganglio-N-tetraosylceramide as a new cell surface marker for murine natural killer (NK) cells. J Immunol. 1980 Jan;124(1):199–201. [PubMed] [Google Scholar]
- van Echten G., Sandhoff K. Ganglioside metabolism. Enzymology, Topology, and regulation. J Biol Chem. 1993 Mar 15;268(8):5341–5344. [PubMed] [Google Scholar]





