ABSTRACT
Biliary tract cancer (BTC) is a rare cancer with poor prognosis, characterized by considerable pathophysiological and molecular heterogeneity. While this makes it difficult to treat, it also provides targeted therapy opportunities. Current standard-of-care is chemotherapy ± immunotherapy, but several targeted agents have recently been approved. The current investigational landscape in BTC emphasizes the importance of biomarker testing at diagnosis. MDM2/MDMX are important negative regulators of the tumor suppressor p53 and provide an additional target in BTC (∼5–8% of tumors are MDM2-amplified). Brigimadlin (BI 907828) is a highly potent MDM2-p53 antagonist that has shown antitumor activity in preclinical studies and promising results in early clinical trials; enrollment is ongoing in a potential registrational trial for patients with BTC.
Keywords: : BI 90782823, biliary tract cancer, brigimadlin, MDM2–p53, plain-language summary, targeted therapy
Plain language summary
Article Highlights.
Biliary tract cancer (BTC), an umbrella term for a number of tumor sub-types, is a rare cancer with a poor prognosis (5-year survival rate: 15% [1]).
It is characterized by considerable pathophysiological and molecular heterogeneity, making it difficult to treat, but providing a number of potentially targetable genetic alterations.
Treatment options for patients with advanced BTC: recent developments
No targeted agents are approved in the first-line setting for patients with BTC; chemotherapy-based regimens with or without immunotherapy are the standard of care.
Clinical trials into alternative immunochemotherapy regimens and FGFR2 inhibitors are ongoing, which may impact on first-line treatment options in the future.
Second-line treatment options are largely chemotherapy-based for patients without targetable mutations, or targeted agents against FGFR2, IDH1, HER2, BRAF V600E, NTRK, DNA damage repair genes, KRAS and RET aberrations, some of which are investigational.
The rationale for targeting MDM2 in patients with BTC
MDM2 is an endogenous negative regulator of p53, hence aberrations of the MDM2 gene can result in inappropriate silencing of wild-type p53, potentially leading to tumorigenesis.
MDM2 amplification is observed in ∼5–8% of BTCs; TP53 mutations are largely mutually exclusive.
The clinical development of MDM2–p53 antagonists
MDM2–p53 antagonists are in clinical development for the treatment of various hematologic and solid tumors.
Brigimadlin, an MDM2–p53 antagonist currently in development in patients with BTC, has shown durable responses and no unexpected toxicities across two phase Ia/Ib studies in patients with solid tumors, including a number of patients with BTC.
Molecular testing for BTC
Comprehensive molecular testing is a key recommended in treatment guidelines for BTC.
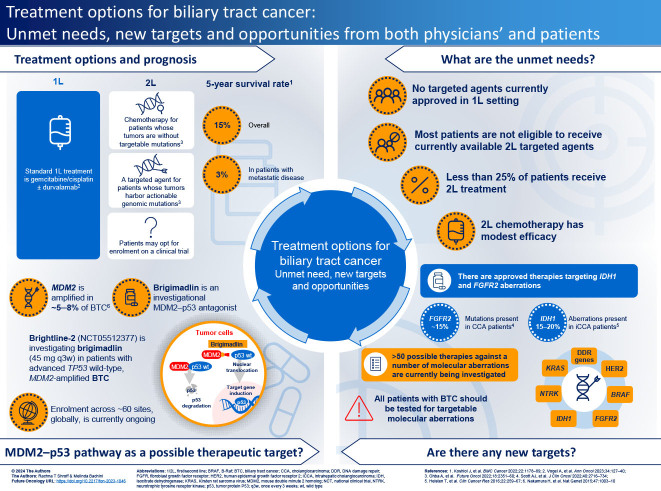
Infographic
Infographic:
A PDF version of this infographic is available as supplemental material.
1. Introduction
Biliary tract cancer (BTC) is an umbrella term for a heterogeneous group of invasive adenocarcinomas, including intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA), gallbladder carcinoma (GC) and ampullary carcinoma (AC). Although it is a relatively rare malignancy, recent epidemiological research in the USA indicates that its incidence, especially that of iCCA, is increasing [1]. Moreover, despite recent treatment advances, prognosis remains discouraging with a 5-year survival rate of only 15%. Prognosis is particularly poor in patients with iCCA (5-year survival: 8.5%), and in patients with distant disease (3%) [1]. There is a clear unmet need, therefore, for improved treatment options for patients with BTC.
BTC is characterized by considerable pathophysiological and molecular heterogeneity. Hence, it is difficult to treat, especially in patients with advanced disease where resection is not possible. Currently approved standard-of-care first-line treatment for patients who are ineligible for resection is chemotherapy with gemcitabine/cisplatin combined with the programmed cell death ligand 1 (PD-L1) inhibitor, durvalumab [2–5]. Additionally, the combination of gemcitabine/cisplatin plus pembrolizumab has recently been approved for locally advanced unresectable or metastatic BTC by the US FDA, with improved overall survival (OS) versus gemcitabine/cisplatin plus placebo shown in the KEYNOTE-966 trial [6,7].
BTC has a high rate of potentially actionable genetic alterations (around 50% of tumors [2]), so second-line treatment options vary from patient to patient depending on whether alterations, such as FGFR2 or IDH1 aberrations, are detected [3]. The recent availability of approved targeted agents, such as the FGFR2 inhibitors, pemigatinib and futibatinib, and the IDH1 inhibitor, ivosidenib, has certainly been a step forward, and these advances reflect progress in the understanding of the molecular pathogenesis of BTC.
Given the availability of targeted agents for BTC, it is important that tumors undergo comprehensive genomic characterization at diagnosis; however, this is not always possible. Many tumor biopsy samples do not provide enough material for genomic testing [8], and repeated invasive biopsies are not always possible or, indeed, advisable, as they can carry a risk of complications for patients. A further problem is that genetic aberrations may be sub-clonal and only present in a proportion of tumor cells and, hence, might not be detected [8]. Even if biopsy material is available, barriers to the use of next-generation sequencing (NGS) platforms may exist in some countries owing to reimbursement and other issues [9].
Notwithstanding the emergence of precision medicine strategies in BTC, the unmet need for additional treatment options is considerable because, currently, only around 50% of patients have targetable molecular aberrations [2]. At present, therefore, the only available second-line treatment options for most patients are chemotherapy regimens, such as FOLFOX (leucovorin [folinic acid], 5-FU, oxaliplatin) [10], CAPIRI (capecitabine, irinotecan) [11], 5-Fu/LV, nal-IRI (leucovorin, 5-FU, and nanoliposomal irinotecan) [12], gemcitabine, S-1 (mostly Japan), or irinotecan monotherapy [2]. Such regimens carry a significant toxicity burden and, accordingly, less than a quarter of patients are deemed sufficiently fit to receive second-line treatment [2]. Furthermore, efficacy benefits with these agents are usually modest [2]. Consequently, intense research is ongoing, both in terms of further molecular characterization of BTC and the clinical development of agents that target specific pathways in second-line and, ultimately, first-line settings (Supplementary Table S1).
In this review article, we summarize advances in the characterization of novel drug targets for the treatment of BTC and highlight important recent and ongoing clinical trials. In particular, we focus on potential strategies for the stimulation of p53-mediated pro-apoptotic signaling in BTC tumor cells. Finally, we discuss the importance of molecular testing and how recent developments in liquid biopsy strategies could increase the detection rate of important molecular aberrations. For each section, we provide a plain language summary of the implications of recent developments for patients and their caregivers.
2. Treatment options for patients with advanced BTC: recent developments
Several comprehensive reviews of current therapeutic options have recently been published and describe how the treatment paradigm for BTC has changed radically with the introduction of targeted agents [2–4]. Figure 1 summarizes an updated approach to the management of BTC in patients with and without targetable mutations, and here we provide a brief overview of the very latest developments in first- and second-line settings.
Figure 1.
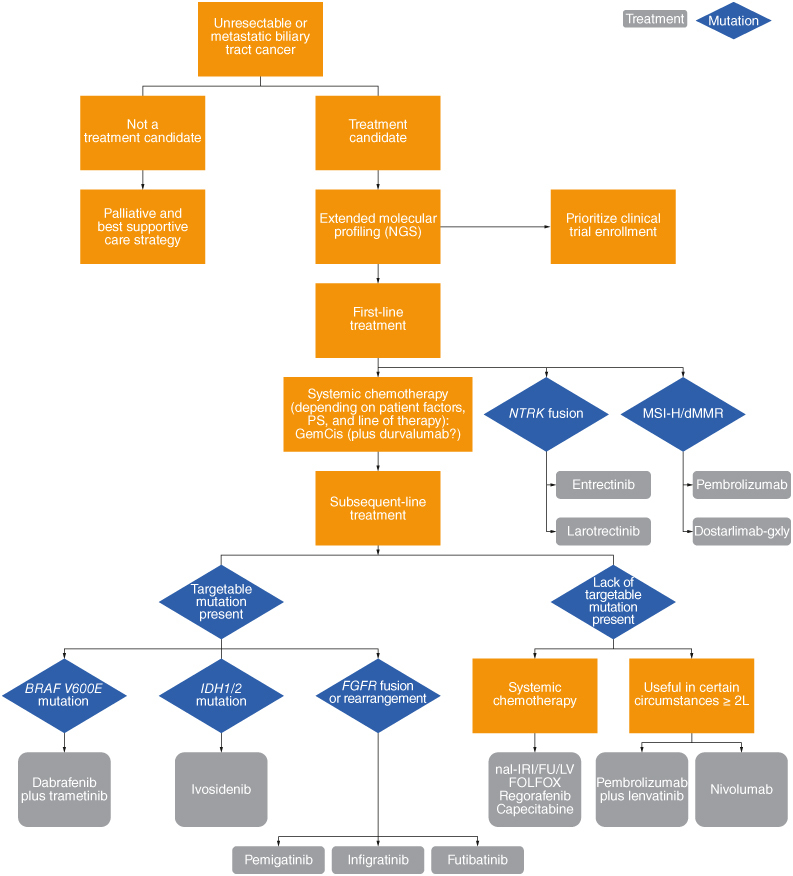
Framework for the approach to managing biliary tract cancer.
2L: Second-line treatment; dMMR: Deficient mismatch repair; MSI-H: Microsatellite instability-high; NGS: Next-generation sequencing; PS: Performance score.
Adapted from Scott et al. 2022 with permission from [3].
2.1. First-line treatment
In September 2022, the immune checkpoint inhibitor, durvalumab, was approved for the first-line treatment of patients with locally advanced or metastatic BTC when combined with gemcitabine and cisplatin [13]. This approval was based on the phase III TOPAZ-1 trial, which demonstrated significant OS benefit with this immunochemotherapy combination versus chemotherapy alone, with median OS of 12.8 versus 11.5 months, respectively (hazard ratio [HR]: 0.80; 95% CI: 0.66–0.97) [14]. OS and PFS generally favored durvalumab in the prespecified subgroups tested, including those based on primary tumor location and PD-L1 expression levels. However, the overall response rate (ORR) with durvalumab combination therapy was modest at 27%, suggesting that BTC may be a relatively immune-resistant tumor type compared with other malignancies.
In October 2023, the immune checkpoint inhibitor pembrolizumab, in combination with gemcitabine/cisplatin, was approved by the FDA for treatment of locally advanced, unresectable or metastatic biliary tract cancer, based on the results of the KEYNOTE-966 trial [7]. In KEYNOTE-966, addition of the immune checkpoint inhibitor, pembrolizumab, to gemcitabine and cisplatin also significantly improved OS versus chemotherapy alone (median 12.7 vs 10.9 months; HR: 0.83; 95% CI: 0.72–0.95) without any new safety signals. However, there was no significant difference in response rate between the two groups [6]. Of note, KEYNOTE-966 had a different design to TOPAZ-1 in terms of maintenance therapy. In TOPAZ-1, patients received up to 8 cycles of gemcitabine plus cisplatin, and then maintenance durvalumab monotherapy [14]. In KEYNOTE-966, patients received gemcitabine as a maintenance therapy with or without pembrolizumab. Another ongoing phase III study is assessing the combination of envafolimab, a checkpoint inhibitor, and gemcitabine/oxaliplatin in patients with BTC (NCT03478488; results expected in 2024).
In addition, triplet chemotherapy regimens are of interest and are under investigation in phase III trials to determine whether additional agents like S-1 [15] or nab-paclitaxel (SWOG S1815; NCT03768414) might improve outcomes with gemcitabine and cisplatin [5]. However, initial results from SWOG S1815 in newly diagnosed patients with advanced BTC showed that there was no statistically significant improvement in median OS with triplet chemotherapy (cisplatin/gemcitabine/nab-paclitaxel) versus gemcitabine/cisplatin (HR: 0.93; 95% CI: 0.74–1.19; p = 0.58) [16].
Currently, no targeted agents are approved in a first-line setting. The FGFR2 inhibitors, futibatinib and pemigatinib, are being assessed as first-line monotherapy versus gemcitabine plus cisplatin in patients with advanced BTC harboring FGFR2 gene rearrangements in two ongoing trials (FOENIX-CCA3, NCT04093362; FIGHT-302, NCT03656536). However, data from these trials are not expected until 2027. Therefore, systemic chemotherapy-based regimens, with or without immunotherapy, will remain standard treatment for patients with advanced disease for the foreseeable future, regardless of the molecular characteristics of the tumor.
2.2. Patient perspective on first-line therapy
The approval of durvalumab in combination with cisplatin and gemcitabine as a first-line treatment (the initial treatment that a person receives after their diagnosis) for BTC has been the only significant change in the standard of care for BTC in many years. More recently, combination therapy with pembrolizumab plus cisplatin and gemcitabine has also been approved for treatment of BTC. However, progress still needs to be made. Several clinical trials are ongoing that are looking at different chemotherapy combinations with or without drugs that target specific aspects of the cancer, such as the immune system drugs mentioned above, or drugs that target cancer-causing mutations (Supplementary Table S1).
The number of trials testing drugs to treat BTC that are currently underway is a promising sign for patients, because it is trials such as these that have led to the approval of durvalumab. There are a lot of opportunities for patients to discuss joining a clinical trial as soon as they are diagnosed, before they have begun another treatment that may exclude them from joining a trial, or while they have only just begun to receive standard treatments. It is important that people with BTC have as many options as possible, as early as possible. To help identify potential treatment options, and define eligibility for appropriate clinical trials, it is important that people are tested for mutations that could be causing the cancer or other molecular abnormalities as soon as possible. This will reveal whether there is a suitable trial available and will help identify the best possible treatment plan.
2.3. Second-line treatment
As mentioned, second-line therapy in BTC can either be chemotherapy-based for patients without targetable mutations, or a targeted agent for patients with a suitable molecular profile; clinical trials are ongoing in both areas. In this section, we briefly summarize information on the main targetable molecular aberrations that have been identified in patients with advanced BTC.
2.4. FGFR2 aberrations
FGFR2 aberrations, including fusions, rearrangements and mutations, are present in approximately 15% of patients with iCCA [17,18]. In 2020, pemigatinib was the first targeted agent to be approved for the treatment of advanced BTC, in patients with refractory cholangiocarcinoma (CCA) harboring FGFR2 fusions or rearrangements [19]. The approval was based on the phase II FIGHT-202 trial which showed that a third of patients responded to treatment in this setting [20]. In September 2022, futibatinib was granted accelerated approval by the FDA for patients with previously treated, unresectable, locally advanced or metastatic iCCA harboring FGFR2 gene fusions or other rearrangements [21]. The approval was based on a phase II trial that demonstrated a response rate of 42% [22]. A third FGFR2 inhibitor, infigratinib, is also approved by the FDA for the treatment of CCA with FGFR2 fusions or other rearrangements [23]. However, further development of this drug has been discontinued due to recruitment difficulties in confirmatory trials [24].
Although the development of these agents for the treatment of BTC was a major advance, several unanswered questions remain, especially regarding mechanisms of resistance [25]. Almost all patients become resistant to treatment with FGFR2 inhibitors within 6–9 months. Therefore, ongoing research is focusing on the molecular mechanisms of resistance and the development of additional treatments that might overcome this resistance. Combination of FGFR2 inhibitors with agents including immune checkpoint inhibitors (NCT05174650), antiangiogenic agents and multikinase inhibitors (NCT04919642) are being investigated [25]. Additionally, next-generation FGFR2 inhibitors with alternative mechanisms of action, such as the irreversible agents gunagratinib (NCT04565275) and RLY-4008 (ReFocus [NCT04526106]) [26], are being assessed in ongoing clinical trials [3]. The phase I/II ReFocus trial is examining RLY-4008 in patients with ICC and other solid tumors harboring FGFR alterations, including mutations, translocations, activation and amplification [27]. Preliminary data demonstrated promising activity at RLY-4008 doses ≥70 mg/day in both FGFR inhibitor-naive (n = 11; ORR: 73%; 6-month progression-free survival [PFS] rate: 100%) and FGFR inhibitor-pretreated patients with FGFR2-altered CCA (n = 14; ORR: 21%; 6-month PFS rate: 43% [28]).
2.5. IDH1 mutations
Overall, IDH1 mutations are present in 15–20% of CCAs [3]. To date, one IDH1 inhibitor, ivosidenib, is approved for the treatment of pretreated IDH-1-mutated CCA, based on the phase III ClarlDHy trial. In this study, ivosidenib significantly improved PFS versus placebo (median 2.7 vs 1.4 months; HR: 0.37; 95% CI: 0.25–0.54) [29], with a trend toward improved OS (median 10.8 vs 9.7 months; HR: 0.69; 95% CI: 0.44–1.10). Importantly, from a patient's perspective, ivosidenib improved health-related quality of life.
As with FGFR2 inhibitors, the next stage in terms of clinical development of IDH1 inhibitors in BTC is a better understanding of resistance mechanisms and an elucidation of improved treatment regimens, including novel combinations. In mouse models of CCA, IDH1 mutations have been shown to support tumor immune evasion by (R)-2-hydroxyglutarate-mediated suppression of CD8+ T-cell activity and tumor cell-autonomous inactivation of TET2 (a DNA demethylase frequently mutated in cancer) [30]. In preclinical experiments, pharmacologic inhibition in IDH1-mutant CCA stimulated CD8+ T-cell recruitment and promoted TET2-activity [30]. Combining IDH1 inhibitors with immune checkpoint inhibitors represents a potentially interesting strategy [31]. Indeed, an ongoing phase II trial is assessing the combination of ivosidenib plus nivolumab in patients with IDH1-mutant tumors (NCT04056910).
Next-generation IDH1 inhibitors are also being investigated with a view to delaying acquired resistance to treatment. For example, LY3410738 is a covalent inhibitor of IDH-1 that is selective of the mutant enzyme with the capacity to inhibit second-site mutation points [32]. In treatment-naive patients with IDH1/2 mutation-positive CCA treated in a phase I study (NCT04521686), LY3410738 in combination with gemcitabine/cisplatin demonstrated a favorable safety profile and early signs of efficacy (ORR: 46%; median PFS not reached after median 4.1 months follow-up; n = 13) [33]. Other potential next-generation IDH1/2 inhibitors are being assessed in patients with BTC, including dasatinib (NCT02428855) and olutasidenib (NCT03684811) [3].
2.6. HER2 aberrations
HER2 overexpression and/or amplification has been identified in 5–20% of CCAs [34]. HER2 is an established drug target in several cancers, including breast cancer [35], gastric cancer [36] and non-small-cell lung cancer (NSCLC) [37]. Several HER2-targeted agents are approved in these settings, including monoclonal antibodies (e.g., trastuzumab, pertuzumab), tyrosine kinase inhibitors (TKIs) (e.g., lapatinib, neratinib) and antibody–drug conjugates (e.g., trastuzumab deruxtecan).
The proof-of-principle of targeting HER2 in BTC has been established in a number of clinical trials. For example, in the phase IIa MyPathway basket trial, the combination of pertuzumab and trastuzumab conferred an ORR of 23%, median PFS of 4 months, and median OS of 10.9 months in 39 patients with previously treated metastatic BTC with HER2 amplification and/or overexpression [38]. Based on these data, the combination is listed in the latest National Comprehensive Cancer Network® (NCCN) guidelines as a category 2A recommendation for HER2-positive tumors [39].
Trastuzumab deruxtecan has demonstrated strong activity in HER2-positive breast, gastric and NSCLC tumors [40–42]. Early data from the ongoing phase II HERB trial have demonstrated that trastuzumab deruxtecan has promising activity and manageable tolerability in patients with HER2-positive BTC. In 22 patients assessed to date, the ORR was 36%, median PFS was 5.1 months, and median OS was 7.1 months [43]. In 41 patients with HER2-positive BTC treated in the phase II DESTINY-PanTumor02 (DP-02) trial, ORR was 22% overall; in patients with centrally confirmed immunohistochemistry (IHC) test scores of 2+ (n = 14) and 3+ (n = 16), the ORR was 0 and 56%, respectively [44].
Following encouraging data from a phase I dose escalation and expansion trial, the bispecific HER2-directed monoclonal antibody, zanidatamab, was assessed in a phase II trial (HERIZON-BTC-01; NCT04466891); in 80 patients with HER2-positive BTC, the ORR was 41% and median duration of response was 12.9 months [45].
Other anti-HER2 agents are currently being assessed in patients with BTC, including the antibody–drug conjugate, A166 (NCT03602079) and the HER2 TKI, tucatinib as an add-on to first-line therapy in patients with HER2-positive tumors (NCT04430738). In an interim analysis of the ongoing phase II basket trial SGNTUC-019 (NCT04579380) of tucatinib in patients with pretreated HER2-positive solid tumors, in 30 patients with BTC, the ORR was 47% and median duration of response was 6 months [46].
2.7. BRAF V600E mutation
The BRAF V600E mutation is present in approximately 3% of patients with iCCA [47]. In a phase II basket trial, the BRAF inhibitor, dabrafenib plus the MEK inhibitor, trametinib demonstrated an encouraging ORR of 47% in 43 pretreated patients with advanced BRAF V600E-positive BTC [48]. Based on these data, dabrafenib combined with trametinib is recommended by the NCCN as a category 2A treatment option for unresectable/metastatic progressive BTC with the BRAF V600E mutation [39]. Therefore, it is important that testing for BRAF V600E is considered in all patients with BTC [48].
2.8. Neurotrophic tropomyosin-related kinase fusions
NTRK fusions are rare in BTCs, occurring in <1% of cases [49]. Nevertheless, as two NTRK inhibitors, larotrectinib and entrectinib, are approved for patients with NTRK fusion-positive solid tumors and have shown strong clinical activity in this setting, it is important that patients with BTC are screened for these aberrations [50]. To date, NTRK inhibitors have been assessed in tumor agnostic clinical trials [51,52]. However, two phase II basket trials that include patients with BTC are ongoing (NCT02568267; NCT02576431).
Entrectinib and larotrectinib are included in the most recent NCCN BTC guidelines as first-line or subsequent-line category 2A treatment options ‘useful in certain circumstances’ for unresectable or metastatic BTC with NTRK gene fusions [39].
2.9. DNA damage repair genes
Aberrations in DNA damage repair genes, including BRCA1 and 2, have been identified in approximately 2% of patients with BTC [8]. As observed in other tumor types, these patients may be more sensitive to treatment with poly (ADP-ribose) polymerase (PARP) inhibitors. Accordingly, an ongoing phase II clinical trial is assessing the PARP inhibitor, olaparib in patients with BTC and mutations in DNA damage repair genes, including BRCA1 and 2 (NCT04042831). Other basket trials are assessing PARP inhibitors such as olaparib, talazoparib, niraparib and rucaparib, either as monotherapy or in combination with immune checkpoint inhibitors [8].
Tumors with mismatch repair deficiency (dMMR) and high levels of microsatellite instability (MSI-H) are known to be sensitive to immune checkpoint inhibitors. In BTC, the frequency of dMMR is approximately 2% in patients with iCCA and 5% in patients with GC [53,54]. In the phase II KEYNOTE-158 trial, 22 patients with iCCA and MSI-H/dMMR were treated with pembrolizumab and achieved an ORR of 40.9%, median PFS of 4.2 months, and median OS of 19.4 months [55]. These findings support the use of pembrolizumab in patients with BTC who lack other therapeutic options and highlight the importance of testing for MSI-H/dMMR. Furthermore, dostarlimab-gxly, another anti-PD-1 therapy, demonstrated durable antitumor activity in patients with dMMR solid, predominantly gastrointestinal, tumors in the phase I GARNET study (NCT02715284) [56], and is included in the NCCN guidelines as a category 2A subsequent line treatment for BTC with MSI-H/dMMR [39]. Dostarlimab plus the PARP inhibitor niraparib are currently being evaluated in recurrent/metastatic BTC in the ongoing phase II NIRADO trial (NCT04779151).
2.10. KRAS mutations
A recent analysis of 1671 patients with BTC identified KRAS G12D and G12C mutations in 5.1 and 1.0% of cases, respectively [8]. Although KRAS has traditionally been regarded as an ‘undruggable’ target, specific KRAS G12C inhibitors, such as sotorasib, have recently been developed, and have shown impressive clinical activity in lung cancer [57]. To date, few patients with BTC have been treated with KRAS G12C inhibitors, but responses have been reported in the CodeBreaK 100 (NCT03600883) and KRYSTAL-1 trials (NCT03785249) [58]. In addition, specific KRAS G12D inhibitors are currently being developed [59]. It is important, therefore, to screen for KRAS mutations in patients with BTC.
2.11. RET fusions
Rare oncogenic receptor tyrosine kinase (RET) fusions have been detected in a range of solid tumors [60]. In a recent phase I/II trial, ARROW, the RET receptor TKI, pralsetinib conferred an ORR of 57%, median PFS of 7 months, and median OS of 14 months in 29 patients with RET-positive solid tumors [60]. Of three patients with CCA, two responded to treatment.
Based on results from the ARROW study, pralsetinib is listed by the NCCN as a category 2b recommendation for patients with RET fusion-positive BTC [39].
2.12. Patient perspective on second-line therapy options
Clinical trials of second-line therapies are available for people with BTC with and without cancer-causing mutations. The main targetable alterations that are being looked at in BTC are those in FGFR2, IDH1, HER2, BRAF V600E, NTRK, DNA damage repair genes, KRAS and RET.
For BTC with FGFR2 alterations, pemigatinib and futibatinib are already approved, and further clinical trials are underway looking at these drugs in combination with other drugs used to treat cancer. New drugs targeting FGFR2 are also being tested. Ivosidenib is approved in people with CCA with IDH1 mutations, and newer IDH1-targeting drugs are being investigated in clinical trials. Research into tumors with FGFR2 and IDH2 alterations is also focused on the ways that cancers can become resistant to these targeted drugs (meaning the drugs stop working effectively) in order to stop resistance from developing. Although NTRK fusions are not common in BTC, larotrectinib and entrectinib are approved for the treatment of cancers with this alteration, including BTC. Results from clinical trials of BTC with HER2 alterations, BRAF V600E mutations, alterations in DNA damage repair genes, KRAS mutations and RET fusions are promising, and multiple trials are underway, particularly for HER2.
This section shows just how many options there are for patients with one of these targets, but even for patients with no targetable mutations, research is still underway. To take full advantage of the potential options for BTC, it is very important to get biomarker testing done so that knowledge can be used to map out the best treatment path possible, regardless of whether a target is present or not. People with BTC should talk to their doctor about their options.
3. The rationale for targeting MDM2 in patients with BTC
The commentary above highlights rapid progress in the identification of driver molecular aberrations in patients with BTC and the appropriate use of targeted therapy. However, as discussed, there is still a large population of patients who do not have molecular aberrations targeted by these treatments. It is extremely important, therefore, to continue the search for other drug targets.
The MDM2–p53 axis is an emerging therapeutic target that aims to activate the p53 protein. This protein is a transcription factor that is widely regarded as one of the most important tumor suppressors in human cancer biology [61–63]. It is activated in response to cellular stress signals, leading to the expression of target genes that can promote cell cycle arrest and apoptosis. It is also considered to have pleotropic antiproliferative effects via transcription-independent mechanisms [61–63].
The critical biological role of p53 is underscored by the fact that TP53, the gene that encodes p53, is mutated in up to 50–60% of human cancers, and represents the most commonly mutated oncogene [64]. Accordingly, attempts to restore the functionality of p53 in tumors has, historically, proved to be one of the ‘holy grails’ of cancer medicine. Nuclear transcription factors are notoriously difficult to target pharmacologically and have long been considered undruggable. Nevertheless, increased understanding of p53 biology and regulation have offered alternative strategies by which p53 may be activated.
Befitting its biological importance as a ‘gatekeeper’ of cell turnover, the activity of p53 is highly regulated in cells by a series of feedback loops [65]. The E3 ubiquitin ligase MDM2 and the related protein MDMX (also known as MDM4) are important negative regulators of p53 [66]. Under normal physiological conditions, MDM2 binds tightly to p53, promoting its ubiquitylation and proteasomal degradation [67]. However, in situations of cellular stress, for example in response to DNA damage, MDM2 becomes phosphorylated and cannot bind to p53, ultimately leading to cell cycle arrest and apoptosis [65].
Given their role as physiological ‘brakes’ on p53-mediated apoptosis, aberrations of the MDM2 or MDMX genes can lead to inappropriate silencing of wild-type p53, potentially leading to tumorigenesis [66]. MDM2 amplification is implicated in the pathogenesis of some cases of BTC [68]. In addition, aberrant activity of MDM2 can be caused by the loss of its negative regulator, p14ARF, due to mutations in the CDKN2A gene, which occur in around 10% of cases of BTC [65].
MDM2 amplification has been detected in approximately 5–8% of BTCs [68,69], with TP53 mutations being mostly mutually exclusive [68]. Prevalence of MDM2 amplification varies depending on the anatomical location of the tumor, ranging from 2–6% in iCCA [70,71], to 16% in AC [72]. Other studies have reported MDM2 amplification in 13–14% of cases of GC [73,74]. MDM2 amplifications are generally mutually exclusive to other genetic aberrations, including FGFR rearrangements, HER2 amplifications and IDH mutations [68]. In principle, therefore, inhibition of MDM2 activity in patients with BTC could restore the p53 pathway, leading to the destruction of tumor cells. Furthermore, MDM2 amplification has been reported as a negative prognostic factor in patients with BTC [65,73,75–77]. Therefore, there is a clear unmet need for effective therapies in this subgroup of patients. Finally, inactivation of p53, either by mutation or due to aberrant activity of endogenous regulators, is thought to be a key mechanism of resistance to gemcitabine, the cornerstone of first-line treatment for BTC [65]. These factors provide strong rationale for exploring MDM2 as a drug target in those patients with BTC with MDM2 amplification.
3.1. Patient perspective of MDM2
Since there are many people with BTC who do not have a mutation that can be treated with the drugs mentioned above, it is very important to continue to search for other alterations in BTC that can be targeted. MDM2 is one of these potential new targets. MDM2 is involved in regulating a protein called p53, which acts to stop cancer from starting. When a cell becomes damaged in a way that might lead to cancer, p53 is responsible for stopping that cell from growing, and if the damage cannot be repaired, p53 is involved in killing that cell so it does not turn into cancer. Often in cancer, p53 is inactivated (mutated or damaged), allowing the cancer to grow. Another way p53 is inactivated is by increased levels of MDM2. The extra MDM2 interacts with p53 and causes it to be destroyed. In cancer cells that have increased MDM2, the inactivation of p53 can leave open the chance for the cancer to grow, because the p53 pathway is not there to stop and kill it. If MDM2 can be blocked from interacting with p53 in cancer cells, then those cancer cells should then be killed. BTC is one of the cancer types that has increased levels of MDM2, and we know that people with BTC that have increased MDM2 have worse outcomes than people with BTC that do not. This shows that MDM2 is an important target for drugs. From a patient perspective, it is encouraging to know that there is another target in BTC, and it emphasizes even more how important it is to have biomarker testing done.
4. The clinical development of MDM2–p53 antagonists
Several small molecule MDM2–p53 antagonists are currently being developed in the clinic for the treatment of various malignancies [78–82].
At this stage, MDM2–p53 antagonists have predominantly been assessed in hematologic malignancies, but some agents such as idasanutlin [80] and ALRN-6924 [78] have been assessed as monotherapy in patients with advanced solid tumors, and have demonstrated tolerability and promising efficacy. MDM2–p53 antagonists are also being assessed in combination with immune checkpoint inhibitors, based on the observation that they may help promote a pro-immunogenic tumor microenvironment and that they synergize with anti-PD-1 antibodies in preclinical models [83]. For example, in an ongoing phase II trial, alrizomadlin (APG-115) plus pembrolizumab is being assessed in patients with various solid tumors, including melanoma, well-differentiated and dedifferentiated liposarcoma and NSCLC, and has demonstrated promising activity [84].
Other novel combination strategies with MDM2–p53 antagonists have been assessed in patients with solid tumors. For example, siremadlin combined with the CDK4/6 inhibitor, ribociclib, demonstrated manageable toxicity and early signs of antitumor activity in patients with dedifferentiated and well-differentiated liposarcoma [85]. All of these recent studies have illustrated the proof-of-concept of targeting MDM2 as a means of restoring the p53 signaling pathway in patients with wild-type TP53.
The MDM2–p53 antagonist milademetan (RAIN-32) was, until recently, one of only two MDM2–p53 antagonists being assessed in solid tumors including BTC [86]. Results from phase I trials demonstrated manageable tolerability and modest antitumor activity in patients with solid tumors [87]. The most common adverse events (AEs) were gastrointestinal (e.g., nausea and decreased appetite) or hematologic (e.g., neutropenia, thrombocytopenia and anemia). Interim data from the MANTRA-2 study, a phase II basket trial, showed promising activity with milademetan in patients with various solid tumors with wild-type TP53 and amplified MDM2, including tumor regression of 29% in one patient with BTC [88]. The safety data were consistent with phase I trials [89]. However, enrollment to MANTRA-2, and plans for phase I/II combination trials, have been suspended (May 2023) after the primary end point was not met in the phase III MANTRA trial of milademetan vs trabectedin in patients with dedifferentiated liposarcoma (median PFS: 3.6 vs 2.2 months; HR: 0.89 [90,91]).
Consequently, the only MDM2–p53 antagonist currently being developed in patients with BTC, to the best of our knowledge, is brigimadlin (BI 907828). This is a highly potent, oral MDM2–p53 antagonist that has shown antitumor activity in preclinical studies, especially in TP53 wild-type, MDM2-amplified dedifferentiated liposarcoma patient-derived xenografts [92]. Preclinical data also suggest that immune modulation contributes to the activity of brigimadlin [93].
Brigimadlin is currently being investigated in two ongoing phase Ia/Ib trials as monotherapy (NCT03449381) or combined with the anti-PD-1 immune checkpoint inhibitor, ezabenlimab (NCT03964233), in patients with advanced TP53-wild-type solid tumors [94,95]. Both trials have dose escalation and dose expansion parts. The monotherapy trial demonstrated that brigimadlin has a long half-life (28–59 h), facilitating an intermittent schedule [96,97]. The recommended dose for expansion was identified as brigimadlin 45 mg tablet orally once every 3 weeks (q3w). At this dose level, the most common treatment-related grade ≥3 AEs were neutropenia (24%), thrombocytopenia (21%) and anemia (12%) [94]. In both the monotherapy and combination studies, patients with wild-type TP53, MDM2-amplified tumors are being recruited into two cohorts: sarcoma or other tumor types, including BTC. To date, 12 patients with BTC have been treated with brigimadlin across the two studies (Figure 2). Six had a best response of PR, and a further four had stable disease (SD) [98]. Preliminary responses appeared to be durable: treatment was administered for over 4 months in ten patients, and four were still receiving brigimadlin at the time of writing. There were no unexpected toxicities. The most common grade 3/4 treatment-related AEs included neutropenia, thrombocytopenia and anemia. Seven patients had dose reductions due to AEs, but no treatment-related AEs led to treatment discontinuation.
Figure 2.
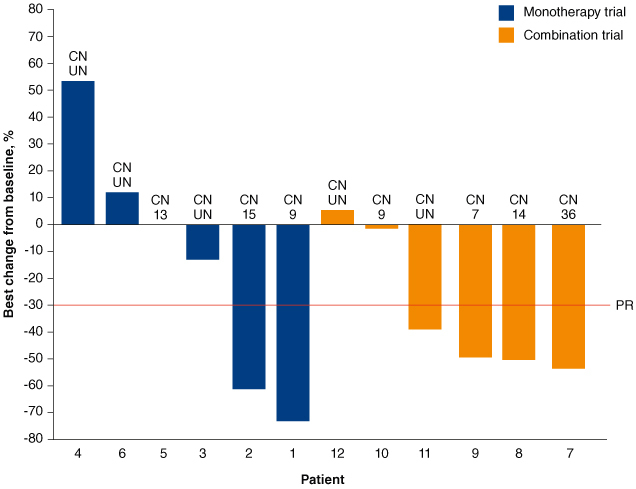
Waterfall plot of BTC patients included in the ongoing brigimadlin monotherapy (NCT03449381) or combination therapy (NCT03964233) trials.
Reused from Yamamoto N, et al. 2024 [98] Licensed under Attribution-NonCommercial-NoDerivatives 4.0 Unported License (https://creativecommons.org/licenses/by-nc-nd/4.0/), with no modifications.
BTC: Biliary tract cancer; CN: Copy number; PR: Partial response; UN: Unknown.
Based on these encouraging results, a phase IIa/IIb, open-label trial of brigimadlin is currently recruiting patients with advanced BTC, pancreatic ductal adenocarcinoma, or other solid tumors across ∼60 sites in multiple countries and regions (Brightline-2; NCT05512377; Figure 3) [99]. All patients will receive brigimadlin 45 mg orally once q3w. Patients with locally advanced/metastatic, unresectable TP53-wild-type, MDM2-amplified disease who have exhausted conventional therapies (or for patients with BTC, a low likelihood of tolerating standard of care) will be eligible. The primary end point is objective response based on central independent review. Secondary end points are duration of response, PFS, OS, disease control, occurrence of AEs and AEs leading to trial drug discontinuation, and patient-reported health-related quality of life.
Figure 3.
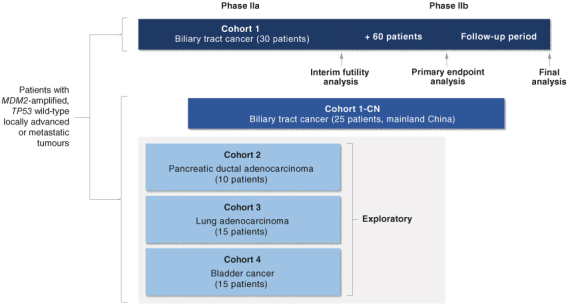
Study design of BRIGHTLINE-2. Reuse from Yoo C et al. 2024 [100]. Licensed under Attribution-NonCommercial-NoDerivatives 4.0 Unported License (https://creativecommons.org/licenses/by-nc-nd/4.0/), with no modifications.
4.1. Patient perspective of brigimadlin & Brightline-2
While several MDM2 blockers are in development, brigimadlin is being evaluated in a number of clinical trials that include patients with BTC. Although the studies are in their early stages, the available results are promising, and brigimadlin appears to have manageable side effects. Based on these results, another clinical trial called Brightline-2 is being done. This is an international trial, with sites in North America, Europe and Asia Pacific. Expenses associated with trial participation will be reimbursed per applicable (local) regulatory requirement(s). Patients who have advanced BTC with normal TP53 and increased MDM2 (checked through biomarker testing) who cannot have surgery, and who have had all available therapies or are not likely to tolerate standard treatments, are able to take part in the study. Other criteria include robust health (fully active or only able to perform non-physically difficult activities, such as light house work or office work), a life expectancy of at least 3 months, biopsy availability, and willingness to give blood samples.
5. Molecular testing for BTC
Although precision medicine remains in its infancy, recent progress in the treatment of BTC represents a paradigm shift in the management of this difficult-to-treat malignancy. Many patients are now receiving, and benefitting from, targeted agents. Moreover, approaches for targeting other aberrations, such as HER2 amplification, BRAF mutations, and NTRK fusions are showing considerable promise in eligible patients.
The bedrock of effective precision medicine is the availability, and appropriate interpretation of, molecular testing for as many patients as possible, as early as possible. The importance of molecular testing is becoming increasingly recognized in contemporary treatment guidelines for BTC. For example, the most recent European Society for Medical Oncology (ESMO) Clinical Practice Guidelines, published in February 2023 recommend comprehensive molecular analysis encompassing testing at the DNA level (NGS panels that, at a minimum, interrogate IDH1, HER2, BRAF and FGFR2), RNA level (to detect FGFR2 and NTRK gene fusions) and protein level (to assess tissue expression of the DNA mismatch repair proteins MLH1, MSH2, MSH6 and PMS2) [5]. The guidelines strongly recommend that results of the molecular testing are discussed with a molecular pathologist. The most recent NCCN BTC Guidelines at time of writing (Version 2 2023) also recommend the molecular testing of unresectable or metastatic tumors for IDH1, FGFR and BRAF V600E mutations, NTRK and RET gene fusions and HER2 amplification or overexpression among other genetic aberrations [39]. Several NGS platforms are commercially available and should be utilized to screen for genetic alterations [9]. Some of these encompass both DNA and RNA, and can therefore detect gene fusions. Also, many of these tests can be utilized to test for MDM2 amplification.
The relationship between expression levels of target aberration and likelihood of response to targeted treatment has not been fully explored in BTC. Data from the phase II DESTINY PanTumor02 trial of trastuzumab deruxtecan in patients with HER2-expressing solid tumors suggest there may be a relationship: in patients with BTC with HER2 IHC 3+ (n = 16), the ORR was 56.3% and median OS was 12.4 months (n = 16); in patients with BTC with HER2 IHC 2+ (n = 14), the ORR was 0% and median OS was 6.0 months [44]. Evaluating expression levels of specific mutations or fusions may be challenging due to their rarity and heterogeneity. Identifying biomarkers for response to targeted treatment is an important challenge that should be addressed in future studies.
From a medical perspective, comprehensive molecular testing should be mandatory for all patients. However, as previously discussed, three main barriers remain: insufficient tumor biopsy material in some cases; inability to undertake repeat biopsies to monitor disease progression mechanisms; potential reimbursement issues. Given the first two barriers, considerable attention has been given to the potential utilization of non-invasive liquid biopsy techniques that examine circulating cell-free DNA (cfDNA) or circulating tumor DNA (ctDNA).
A recent study analyzed the tumor profile of 1671 patients with advanced BTC by screening cfDNA samples, utilizing the FDA-approved Guardant360 CDx NGS platform [8]. Targetable genetic alterations were identified in 44% of patients. Importantly, concordance rates between cfDNA and tumor samples were high for some aberrations, including IDH1 (87%) and BRAF V600E (100%). Accordingly, the data support cfDNA as a means of reliably detecting clinically actionable mutations in some cases. However, the concordance rate for other aberrations, like FGFR2 fusions (18%), was much lower. This probably reflects the fact that the chosen NGS platform utilizes a DNA-based hybrid capture methodology. Analysis of cell-free RNA is probably required to detect fusions, but such an approach is currently limited by the instability of RNA in blood. The liquid biopsy strategy has shown promise in identifying putative resistance mechanisms to targeted therapies. For example, some patients treated with FGFR2 inhibitors developed a tertiary mutation (FGF2R C492) that blocked interaction between the drug and the receptor. In a recent study in China, NGS using the NextSeq® 500 (Illumina®) platform was undertaken on blood ctDNA and/or tumor tissue samples in 545 patients with metastatic BTC [101]. Of the 545 patients who provided paired blood and tumor tissue samples, ctDNA was detected in the blood in 520 (95%). Overall, the most frequent genomic alterations in ctDNA samples were TP53 (35%) and KRAS (20%), and other identified driver genes were EGFR (16%) and CDKN2A (10%). Subgroup analysis of patients with CCA (n = 105) showed that TP53 was the most frequently altered gene, followed by KRAS and EGFR, while ctDNA samples from patients with gallbladder subtypes (n = 37) were enriched for TP53, CDK2NA and EGFR mutations. The mutation frequencies seen in ctDNA were similar to those seen in tissue samples for most genes. Overall, these recent findings illustrate the potential of liquid biopsy for informing clinical decisions, although it remains an important area for technological improvements and requires further refinement in terms of incorporation into day-to-day practice.
5.1. Patient perspective of biomarker testing & cfDNA/ctDNA strategies
There is a large amount of research being done into the genetic changes in BTC and how to target them with anticancer drugs. As a person with BTC, the ideal situation is to have as many options as possible, and having biomarker testing done at diagnosis and potentially throughout the treatment journey will make that possible. It is important to make sure that enough of the cancer tissue is taken during the first biopsy so that the procedure does not have to be repeated. However, results from early research into using blood samples for biomarker testing suggest that this much less invasive method of sampling could be a potential option in the future if further testing is required. Note that for those with health insurance, more companies are beginning to cover biomarker testing, and often testing companies will have patient assistance programs if cost is an issue.
For further information on the importance of biomarker testing, please see the Biomarkers Matter campaign from the Cholangiocarcinoma Foundation [102].
6. Conclusion
Although the current standard of care for the treatment of patients with BTC is chemotherapy with or without immunotherapy, advances are being made in the development of targeted agents against a number of genetic aberrations found in BTC. More than 50 active clinical trials are assessing targeted therapies in biomarker-selected patients with BTC [8]. MDM2–p53 antagonists are one class of targeted therapy in development, of which brigimadlin is currently in development for the treatment of patients with BTC. Given the emergence of molecular aberrations for targetable therapies, patients with BTC should be routinely tested. However, it is important to note that treatment options are still available for those patients without a targetable mutation.
7. Future perspective
All patients with BTC should undergo comprehensive genomic analysis, including testing for MDM2, FGFR2, IDH1, HER2, BRAF V600E, NTRK, DNA damage repair genes, KRAS and RET aberrations. Patients with targetable alterations may be eligible for clinical trials or approved targeted treatments. In the future, targeted therapy tailored to the tumor characteristics of individual patients could complement effective immunochemotherapy and significantly improve outcomes in this difficult-to-treat group of malignancies.
Supplementary Material
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Funding Statement
R Shroff reports: advisory council or committee: AstraZeneca, Boehringer Ingelheim, Clovis, Genentech, Incyte, Merck, QED Therapeutics, Servier, Taiho, Zymeworks Bipharm, Astellas, Ability Pharm. Consulting fee: SYROS, Hookipa Pharm., AbbVie. M Bachini reports: employment – Cholangiocarcinoma Foundation. Advisory Council – Taiho Pharmaceutical. Honoraria from AstraZeneca, Boehringer Ingelheim, Kinnate, and Relay Therapeutics.
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/14796694.2024.2340959
Author contributions
The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development and have approved the final version.
Financial disclosure
R Shroff reports: advisory council or committee: AstraZeneca, Boehringer Ingelheim, Clovis, Genentech, Incyte, Merck, QED Therapeutics, Servier, Taiho, Zymeworks Bipharm, Astellas, Ability Pharm. Consulting fee: SYROS, Hookipa Pharm., AbbVie. M Bachini reports: employment – Cholangiocarcinoma Foundation. Advisory Council – Taiho Pharmaceutical. Honoraria from AstraZeneca, Boehringer Ingelheim, Kinnate, and Relay Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by S Kirkham of Ashfield MedComms, and L Pritchard, an Inizio Company, and funded by Boehringer Ingelheim.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Koshiol J, Yu B, Kabadi SM, et al. Epidemiologic patterns of biliary tract cancer in the United States: 2001–2015. BMC Cancer. 2022;22(1):1178. doi: 10.1186/s12885-022-10286-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti S, Kamgar M, Mahipal A. Targeted therapies in advanced biliary tract cancer: an evolving paradigm. Cancers. 2020;12(8):2039. doi: 10.3390/cancers12082039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott AJ, Sharman R, Shroff RT. Precision medicine in biliary tract cancer. J Clin Oncol. 2022;40(24):2716–2734. doi: 10.1200/JCO.21.02576 [DOI] [PubMed] [Google Scholar]; •• Examines biomarker assessment in biliary tract cancer and provides practical guidance for the use of targeted therapies.
- 4.Rizzo A, Ricci AD, Cusmai A, et al. Systemic treatment for metastatic biliary tract cancer: state of the art and a glimpse to the future. Curr Oncol. 2022;29(2):551–564. doi: 10.3390/curroncol29020050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel A, Bridgewater J, Edeline J, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127–140. doi: 10.1016/j.annonc.2022.10.506 [DOI] [PubMed] [Google Scholar]
- 6.Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, Phase III trial. Lancet. 2023;401(10391):1853–1865. doi: 10.1016/S0140-6736(23)00727-4 [DOI] [PubMed] [Google Scholar]; • The phase III KEYNOTE-966 trial compared the efficacy of immune checkpoint inhibitor pembrolizumab in combination with gemcitabine/cisplatin versus placebo plus gemcitabine/cisplatin in previously untreated unresectable, locally advanced or metastatic biliary tract cancer.
- 7.FDA approves pembrolizumab with chemotherapy for biliary tract cancer [Internet] . U.S. Food and Drug Administration. 2023 Oct 31. [cited 2023 Nov 22]. Available from: www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-chemotherapy-biliary-tract-cancer
- 8.Berchuck JE, Facchinetti F, DiToro DF, et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann Oncol. 2022;33(12):1269–1283. doi: 10.1016/j.annonc.2022.09.150 [DOI] [PubMed] [Google Scholar]
- 9.Malka D, Siebenhüner AR, Mertens JC, et al. The importance of molecular testing in the treatment of cholangiocarcinoma. EMJ Oncol. 2020;8(1):82–94. [Google Scholar]
- 10.Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a Phase III, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Tu X, Zhao P, et al. A randomised Phase II study of second-line XELIRI regimen versus irinotecan monotherapy in advanced biliary tract cancer patients progressed on gemcitabine and cisplatin. Br J Cancer. 2018;119(3):291–295. doi: 10.1038/s41416-018-0138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allo G, Can AD, Wahba R, et al. Nanoliposomal irinotecan in combination with leucovorin and 5-fluorouracil in advanced biliary tract cancers. Mol Clin Oncol. 2022;16(2):52. doi: 10.3892/mco.2021.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA approves durvalumab for locally advanced or metastatic biliary tract cancer [Internet] . U.S. Food and Drug Administration. 2022 Sep 2. [cited 2023 Nov 22]. Available from: www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-locally-advanced-or-metastatic-biliary-tract-cancer
- 14.Oh D-Y, He AR, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):EVIDoa2200015. doi: 10.1056/EVIDoa2200015 [DOI] [PubMed] [Google Scholar]
- 15.Ioka T, Kanai M, Kobayashi S, et al. Randomized Phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401-MITSUBA). J Hepatobiliary Pancreat Sci. 2023;30(1):102–110. doi: 10.1002/jhbp.1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shroff RT, Guthrie KA, Scott AJ, et al. SWOG 1815: a Phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol. 2023;41(Suppl. 4):LBA490. doi: 10.1200/JCO.2023.41.4_suppl.LBA490 [DOI] [Google Scholar]
- 17.Helsten T, Elkin S, Arthur E, et al. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016;22(1):259–267. doi: 10.1158/1078-0432.CCR-14-3212 [DOI] [PubMed] [Google Scholar]
- 18.Goyal L, Kongpetch S, Crolley VE, et al. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat Rev. 2021;95:102170. doi: 10.1016/j.ctrv.2021.102170 [DOI] [PubMed] [Google Scholar]
- 19.FDA approves first targeted treatment for patients with cholangiocarcinoma, a cancer of bile ducts [Internet] . U.S. Food and Drug Administration. 2020 Apr 17. [cited 2023 Nov 22]. Available from: www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-treatment-patients-cholangiocarcinoma-cancer-bile-ducts
- 20.Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, Phase II study. Lancet Oncol. 2020;21(5):671–684. doi: 10.1016/S1470-2045(20)30109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FDA grants accelerated approval to futibatinib for cholangiocarcinoma [Internet] . U.S. Food and Drug Administration. 2022 Sep 30. [cited 2023 Nov 22]. Available from: www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-futibatinib-cholangiocarcinoma
- 22.Highlights of Prescribing Information LYTGOBI® (futibatinib) [Internet] . 2022 Sept. [cited 2023 Nov 22]. Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2022/214801s000lbl.pdf
- 23.FDA grants accelerated approval to infigratinib for metastatic cholangiocarcinoma [Internet] . U.S. Food and Drug Administration. 2021 May 28. [cited 2023 Nov 22]. Available from: www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma
- 24.Important information . Truseltiq® (Infigratinib) capsules. Notice of permanent discontinuation of distribution [Internet]. CCA News. 2022 Oct 11. [cited 2023 Nov 22]. Available from: www.ccanewsonline.com/web-exclusives/press-releases/october-10-2022-truseltiq
- 25.Gadaleta-Caldarola G, Rizzo A, Dadduzio V, et al. Pemigatinib in intrahepatic cholangiocarcinoma: a work in progress. Curr Oncol. 2022;29(10):7925–7931. doi: 10.3390/curroncol29100626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbiah V, Sahai V, Maglic D, et al. RLY-4008, the first highly selective FGFR2 inhibitor with activity across FGFR2 alterations and resistance mutations. Cancer Discov. 2023;13(9):2012–2031. doi: 10.1158/2159-8290.CD-23-0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.REFOCUS: a first-in-human study of highly selective FGFR2 inhibitor, RLY-4008, in patients with ICC and other advanced solid tumors . ClinicalTrials.gov Identifier: NCT04526106 [Internet] U.S. National Library of Medicine. 2020. [cited 2023 Nov 22]. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT04526106
- 28.Borad MJ, Schram AM, Kim RD, et al. Updated dose escalation results for ReFocus, a first-in-human study of highly selective FGFR2 inhibitor RLY-4008 in cholangiocarcinoma and other solid tumors. Slides presented at: ASCO Annual Meeting; 2023 June 2–6; Chicago, IL. doi: 10.1200/JCO.2023.41.16_suppl.4009 [DOI] [Google Scholar]
- 29.Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, Phase III study. Lancet Oncol. 2020;21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu MJ, Shi L, Dubrot J, et al. Mutant IDH inhibits IFNgamma-TET2 signaling to promote immunoevasion and tumor maintenance in cholangiocarcinoma. Cancer Discov. 2022;12(3):812–835. doi: 10.1158/2159-8290.CD-21-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandi G, Rizzo A. IDH inhibitors and immunotherapy for biliary tract cancer: a marriage of convenience? Int J Mol Sci. 2022;23(18):10869. doi: 10.3390/ijms231810869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumbly V, Landry I, Rizzo V. Ivosidenib for IDH1 mutant cholangiocarcinoma: a narrativereview. Cureus. 2022;14(1):e21018. doi: 10.7759/cureus.21018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harding J, Ikeda M, Goyal L, et al. A first-in-human Phase I study of LY3410738, a covalent inhibitor of mutant IDH1 and IDH2, as monotherapy and in combination with cisplatin and gemcitabine in advanced IDH-mutant cholangiocarcinoma. Poster session presented at: ESMO World Congress on Gastrointestinal Cancer; 2023 Jun 28-Jul 1; Barcelona, Spain. Available from: www.postersessiononline.eu/173580348_eu/congresos/WCGIC2023/aula/-SO_1_WCGIC2023.pdf [Google Scholar]
- 34.Ohba A, Morizane C, Ueno M, et al. Multicenter Phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Future Oncol. 2022;18(19):2351–2360. doi: 10.2217/fon-2022-0214 [DOI] [PubMed] [Google Scholar]
- 35.Larionov AA. Current therapies for human epidermal growth factor receptor 2-positive metastatic breast cancer patients. Front Oncol. 2018;8:89. doi: 10.3389/fonc.2018.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a Phase III, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 37.Riudavets M, Sullivan I, Abdayem P, et al. Targeting HER2 in non-small-cell lung cancer (NSCLC): a glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open. 2021;6(5):100260. doi: 10.1016/j.esmoop.2021.100260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Javle M, Borad MJ, Azad NS, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, Phase IIa, multiple basket study. Lancet Oncol. 2021;22(9):1290–1300. doi: 10.1016/S1470-2045(21)00336-3 [DOI] [PubMed] [Google Scholar]
- 39.Benson AB, D'Angelica MI, Abrams T, et al. NCCN guidelines insights: biliary tract cancers, Version 2.2023. J Natl Compr Canc Netw. 2023;21(7):694–704. doi: 10.6004/jnccn.2023.0035 [DOI] [PubMed] [Google Scholar]
- 40.Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386(3):241–251. doi: 10.1056/NEJMoa2112431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–2430. doi: 10.1056/NEJMoa2004413 [DOI] [PubMed] [Google Scholar]
- 43.Ohba A, Morizane C, Kawamoto Y, et al. Circulating tumor DNA (ctDNA) analyses in patients with HER2-positive biliary tract cancer (BTC) treated with trastuzumab deruxtecan (T-DXd): exploratory results from the HERB trial. J Clin Oncol. 2023;41(Suppl. 16):4097. doi: 10.1200/JCO.2023.41.16_suppl.409737099736 [DOI] [Google Scholar]
- 44.Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 Phase II trial. J Clin Oncol. 2024;42(1):47–58. doi: 10.1200/JCO.23.02005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harding JJ, Fan J, Oh DY, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, Phase IIb study. Lancet Oncol. 2023;24(7):772–782. doi: 10.1016/S1470-2045(23)00242-5 [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Mizuno N, Sunakawa Y, et al. Tucatinib and trastuzumab for previously treated human epidermal growth factor receptor 2-positive metastatic biliary tract cancer (SGNTUC-019): a Phase II basket study. J Clin Oncol. 2023;41(36):5569–5578. doi: 10.1200/JCO.23.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goeppert B, Frauenschuh L, Renner M, et al. BRAF V600E-specific immunohistochemistry reveals low mutation rates in biliary tract cancer and restriction to intrahepatic cholangiocarcinoma. Mod Pathol. 2014;27(7):1028–1034. doi: 10.1038/modpathol.2013.206 [DOI] [PubMed] [Google Scholar]
- 48.Subbiah V, Lassen U, Élez E, et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): a Phase II, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21(9):1234–1243. doi: 10.1016/S1470-2045(20)30321-1 [DOI] [PubMed] [Google Scholar]
- 49.Ray EM, Strassle PD, Gaber CE, et al. Predicting nodal positivity in women with hormone receptor-positive (HR+), early stage breast cancer (ESBC). J Clin Oncol. 2020;38(Suppl. 15):574. doi: 10.1200/JCO.2020.38.15_suppl.574 [DOI] [Google Scholar]
- 50.Boilève A, Verlingue L, Hollebecque A, et al. Rare cancer, rare alteration: the case of NTRK fusions in biliary tract cancers. Expert Opin Investig Drugs. 2021;30(4):401–409. doi: 10.1080/13543784.2021.1896703 [DOI] [PubMed] [Google Scholar]
- 51.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. doi: 10.1056/NEJMoa1714448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three Phase I–II trials. Lancet Oncol. 2020;21(2):271–282. doi: 10.1016/S1470-2045(19)30691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Werneck Krauss Silva V, Askan G, Daniel TD, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol. 2016;5(5):62. doi: 10.21037/cco.2016.10.04 [DOI] [PubMed] [Google Scholar]
- 54.Yu J, Zhang X, Huang Q, et al. Rare DNA mismatch repair-related protein loss in patients with intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma and their response to immunotherapy. Cancer Manag Res. 2021;13:4283–4290. doi: 10.2147/CMAR.S304281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maio M, Ascierto PA, Manzyuk L, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the Phase II KEYNOTE-158 study. Ann Oncol. 2022;33(9):929–938. doi: 10.1016/j.annonc.2022.05.519 [DOI] [PubMed] [Google Scholar]
- 56.Andre T, Berton D, Curigliano G, et al. Safety and efficacy of anti-PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: Results from GARNET study. J Clin Oncol. 2021;39(Suppl. 3):9. doi: 10.1200/JCO.2021.39.3_suppl.9 [DOI] [Google Scholar]
- 57.Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371–2381. doi: 10.1056/NEJMoa2103695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kabbara KW, Cannon T, Winer A, et al. Molecular pathogenesis of cholangiocarcinoma: implications for disease classification and therapy. Oncology (Williston Park, NY). 2022;36(8):492–498. doi: 10.46883/2022.25920971 [DOI] [PubMed] [Google Scholar]
- 59.Mao Z, Xiao H, Shen P, et al. KRAS(G12D) can be targeted by potent inhibitors via formation of salt bridge. Cell Discov. 2022;8(1):5. doi: 10.1038/s41421-021-00368-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subbiah V, Cassier PA, Siena S, et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the Phase I/II ARROW trial. Nat Med. 2022;28(8):1640–1645. doi: 10.1038/s41591-022-01931-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hassin O, Oren M. Drugging p53 in cancer: one protein, many targets. Nat Rev Drug Discov. 2022;22(2):127–144. doi: 10.1038/s41573-022-00571-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munisamy M, Mukherjee N, Thomas L, et al. Therapeutic opportunities in cancer therapy: targeting the p53-MDM2/MDMX interactions. Am J Cancer Res. 2021;11(12):5762–5781. [PMC free article] [PubMed] [Google Scholar]
- 63.Hu J, Cao J, Topatana W, et al. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J Hematol Oncol. 2021;14(1):157. doi: 10.1186/s13045-021-01169-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baugh EH, Ke H, Levine AJ, et al. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018;25(1):154–160. doi: 10.1038/cdd.2017.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu CE, Pan YR, Yeh CN, et al. Targeting P53 as a future strategy to overcome gemcitabine resistance in biliary tract cancers. Biomolecules. 2020;10(11):1474. doi: 10.3390/biom10111474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wade M, Wang YV, Wahl GM. The p53 orchestra: mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20(5):299–309. doi: 10.1016/j.tcb.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oliner JD, Pietenpol JA, Thiagalingam S, et al. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362(6423):857–860. doi: 10.1038/362857a0 [DOI] [PubMed] [Google Scholar]
- 68.Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003–1010. doi: 10.1038/ng.3375 [DOI] [PubMed] [Google Scholar]; •• This study molecularly characterized tumors from 260 patients with biliary tract cancer, detecting a broad range of genomic alterations that represented potential therapeutic targets, including TP53.
- 69.Bouattour M, Valle JW, Vogel A, et al. Characterization of long-term survivors in the TOPAZ-1 study of durvalumab or placebo plus gemcitabine and cisplatin in advanced biliary tract cancer. J Clin Oncol. 2023;41(Suppl. 4):531. doi: 10.1200/JCO.2023.41.4_suppl.531 [DOI] [Google Scholar]
- 70.Pu X, Zhu L, Li F, et al. Target molecular treatment markers in intrahepatic cholangiocarcinoma based on Chinese population. Pathol Res Pract. 2020;216(9):153116. doi: 10.1016/j.prp.2020.153116 [DOI] [PubMed] [Google Scholar]
- 71.Kendre G, Murugesan K, Brummer T, et al. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J Hepatol. 2023;78(3):614–626. doi: 10.1016/j.jhep.2022.11.030 [DOI] [PubMed] [Google Scholar]
- 72.Wong W, Lowery MA, Berger MF, et al. Ampullary cancer: evaluation of somatic and germline genetic alterations and association with clinical outcomes. Cancer. 2019;125(9):1441–1448. doi: 10.1002/cncr.31951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim SJ, Akita M, Sung YN, et al. MDM2 amplification in intrahepatic cholangiocarcinomas: its relationship with large-duct type morphology and uncommon KRAS mutations. Am J Surg Pathol. 2018;42(4):512–521. doi: 10.1097/PAS.0000000000001006 [DOI] [PubMed] [Google Scholar]
- 74.D'Afonseca V, Arencibia AD, Echeverria-Vega A, et al. Identification of altered genes in gallbladder cancer as potential driver mutations for diagnostic and prognostic purposes: a computational approach. Cancer Inform. 2020;19:1176935120922154. doi: 10.1177/1176935120922154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horie S, Endo K, Kawasaki H, et al. Overexpression of MDM2 protein in intrahepatic cholangiocarcinoma: relationship with p53 overexpression, Ki-67 labeling, and clinicopathological features. Virchows Arch. 2000;437(1):25–30. doi: 10.1007/s004280000201 [DOI] [PubMed] [Google Scholar]
- 76.Wattanawongdon W, Simawaranon Bartpho T, Tongtawee T. Expression of CD44 and MDM2 in cholangiocarcinoma is correlated with poor clinicopathologic characteristics. Int J Clin Exp Pathol. 2019;12(10):3961–3967. [PMC free article] [PubMed] [Google Scholar]
- 77.Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24(17):4154–4161. doi: 10.1158/1078-0432.CCR-18-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saleh MN, Patel MR, Bauer TM, et al. Phase I trial of ALRN-6924, a dual inhibitor of MDMX and MDM2, in patients with solid tumors and lymphomas bearing wild-type TP53. Clin Cancer Res. 2021;27(19):5236–5247. doi: 10.1158/1078-0432.CCR-21-0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konopleva M, Martinelli G, Daver N, et al. MDM2 inhibition: an important step forward in cancer therapy. Leukemia. 2020;34(11):2858–2874. doi: 10.1038/s41375-020-0949-z [DOI] [PubMed] [Google Scholar]
- 80.Italiano A, Miller WH Jr, Blay JY, et al. Phase I study of daily and weekly regimens of the orally administered MDM2 antagonist idasanutlin in patients with advanced tumors. Invest New Drugs. 2021;39(6):1587–1597. doi: 10.1007/s10637-021-01141-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gluck WL, Gounder MM, Frank R, et al. Phase I study of the MDM2 inhibitor AMG 232 in patients with advanced P53 wild-type solid tumors or multiple myeloma. Invest New Drugs. 2020;38(3):831–843. doi: 10.1007/s10637-019-00840-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stein EM, DeAngelo DJ, Chromik J, et al. Results from a first-in-human Phase I study of siremadlin (HDM201) in patients with advanced wild-type TP53 solid tumors and acute leukemia. Clin Cancer Res. 2022;28(5):870–881. doi: 10.1158/1078-0432.CCR-21-1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fang DD, Tang Q, Kong Y, et al. MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment. J Immunother Cancer. 2019;7(1):327. doi: 10.1186/s40425-019-0750-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McKean M, Tolcher AW, Reeves JA, et al. Newly updated activity results of alrizomadlin (APG-115), a novel MDM2/p53 inhibitor, plus pembrolizumab: Phase II study in adults and children with various solid tumors. J Clin Oncol. 2022;40(Suppl. 16):9517. doi: 10.1200/JCO.2022.40.16_suppl.9517 [DOI] [Google Scholar]
- 85.Abdul Razak AR, Bauer S, Suarez C, et al. Co-targeting of MDM2 and CDK4/6 with siremadlin and ribociclib for the treatment of patients with well-differentiated or dedifferentiated liposarcoma: results from a proof-of-concept, Phase Ib study. Clin Cancer Res. 2022;28(6):1087–1097. doi: 10.1158/1078-0432.CCR-21-1291 [DOI] [PubMed] [Google Scholar]
- 86.Gounder MM, Bauer TM, Schwartz GK, et al. A first-in-human Phase I study of milademetan, an MDM2 inhibitor, in patients with advanced liposarcoma, solid tumors, or lymphomas. J. Clin. Oncol. 2023;41(9):1714–1724. doi: 10.1200/JCO.22.01285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takahashi S, Fujiwara Y, Nakano K, et al. Safety and pharmacokinetics of milademetan, a MDM2 inhibitor, in Japanese patients with solid tumors: a Phase I study. Cancer Sci. 2021;112(6):2361–2370. doi: 10.1111/cas.14875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rain therapeutics provides interim analysis of Phase II basket trial of Milademetan for MDM2-amplified advanced solid tumors (MANTRA-2) [Internet] . Rain Therapeutics. 2022 Nov 4. [cited 2023 Nov 22]. Available from: www.rainoncology.com/news-press-releases/rain-therapeutics-provides-interim-analysis-of-phase-2-basket-trial-of-milademetan-for-mdm2-amplified-advanced-solid-tumors-mantra-2
- 89.Milademetan shows early activity in MDM2-amplified advanced solid tumors [Internet] . OncLive©. 2022 Nov 10. [cited 2023 Nov 22]. Available from: www.onclive.com/view/milademetan-shows-early-activity-in-mdm2-amplified-advanced-solid-tumors
- 90.Press release Rain Oncology announces topline results from Phase III MANTRA trial of milademetan for the treatment of dedifferentiated liposarcoma [Internet] . Rain Oncology. 2023 May 22. [cited 2023 Nov 22]. Available from: www.rainoncology.com/news-press-releases/rain-oncology-announces-topline-results-from-phase-3-mantra-trial-of-milademetan-for-the-treatment-of-dedifferentiated-liposarcoma
- 91.Press release Rain Oncology provides company update and outlines strategic priorities of milademetan clinical programs [Internet] . Rain Oncology. 2023 May 30. [cited 2023 Nov 22]. Available from: www.rainoncology.com/news-press-releases/rain-oncology-provides-company-update-and-outlines-strategic-priorities-of-milademetan-clinical-programs
- 92.Cornillie J, Wozniak A, Li H, et al. Anti-tumor activity of the MDM2-TP53 inhibitor BI-907828 in dedifferentiated liposarcoma patient-derived xenograft models harboring MDM2 amplification. Clin Transl Oncol. 2020;22(4):546–554. doi: 10.1007/s12094-019-02158-z [DOI] [PubMed] [Google Scholar]
- 93.Rudolph D, Reschke M, Blake S, et al. BI 907828: a novel, potent MDM2 inhibitor that induces antitumor immunologic memory and acts synergistically with an anti-PD-1 antibody in syngeneic mouse models of cancer. Cancer Res. 2018;78(Suppl. 13):4866. doi: 10.1158/1538-7445.AM2018-4866 [DOI] [Google Scholar]
- 94.Gounder MM, Yamamoto N, Patel MR, et al. A Phase Ia/Ib, dose-escalation/expansion study of the MDM2-p53 antagonist BI 907828 in patients with solid tumors, including advanced/metastatic liposarcoma (LPS). J Clin Oncol. 2022;40(Suppl. 16):3004. doi: 10.1200/JCO.2022.40.16_suppl.3004 [DOI] [Google Scholar]
- 95.Yamamoto N, Hafez N, Tolcher AW, et al. A Phase Ia/Ib, dose-escalation/expansion study of BI 907828 in combination with BI 754091 (ezabenlimab) and BI 754111 in patients (pts) with advanced solid tumors. J Clin Oncol. 2022;40(Suppl. 16):3095. doi: 10.1200/JCO.2022.40.16_suppl.309535709436 [DOI] [Google Scholar]
- 96.LoRusso P, Gounder MM, Patel MR, et al. A Phase I dose-escalation study of the MDM2-p53 antagonist BI 907828 in patients (pts) with advanced solid tumors. J Clin Oncol. 2021;39(Suppl. 15):3016. doi: 10.1200/JCO.2021.39.15_suppl.3016 [DOI] [Google Scholar]
- 97.LoRusso P, Yamamoto N, Patel MR, et al. The MDM2-p53 antagonist brigimadlin (BI 907828) in patients with advanced or metastatic solid tumors: results of a phase ia, first-in-human, dose-escalation study. Cancer Discov. 2023;13(8):1802–1813. doi: 10.1158/2159-8290.CD-23-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamamoto N, Tolcher A, Hafez N, et al. Efficacy and Safety of the MDM2–p53 Antagonist Brigimadlin (BI 907828) in Patients with Advanced Biliary Tract Cancer: A Case Series. Onco Targets Ther. 2024;17:267–280. doi: 10.2147/OTT.S440979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoo C, Lamarca A, Choi HJ, et al. Brightline-2: a Phase IIa/IIb trial of brigimadlin (BI 907828) in advanced biliary tract cancer, pancreatic ductal adenocarcinoma or other solid tumors. Future Oncol. 2024. [cited 2023 Nov 22]. doi: 10.2217/fon-2023-0963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoo C, Lamarca A, Choi HJ, et al. Brightline-2: a Phase IIa/IIb trial of brigimadlin (BI 907828) in advanced biliary tract cancer, pancreatic ductal adenocarcinoma or other solid tumors. Future Oncol. 2024. doi: 10.2217/fon-2023-0963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen C, Wang T, Yang M, et al. Genomic profiling of blood-derived circulating tumor DNA from patients with advanced biliary tract cancer. Pathol Oncol Res. 2021;27:1609879. doi: 10.3389/pore.2021.1609879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Biomarkers Matter [Internet] . Cholangiocarcinoma Foundation. 2022. [cited 2023 Nov 22]. Available from: https://cholangiocarcinoma.org/mutationsmatter/biomarkers-matter/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.