Abstract
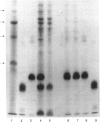
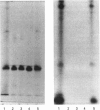
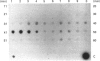
Quantitative isolation of bovine prolactin was accomplished by immunoaffinity chromatography using clonal antibody as the stationary ligand. The phosphorylated and non-phosphorylated (native) prolactins contained in the immunopurified preparations were separated by chromatofocusing. Isolates from individual pituitaries revealed that phosphorylated prolactin represented between 20 and 80% of the total prolactin. The stoichiometry of phosphate in phosphorylated prolactin was 1.4:1 when determined by amino acid analysis after preparation of the S-ethylcysteine derivative. One major phosphorylation site, serine-90, and two minor sites, serine-26 and -34, were determined by mapping and sequencing studies. Serine-90 was conserved in prolactins, growth hormones and placental lactogens. Serine-26 and -34 were conserved in prolactins, but were not found in growth hormones or placental lactogens. Absorption spectroscopy of the aromatic amino acid residues indicated that phosphorylation of prolactin was associated with a unique structural conformation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Meguid S. S., Shieh H. S., Smith W. W., Dayringer H. E., Violand B. N., Bentle L. A. Three-dimensional structure of a genetically engineered variant of porcine growth hormone. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6434–6437. doi: 10.1073/pnas.84.18.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams T. E., Baker L., Brandon M. R. Cloning and nucleotide sequence of an ovine prolactin cDNA. Nucleic Acids Res. 1989 Jan 11;17(1):440–440. doi: 10.1093/nar/17.1.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Rossmann M. G., Johnson J. E. A four-helical super-secondary structure. Biochem Biophys Res Commun. 1977 Mar 7;75(1):83–86. doi: 10.1016/0006-291x(77)91292-x. [DOI] [PubMed] [Google Scholar]
- Asawaroengchai H., Russell S. M., Nicoll C. S. Electrophoretically separable forms of rat prolactin with different bioassay and radioimmunoassay activities. Endocrinology. 1978 Feb;102(2):407–414. doi: 10.1210/endo-102-2-407. [DOI] [PubMed] [Google Scholar]
- Brooks C. L., Kim B. G., Aphale P., Kleeman B. E., Johnson G. C. Phosphorylated variant of bovine prolactin. Mol Cell Endocrinol. 1990 Jun 18;71(2):117–123. doi: 10.1016/0303-7207(90)90248-7. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Shine J., Baxter J. D., Martial J. A. Human prolactin. cDNA structural analysis and evolutionary comparisons. J Biol Chem. 1981 Apr 25;256(8):4007–4016. [PubMed] [Google Scholar]
- Cooke N. E., Ray J., Emery J. G., Liebhaber S. A. Two distinct species of human growth hormone-variant mRNA in the human placenta predict the expression of novel growth hormone proteins. J Biol Chem. 1988 Jun 25;263(18):9001–9006. [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Phosphorylation of pro-adrenocorticotropin/endorphin-derived peptides. J Biol Chem. 1982 May 10;257(9):4907–4915. [PubMed] [Google Scholar]
- Fellows R. E., Jr, Rogol A. D. Structural studies on bovine growth hormone. I. Isolation and characterization of cyanogen bromide fragments. J Biol Chem. 1969 Mar 25;244(6):1567–1575. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gubbins E. J., Maurer R. A., Lagrimini M., Erwin C. R., Donelson J. E. Structure of the rat prolactin gene. J Biol Chem. 1980 Sep 25;255(18):8655–8662. [PubMed] [Google Scholar]
- Guyda J. H. Heterogeneity of human growth hormone and prolactin secreted in vitro: Immunoassay and radioreceptor assay correlations. J Clin Endocrinol Metab. 1975 Nov;41(5):953–967. doi: 10.1210/jcem-41-5-953. [DOI] [PubMed] [Google Scholar]
- Hampson R. K., Rottman F. M. Alternative processing of bovine growth hormone mRNA: nonsplicing of the final intron predicts a high molecular weight variant of bovine growth hormone. Proc Natl Acad Sci U S A. 1987 May;84(9):2673–2677. doi: 10.1073/pnas.84.9.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro L. S., Lee D. W., Singh R. N., Bee G., Markoff E., Lewis U. J. Glycosylated human prolactin: alterations in glycosylation pattern modify affinity for lactogen receptor and values in prolactin radioimmunoassay. J Clin Endocrinol Metab. 1990 Aug;71(2):379–383. doi: 10.1210/jcem-71-2-379. [DOI] [PubMed] [Google Scholar]
- Haro L. S., Talamantes F. J. Secreted mouse prolactin (PRL) and stored ovine PRL. II. Role of amides in receptor binding and immunoreactivity. Endocrinology. 1985 Jan;116(1):353–358. doi: 10.1210/endo-116-1-353. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hirt H., Kimelman J., Birnbaum M. J., Chen E. Y., Seeburg P. H., Eberhardt N. L., Barta A. The human growth hormone gene locus: structure, evolution, and allelic variations. DNA. 1987 Feb;6(1):59–70. doi: 10.1089/dna.1987.6.59. [DOI] [PubMed] [Google Scholar]
- Ho T. W., Greenan J. R., Walker A. M. Mammotroph autoregulation: the differential roles of the 24K isoforms of prolactin. Endocrinology. 1989 Mar;124(3):1507–1514. doi: 10.1210/endo-124-3-1507. [DOI] [PubMed] [Google Scholar]
- Karatzas C. N., Zadworny D., Kuhnlein U. Nucleotide sequence of turkey prolactin. Nucleic Acids Res. 1990 May 25;18(10):3071–3071. doi: 10.1093/nar/18.10.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Shimokawa N., Kato T., Hirai T., Yoshihama K., Kawai H., Hattori M., Ezashi T., Shimogori Y., Wakabayashi K. Porcine growth hormone: molecular cloning of cDNA and expression in bacterial and mammalian cells. Biochim Biophys Acta. 1990 Apr 6;1048(2-3):290–293. doi: 10.1016/0167-4781(90)90069-e. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LI C. H., ASH L. The nitrogen terminal end-groups of hypophyseal growth hormone. J Biol Chem. 1953 Jul;203(1):419–424. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson D. M., Gala R. R., Chin M. L., Haisenleder D. H. Size heterogeneity of plasma prolactin in the rat: TRH and serotonin-induced changes. Life Sci. 1980 Sep 29;27(13):1147–1151. doi: 10.1016/0024-3205(80)90465-8. [DOI] [PubMed] [Google Scholar]
- Lehrman S. R., Lahm H. W., Miedel M. C., Hulmes J. D., Li C. H. Primary structure of equine pituitary prolactin. Int J Pept Protein Res. 1988 Jun;31(6):544–554. doi: 10.1111/j.1399-3011.1988.tb00913.x. [DOI] [PubMed] [Google Scholar]
- Lewis U. J., Singh R. N., Lewis L. J., Seavey B. K., Sinha Y. N. Glycosylated ovine prolactin. Proc Natl Acad Sci U S A. 1984 Jan;81(2):385–389. doi: 10.1073/pnas.81.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis U. J. Variants of growth hormone and prolactin and their posttranslational modifications. Annu Rev Physiol. 1984;46:33–42. doi: 10.1146/annurev.ph.46.030184.000341. [DOI] [PubMed] [Google Scholar]
- Li C. H., Oosthuizen M. M., Chung D. Primary structure of elephant pituitary prolactin. Int J Pept Protein Res. 1989 Jan;33(1):67–69. doi: 10.1111/j.1399-3011.1989.tb00684.x. [DOI] [PubMed] [Google Scholar]
- Liberti J. P., Antoni B. A., Chlebowski J. F. Naturally-occurring pituitary growth hormone is phosphorylated. Biochem Biophys Res Commun. 1985 Apr 30;128(2):713–720. doi: 10.1016/0006-291x(85)90105-6. [DOI] [PubMed] [Google Scholar]
- Liberti J. P., Joshi G. S. Synthesis and secretion of phosphorylated growth hormone by rat pituitary glands in vitro. Biochem Biophys Res Commun. 1986 Jun 13;137(2):806–812. doi: 10.1016/0006-291x(86)91151-4. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Talamantes F. Nucleotide sequence of mouse prolactin and growth hormone mRNAs and expression of these mRNAs during pregnancy. J Biol Chem. 1985 Aug 15;260(17):9574–9579. [PubMed] [Google Scholar]
- Martial J. A., Hallewell R. A., Baxter J. D., Goodman H. M. Human growth hormone: complementary DNA cloning and expression in bacteria. Science. 1979 Aug 10;205(4406):602–607. doi: 10.1126/science.377496. [DOI] [PubMed] [Google Scholar]
- Meyer H. E., Hoffmann-Posorske E., Korte H., Heilmeyer L. M., Jr Sequence analysis of phosphoserine-containing peptides. Modification for picomolar sensitivity. FEBS Lett. 1986 Aug 11;204(1):61–66. doi: 10.1016/0014-5793(86)81388-6. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Martial J. A., Baxter J. D. Molecular cloning of DNA complementary to bovine growth hormone mRNA. J Biol Chem. 1980 Aug 25;255(16):7521–7524. [PubMed] [Google Scholar]
- Nyberg F., Roos P., Wide L. Human pituitary prolactin: isolation and characterization of three isohormones with different bioassay and radioimmunoassay activities. Biochim Biophys Acta. 1980 Oct 21;625(2):255–265. doi: 10.1016/0005-2795(80)90289-5. [DOI] [PubMed] [Google Scholar]
- Oetting W. S., Tuazon P. T., Traugh J. A., Walker A. M. Phosphorylation of prolactin. J Biol Chem. 1986 Feb 5;261(4):1649–1652. [PubMed] [Google Scholar]
- Oetting W. S., Walker A. M. Differential isoform distribution between stored and secreted prolactin. Endocrinology. 1986 Sep;119(3):1377–1381. doi: 10.1210/endo-119-3-1377. [DOI] [PubMed] [Google Scholar]
- Orian J. M., O'Mahoney J. V., Brandon M. R. Cloning and sequencing of the ovine growth hormone gene. Nucleic Acids Res. 1988 Sep 26;16(18):9046–9046. doi: 10.1093/nar/16.18.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F. M., Chang W. C. Cloning and sequencing of bullfrog growth hormone complementary DNA. Biochim Biophys Acta. 1988 Jul 13;950(2):238–242. doi: 10.1016/0167-4781(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Sasavage N. L., Nilson J. H., Horowitz S., Rottman F. M. Nucleotide sequence of bovine prolactin messenger RNA. Evidence for sequence polymorphism. J Biol Chem. 1982 Jan 25;257(2):678–681. [PubMed] [Google Scholar]
- Sasavage N. L., Smith M., Gillam S., Woychik R. P., Rottman F. M. Variation in the polyadenylylation site of bovine prolactin mRNA. Proc Natl Acad Sci U S A. 1982 Jan;79(2):223–227. doi: 10.1073/pnas.79.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler L. A., Shimomura K., Kessler M. A., Zieler C. G., Bremel R. D. Bovine placental lactogen: molecular cloning and protein structure. Biochemistry. 1988 Nov 1;27(22):8443–8448. doi: 10.1021/bi00422a022. [DOI] [PubMed] [Google Scholar]
- Schulz-Aellen M. F., Schmid E., Movva R. N. Nucleotide sequence of porcine preprolactin cDNA. Nucleic Acids Res. 1989 Apr 25;17(8):3295–3295. doi: 10.1093/nar/17.8.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Shine J., Martial J. A., Baxter J. D., Goodman H. M. Nucleotide sequence and amplification in bacteria of structural gene for rat growth hormone. Nature. 1977 Dec 8;270(5637):486–494. doi: 10.1038/270486a0. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Sias S., Adelman J., de Boer H. A., Hayflick J., Jhurani P., Goeddel D. V., Heyneker H. L. Efficient bacterial expression of bovine and porcine growth hormones. DNA. 1983;2(1):37–45. doi: 10.1089/dna.1.1983.2.37. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H. The human growth hormone gene family: nucleotide sequences show recent divergence and predict a new polypeptide hormone. DNA. 1982;1(3):239–249. doi: 10.1089/dna.1.1982.1.239. [DOI] [PubMed] [Google Scholar]
- Shine J., Seeburg P. H., Martial J. A., Baxter J. D., Goodman H. M. Construction and analysis of recombinant DNA for human chorionic somatomammotropin. Nature. 1977 Dec 8;270(5637):494–499. doi: 10.1038/270494a0. [DOI] [PubMed] [Google Scholar]
- Simpson D. L., Hranisavljevic J., Davidson E. A. Elimination and sulfite addition as a means of localization and identification of substituted seryl and threonyl residues in proteins and proteoglycans. Biochemistry. 1972 May 9;11(10):1849–1856. doi: 10.1021/bi00760a019. [DOI] [PubMed] [Google Scholar]
- Sinha Y. N., Baxter S. R. Identification of a nonimmunoreactive but highly bioactive form of prolactin in the mouse pituitary by gel electrophoresis. Biochem Biophys Res Commun. 1979 Jan 30;86(2):325–330. doi: 10.1016/0006-291x(79)90869-6. [DOI] [PubMed] [Google Scholar]
- Sinha Y. N. Molecular size variants of prolactin and growth hormone in mouse serum: strain differences and alterations of concentrations by physiological and pharmacological stimuli. Endocrinology. 1980 Dec;107(6):1959–1969. doi: 10.1210/endo-107-6-1959. [DOI] [PubMed] [Google Scholar]
- Smith A. I., Funder J. W. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev. 1988 Feb;9(1):159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- Strickland T. W., Pierce J. G. Glycosylation of ovine prolactin during cell-free biosynthesis. Endocrinology. 1985 Apr;116(4):1295–1298. doi: 10.1210/endo-116-4-1295. [DOI] [PubMed] [Google Scholar]
- Watahiki M., Tanaka M., Masuda N., Sugisaki K., Yamamoto M., Yamakawa M., Nagai J., Nakashima K. Primary structure of chicken pituitary prolactin deduced from the cDNA sequence. Conserved and specific amino acid residues in the domains of the prolactins. J Biol Chem. 1989 Apr 5;264(10):5535–5539. [PubMed] [Google Scholar]
- Yamano Y., Oyabayashi K., Okuno M., Yato M., Kioka N., Manabe E., Hashi H., Sakai H., Komano T., Utsumi K. Cloning and sequencing of cDNA that encodes goat growth hormone. FEBS Lett. 1988 Feb 15;228(2):301–304. doi: 10.1016/0014-5793(88)80020-6. [DOI] [PubMed] [Google Scholar]
- Zakin M. M., Poskus E., Langton A. A., Ferrara P., Santomé J. A., Dellacha J. M., Paladini A. C. Primary structure of equine growth hormone. Int J Pept Protein Res. 1976;8(5):435–444. doi: 10.1111/j.1399-3011.1976.tb02523.x. [DOI] [PubMed] [Google Scholar]