Abstract
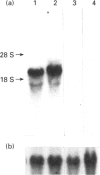
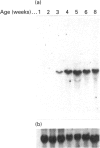
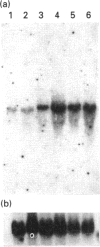
A sandwich e.l.i.s.a. method was developed to examine the distribution of lactoferrin in mouse reproductive tract. The lactoferrin concentration was found to be much higher in oviduct, uterus, vagina, vas deferens or epididymis than in serum, but the concentration in ovary, testis, seminal vesicle, prostate or coagulating gland was comparable with that in serum. The existence of lactoferrin in male sexual organs was confirmed by Western-blot analyses for tissue proteins. Lactoferrin in male sexual organs was shown to have a molecular mass similar to that of the deglycosylated form of lactoferrin purified from mouse uterine luminal fluid. Northern-blot analyses for total RNA prepared from male sexual organs indicated that only epididymis contained the lactoferrin mRNA. The lactoferrin mRNA was found in the prepubertal period and increased with the growth of epididymis. The mRNA level in prepubertal epididymis could be stimulated by 17 beta-oestradiol, but was not influenced by testosterone.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M., Skutelsky E. Affinity cytochemistry: the localization of lectin and antibody receptors on erythrocytes via the avidin-biotin complex. FEBS Lett. 1976 Oct 1;68(2):240–244. doi: 10.1016/0014-5793(76)80445-0. [DOI] [PubMed] [Google Scholar]
- Bellamy W., Takase M., Yamauchi K., Wakabayashi H., Kawase K., Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992 May 22;1121(1-2):130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- Brenner R. M., West N. B., McClellan M. C. Estrogen and progestin receptors in the reproductive tract of male and female primates. Biol Reprod. 1990 Jan;42(1):11–19. doi: 10.1095/biolreprod42.1.11. [DOI] [PubMed] [Google Scholar]
- Brock J. H. Lactoferrin in human milk: its role in iron absorption and protection against enteric infection in the newborn infant. Arch Dis Child. 1980 Jun;55(6):417–421. doi: 10.1136/adc.55.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D. E. Effect of androgens on protein synthesis and secretion in various regions of the rat epididymis, as analysed by two-dimensional gel electrophoresis. Mol Cell Endocrinol. 1983 Mar;29(3):255–270. doi: 10.1016/0303-7207(83)90016-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Connell C. J., Donjacour A. A morphological study of the epididymides of control and estradiol-treated prepubertal dogs. Biol Reprod. 1985 Nov;33(4):951–969. doi: 10.1095/biolreprod33.4.951. [DOI] [PubMed] [Google Scholar]
- Crouch S. P., Slater K. J., Fletcher J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood. 1992 Jul 1;80(1):235–240. [PubMed] [Google Scholar]
- Delongeas J. L., Gelly J. L. Differentiation of the rat epididymis after withdrawal of androgen. Cell Tissue Res. 1985;241(3):657–662. doi: 10.1007/BF00214588. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Gentile P., Broxmeyer H. E. Suppression of mouse myelopoiesis by administration of human lactoferrin in vivo and the comparative action of human transferrin. Blood. 1983 May;61(5):982–993. [PubMed] [Google Scholar]
- Goodman S. A., Young L. G. Immunological identification of lactoferrin as a shared antigen on radioiodinated human sperm surface and in radioiodinated human seminal plasma. J Reprod Immunol. 1981 Jun;3(2):99–108. doi: 10.1016/0165-0378(81)90014-0. [DOI] [PubMed] [Google Scholar]
- Jones R., Brown C. R., Von Glós K. I., Parker M. G. Hormonal regulation of protein synthesis in the rat epididymis. Characterization of androgen-dependent and testicular fluid-dependent proteins. Biochem J. 1980 Jun 15;188(3):667–676. doi: 10.1042/bj1880667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Liu Y. H., Teng C. T. Characterization of estrogen-responsive mouse lactoferrin promoter. J Biol Chem. 1991 Nov 15;266(32):21880–21885. [PubMed] [Google Scholar]
- Liu Y., Teng C. T. Estrogen response module of the mouse lactoferrin gene contains overlapping chicken ovalbumin upstream promoter transcription factor and estrogen receptor-binding elements. Mol Endocrinol. 1992 Mar;6(3):355–364. doi: 10.1210/mend.6.3.1584212. [DOI] [PubMed] [Google Scholar]
- Lu L., Hangoc G., Oliff A., Chen L. T., Shen R. N., Broxmeyer H. E. Protective influence of lactoferrin on mice infected with the polycythemia-inducing strain of Friend virus complex. Cancer Res. 1987 Aug 1;47(15):4184–4188. [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Lactoferrin in milk from different species. Comp Biochem Physiol B. 1971 May 15;39(1):119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- McMaster M. T., Teng C. T., Dey S. K., Andrews G. K. Lactoferrin in the mouse uterus: analyses of the preimplantation period and regulation by ovarian steroids. Mol Endocrinol. 1992 Jan;6(1):101–111. doi: 10.1210/mend.6.1.1738363. [DOI] [PubMed] [Google Scholar]
- Metz-Boutigue M. H., Jollès J., Mazurier J., Schoentgen F., Legrand D., Spik G., Montreuil J., Jollès P. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984 Dec 17;145(3):659–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Teng C. T., Beckman W. C., Jr, Jefferson W. N., Hanson R. B., Miller J. V., McLachlan J. A. Fluctuations of lactoferrin protein and messenger ribonucleic acid in the reproductive tract of the mouse during the estrous cycle. Biol Reprod. 1992 Nov;47(5):903–915. doi: 10.1095/biolreprod47.5.903. [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist M. C., Eller B. C., Danzo B. J. The effects of estradiol, tamoxifen, and testosterone on the weights and histology of the epididymis and accessory sex organs of sexually immature rabbits. Endocrinology. 1983 Nov;113(5):1703–1715. doi: 10.1210/endo-113-5-1703. [DOI] [PubMed] [Google Scholar]
- Pentecost B. T., Teng C. T. Lactotransferrin is the major estrogen inducible protein of mouse uterine secretions. J Biol Chem. 1987 Jul 25;262(21):10134–10139. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rado T. A., Bollekens J., St Laurent G., Parker L., Benz E. J., Jr Lactoferrin biosynthesis during granulocytopoiesis. Blood. 1984 Nov;64(5):1103–1109. [PubMed] [Google Scholar]
- Schleicher G., Drews U., Stumpf W. E., Sar M. Differential distribution of dihydrotestosterone and estradiol binding sites in the epididymis of the mouse. An autoradiographic study. Histochemistry. 1984;81(2):139–147. doi: 10.1007/BF00490107. [DOI] [PubMed] [Google Scholar]
- Sun E. L., Flickinger C. J. Development of cell types and of regional differences in the postnatal rat epididymis. Am J Anat. 1979 Jan;154(1):27–55. doi: 10.1002/aja.1001540104. [DOI] [PubMed] [Google Scholar]
- Sun E. L., Flickinger C. J. Proliferative activity in the rat epididymis during postnatal development. Anat Rec. 1982 Jun;203(2):273–284. doi: 10.1002/ar.1092030209. [DOI] [PubMed] [Google Scholar]
- Sánchez L., Calvo M., Brock J. H. Biological role of lactoferrin. Arch Dis Child. 1992 May;67(5):657–661. doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber P. F., Zaneveld L. J., Propping D., Schumacher G. F. Components of human split ejaculates. I. Spermatozoa, fructose, immunoglobulins, albumin, lactoferrin, transferrin and other plasma proteins. J Reprod Fertil. 1975 May;43(2):249–267. doi: 10.1530/jrf.0.0430249. [DOI] [PubMed] [Google Scholar]
- Teng C. T., Pentecost B. T., Chen Y. H., Newbold R. R., Eddy E. M., McLachlan J. A. Lactotransferrin gene expression in the mouse uterus and mammary gland. Endocrinology. 1989 Feb;124(2):992–999. doi: 10.1210/endo-124-2-992. [DOI] [PubMed] [Google Scholar]
- Teng C. T., Walker M. P., Bhattacharyya S. N., Klapper D. G., DiAugustine R. P., McLachlan J. A. Purification and properties of an oestrogen-stimulated mouse uterine glycoprotein (approx. 70 kDa). Biochem J. 1986 Dec 1;240(2):413–422. doi: 10.1042/bj2400413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmer D. K., Wrona M. A., Hughes C. L., Nelson K. G. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology. 1992 Sep;131(3):1458–1466. doi: 10.1210/endo.131.3.1505477. [DOI] [PubMed] [Google Scholar]
- Zucali J. R., Broxmeyer H. E., Levy D., Morse C. Lactoferrin decreases monocyte-induced fibroblast production of myeloid colony-stimulating activity by suppressing monocyte release of interleukin-1. Blood. 1989 Oct;74(5):1531–1536. [PubMed] [Google Scholar]
- van Beurden-Lamers W. M., Brinkmann A. O., Mulder E., van der Molen H. J. High-affinity binding of oestradiol-17beta by cytosols from testis interstitial tissue, pituitary, adrenal, liver and accessory sex glands of the male rat. Biochem J. 1974 Jun;140(3):495–502. doi: 10.1042/bj1400495. [DOI] [PMC free article] [PubMed] [Google Scholar]