Abstract
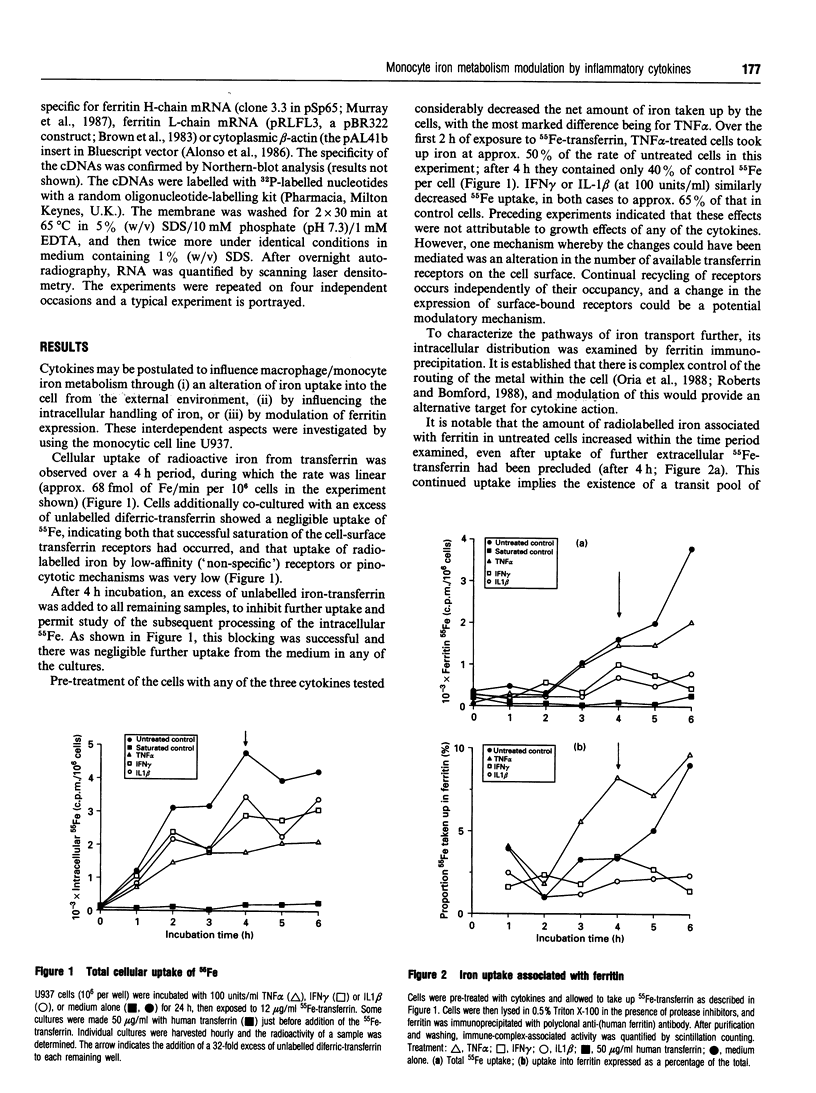
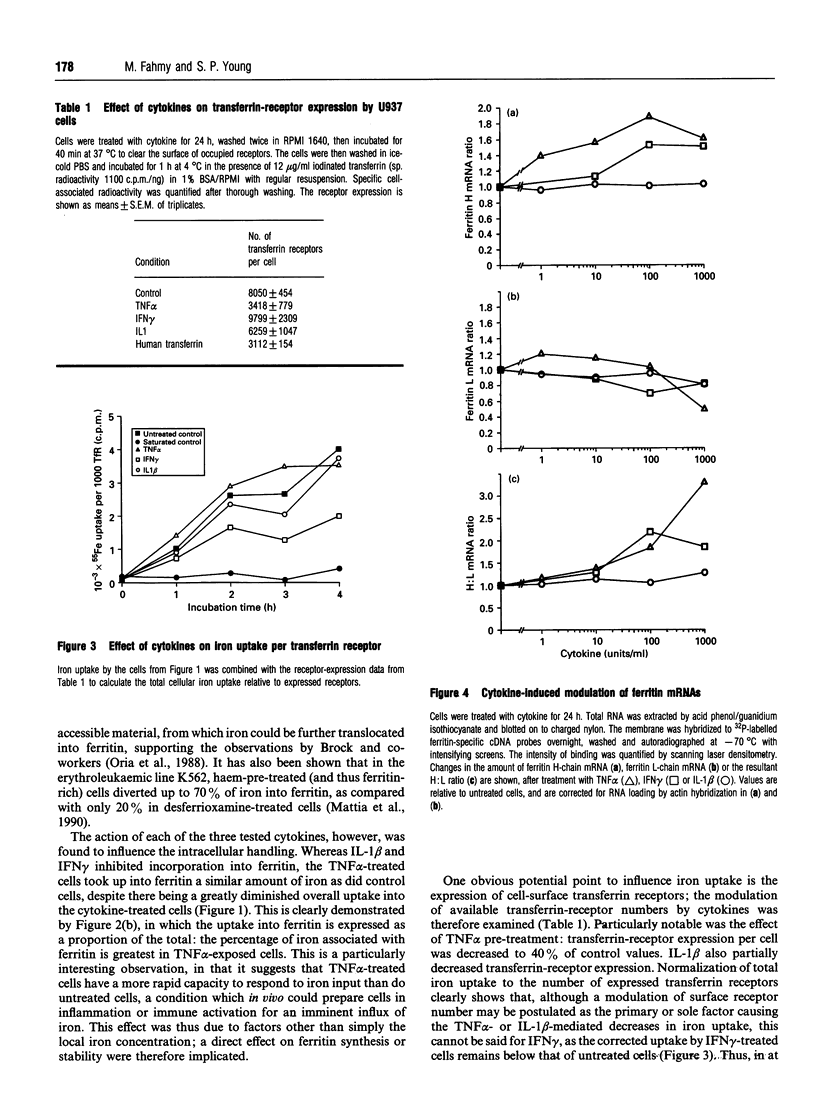
We have investigated the effects of the pro-inflammatory cytokines interleukin 1 beta (IL-1 beta), tumour necrosis factor alpha (TNF alpha) and interferon gamma (IFN gamma) on the iron metabolism of the human monocytic cell line U937. Cells were treated with each cytokine for up to 24 h, and then iron uptake from diferric transferrin was determined. The intracellular distribution of this iron, the expression of the transferrin receptor and levels of mRNA for the two ferritin subunits were also studied. IL-1 beta, TNF alpha and IFN gamma all decreased transferrin-iron uptake into cells, and all three cytokines had effects on the proportion of iron associated with ferritin. With TNF alpha there was a marked enhancement of the fraction incorporated into ferritin. Transferrin-receptor expression was diminished by TNF alpha and IL-1 beta, but not IFN gamma, suggesting different effector mechanisms. Both TNF alpha and IFN gamma increased the amount of cellular mRNA for ferritin H-chain, but not the L-chain; IL-1 beta affected mRNA for neither ferritin. These data demonstrate that cytokines, which can be present at high concentrations in inflammation, have the capacity to affect macrophage iron uptake, transferrin receptor expression, intracellular iron handling and the relative abundance of ferritin-subunit mRNA, and may therefore be important mediators in the observed perturbations of iron metabolism in inflammatory diseases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman T. G., Arosio P., Drysdale J. W. Multiple subunits in human ferritins: evidence for hybrid molecules. Biochem Biophys Res Commun. 1975 Apr 21;63(4):1056–1062. doi: 10.1016/0006-291x(75)90676-2. [DOI] [PubMed] [Google Scholar]
- Alvarez-Hernández X., Felstein M. V., Brock J. H. The relationship between iron release, ferritin synthesis and intracellular iron distribution in mouse peritoneal macrophages. Evidence for a reduced level of metabolically available iron in elicited macrophages. Biochim Biophys Acta. 1986 Apr 29;886(2):214–222. doi: 10.1016/0167-4889(86)90139-4. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgegård G., Caro J. Increased ferritin synthesis and iron uptake in inflammatory mouse macrophages. Scand J Haematol. 1984 Jul;33(1):43–48. doi: 10.1111/j.1600-0609.1984.tb02208.x. [DOI] [PubMed] [Google Scholar]
- Bolann B. J., Ulvik R. J. On the limited ability of superoxide to release iron from ferritin. Eur J Biochem. 1990 Nov 13;193(3):899–904. doi: 10.1111/j.1432-1033.1990.tb19415.x. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Brock J. H., Esparza I., Logie A. C. The nature of iron released by resident and stimulated mouse peritoneal macrophages. Biochim Biophys Acta. 1984 Jan 24;797(1):105–111. doi: 10.1016/0304-4165(84)90388-x. [DOI] [PubMed] [Google Scholar]
- Brock J. H. The effect of iron and transferrin on the response of serum-free cultures of mouse lymphocytes to concanavalin A and lipopolysaccharide. Immunology. 1981 Jun;43(2):387–392. [PMC free article] [PubMed] [Google Scholar]
- Brown A. J., Leibold E. A., Munro H. N. Isolation of cDNA clones for the light subunit of rat liver ferritin: evidence that the light subunit is encoded by a multigene family. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1265–1269. doi: 10.1073/pnas.80.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazenbalk G. D., Wadsworth H. L., Rapoport B. Transcriptional regulation of ferritin H messenger RNA levels in FRTL5 rat thyroid cells by thyrotropin. J Biol Chem. 1990 Jan 15;265(2):666–670. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Edbrooke M. R., Burt D. W., Cheshire J. K., Woo P. Identification of cis-acting sequences responsible for phorbol ester induction of human serum amyloid A gene expression via a nuclear factor kappaB-like transcription factor. Mol Cell Biol. 1989 May;9(5):1908–1916. doi: 10.1128/mcb.9.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza I., Brock J. H. Release of iron by resident and stimulated mouse peritoneal macrophages following ingestion and degradation of transferrin-antitransferrin immune complexes. Br J Haematol. 1981 Dec;49(4):603–614. doi: 10.1111/j.1365-2141.1981.tb07270.x. [DOI] [PubMed] [Google Scholar]
- Finch C. A., Deubelbeiss K., Cook J. D., Eschbach J. W., Harker L. A., Funk D. D., Marsaglia G., Hillman R. S., Slichter S., Adamson J. W. Ferrokinetics in man. Medicine (Baltimore) 1970 Jan;49(1):17–53. doi: 10.1097/00005792-197001000-00002. [DOI] [PubMed] [Google Scholar]
- Galbraith R. M., Galbraith G. M. Expression of transferrin receptors on mitogen-stimulated human peripheral blood lymphocytes: relation to cellular activation and related metabolic events. Immunology. 1981 Dec;44(4):703–710. [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T., Bitterman P. B., Mornex J. F., Crystal R. G. Expression of the transferrin receptor gene during the process of mononuclear phagocyte maturation. J Immunol. 1986 Feb 15;136(4):1339–1345. [PubMed] [Google Scholar]
- Iacopetta B. J., Rothenberger S., Kühn L. C. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988 Aug 12;54(4):485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Imamura K., Spriggs D., Kufe D. Expression of tumor necrosis factor receptors on human monocytes and internalization of receptor bound ligand. J Immunol. 1987 Nov 1;139(9):2989–2992. [PubMed] [Google Scholar]
- Konijn A. M., Hershko C. Ferritin synthesis in inflammation. I. Pathogenesis of impaired iron release. Br J Haematol. 1977 Sep;37(1):7–16. [PubMed] [Google Scholar]
- Lamb J. E., Ray F., Ward J. H., Kushner J. P., Kaplan J. Internalization and subcellular localization of transferrin and transferrin receptors in HeLa cells. J Biol Chem. 1983 Jul 25;258(14):8751–8758. [PubMed] [Google Scholar]
- Lee D. S., Griffiths B. W. Comparative studies of Iodo-bead and chloramine-T methods for the radioiodination of human alpha-fetoprotein. J Immunol Methods. 1984 Nov 16;74(1):181–189. doi: 10.1016/0022-1759(84)90379-x. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Levi S., Luzzago A., Cesareni G., Cozzi A., Franceschinelli F., Albertini A., Arosio P. Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J Biol Chem. 1988 Dec 5;263(34):18086–18092. [PubMed] [Google Scholar]
- Mattia E., den Blaauwen J., van Renswoude J. Role of protein synthesis in the accumulation of ferritin mRNA during exposure of cells to iron. Biochem J. 1990 Apr 15;267(2):553–555. doi: 10.1042/bj2670553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May W. S., Jr, Sahyoun N., Wolf M., Cuatrecasas P. Role of intracellular calcium mobilization in the regulation of protein kinase C-mediated membrane processes. Nature. 1985 Oct 10;317(6037):549–551. doi: 10.1038/317549a0. [DOI] [PubMed] [Google Scholar]
- Miller L. L., Miller S. C., Torti S. V., Tsuji Y., Torti F. M. Iron-independent induction of ferritin H chain by tumor necrosis factor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4946–4950. doi: 10.1073/pnas.88.11.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. T., White K., Munro H. N. Conservation of ferritin heavy subunit gene structure: implications for the regulation of ferritin gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7438–7442. doi: 10.1073/pnas.84.21.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllner E. W., Neupert B., Kühn L. C. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989 Jul 28;58(2):373–382. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oria R., Alvarez-Hernández X., Licéaga J., Brock J. H. Uptake and handling of iron from transferrin, lactoferrin and immune complexes by a macrophage cell line. Biochem J. 1988 May 15;252(1):221–225. doi: 10.1042/bj2520221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L. W., Alpert E., Isselbacher K. J., Drysdale J. W. Abnormality in tissue isoferritin distribution in idiopathic haemochromatosis. Nature. 1974 Jul 26;250(464):333–335. doi: 10.1038/250333a0. [DOI] [PubMed] [Google Scholar]
- Powell L. W., Alpert E., Isselbacher K. J., Drysdale J. W. Human isoferritins: organ specific iron and apoferritin distribution. Br J Haematol. 1975 May;30(1):47–55. doi: 10.1111/j.1365-2141.1975.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Roberts S., Bomford A. Chelation of transferrin iron by desferrioxamine in K562 cells. The partition of iron between ferrioxamine and ferritin. Biochem J. 1988 Sep 15;254(3):869–875. doi: 10.1042/bj2540869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Tang C. K., Kaptain S., Burgess W. H., Haile D. J., Samaniego F., McBride O. W., Harford J. B., Klausner R. D. Cloning of the cDNA encoding an RNA regulatory protein--the human iron-responsive element-binding protein. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7958–7962. doi: 10.1073/pnas.87.20.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Nishisato T., Grasso J. A., Aisen P. Interaction of transferrin with iron-loaded rat peritoneal macrophages. Br J Haematol. 1986 Feb;62(2):275–286. doi: 10.1111/j.1365-2141.1986.tb02930.x. [DOI] [PubMed] [Google Scholar]
- Schiaffonati L., Rappocciolo E., Tacchini L., Bardella L., Arosio P., Cozzi A., Cantu G. B., Cairo G. Mechanisms of regulation of ferritin synthesis in rat liver during experimental inflammation. Exp Mol Pathol. 1988 Apr;48(2):174–181. doi: 10.1016/0014-4800(88)90054-8. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Mitomo K., Watanabe T., Okamoto S., Yamamoto K. Involvement of a NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol Cell Biol. 1990 Feb;10(2):561–568. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taetle R., Honeysett J. M. Gamma-interferon modulates human monocyte/macrophage transferrin receptor expression. Blood. 1988 Jun;71(6):1590–1595. [PubMed] [Google Scholar]
- Torti S. V., Kwak E. L., Miller S. C., Miller L. L., Ringold G. M., Myambo K. B., Young A. P., Torti F. M. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988 Sep 5;263(25):12638–12644. [PubMed] [Google Scholar]
- Tosato G., Jones K. D. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood. 1990 Mar 15;75(6):1305–1310. [PubMed] [Google Scholar]
- Wagstaff M., Worwood M., Jacobs A. Properties of human tissue isoferritins. Biochem J. 1978 Sep 1;173(3):969–977. doi: 10.1042/bj1730969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Wei Y., Miller S. C., Tsuji Y., Torti S. V., Torti F. M. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990 May 31;169(1):289–296. doi: 10.1016/0006-291x(90)91466-6. [DOI] [PubMed] [Google Scholar]
- Worwood M., Hourahane D., Jones B. M. Accumulation and release of isoferritins during incubation in vitro of human peripheral blood mononuclear cells. Br J Haematol. 1984 Jan;56(1):31–43. doi: 10.1111/j.1365-2141.1984.tb01269.x. [DOI] [PubMed] [Google Scholar]
- Young S. P., Garner C. Delivery of iron to human cells by bovine transferrin. Implications for the growth of human cells in vitro. Biochem J. 1990 Jan 15;265(2):587–591. doi: 10.1042/bj2650587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. F., Koerner T. J., Adams D. O. Gene regulation in macrophage activation: differential regulation of genes encoding for tumor necrosis factor, interleukin-1, JE, and KC by interferon-gamma and lipopolysaccharide. J Leukoc Biol. 1990 Nov;48(5):412–419. doi: 10.1002/jlb.48.5.412. [DOI] [PubMed] [Google Scholar]


