Abstract
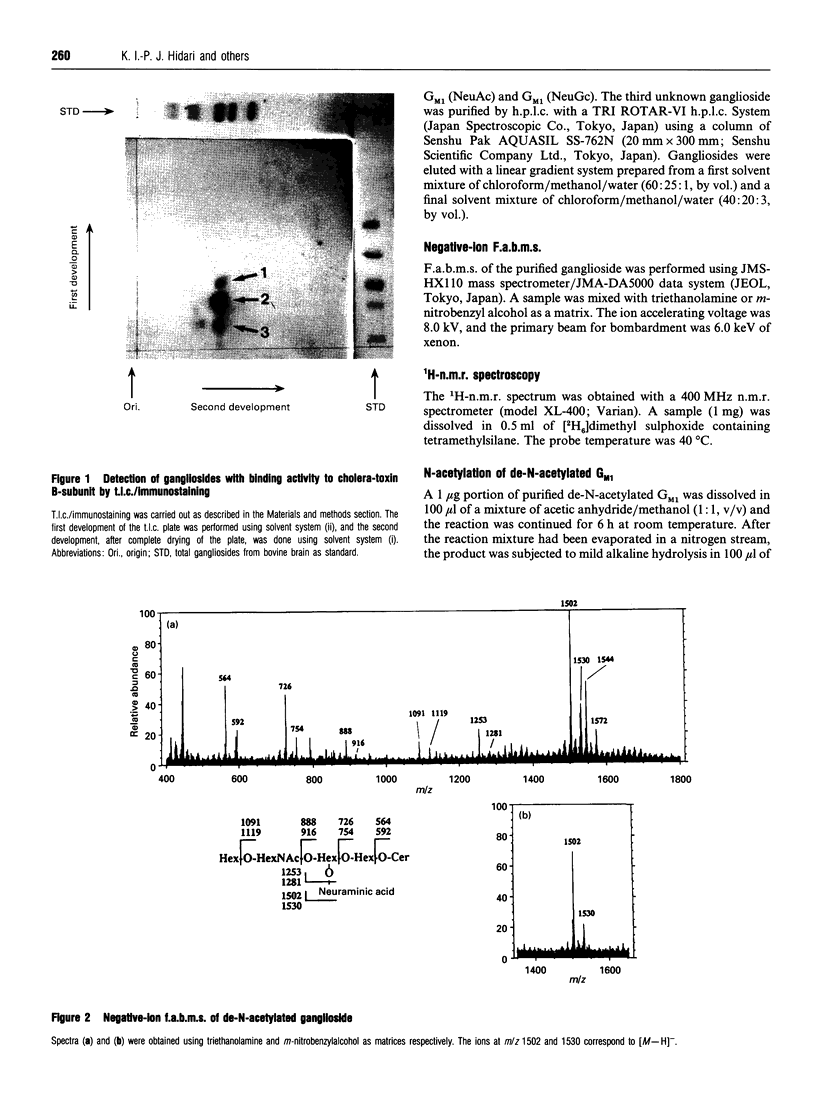
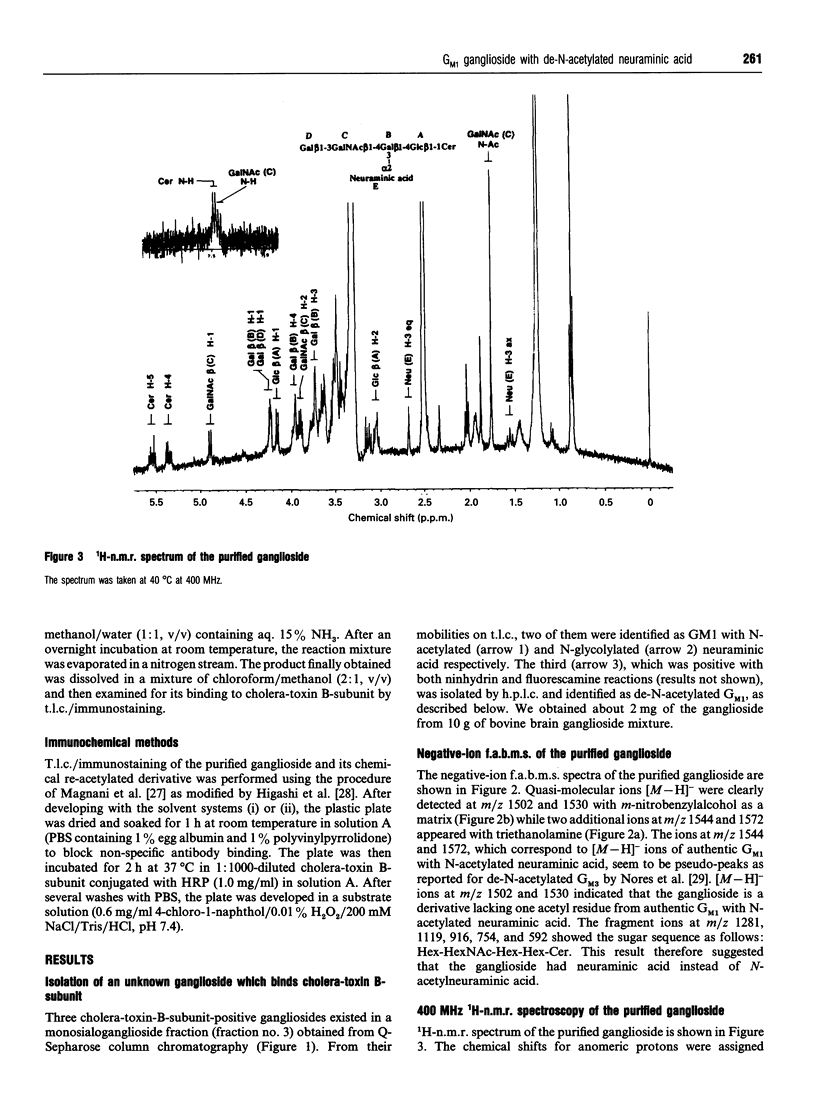
A novel ganglioside which binds cholera-toxin B-subunit was purified from bovine brain by an h.p.l.c. system using an Aquasil column subsequent to Q-Sepharose column chromatography. T.l.c./immunostaining showed that the isolated ganglioside had about 60% of the binding reactivity of the authentic ganglioside GM1 for cholera-toxin B-subunit. On h.p.l.c., this ganglioside migrated between ganglioside GD1a and GD1b, and was found to give positive reactions with ninhydrin and fluorescamine reagents which specifically react with amino groups. The presence of a free amino group was further confirmed by chemical re-N-acetylation. The N-acetylated product had an identical RF value on h.p.l.c. and similar reactivity with cholera-toxin B-subunit as the authentic GM1. H.p.t.l.c., t.l.c./immunostaining, negative-ion fast-atom-bombardment (f.a.b.)-m.s., and 1H-n.m.r. spectroscopy of the novel ganglioside unequivocally demonstrated that it has the basal structure of GM1 with de-N-acetylated neuraminic acid instead of N-acetylneuraminic acid. In the present study we report for the first time that a ganglioside derivative containing de-N-acetylated neuraminic acid, de-N-acetylated GM1, exists in natural brain tissues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Hirabayashi Y., Kon K., Inagaki F., Tate S., Whittaker V. P. A trisialoganglioside containing a sialyl alpha 2-6 N-acetylgalactosamine residue is a cholinergic-specific antigen, Chol-1 alpha. J Biochem. 1992 Mar;111(3):287–290. doi: 10.1093/oxfordjournals.jbchem.a123751. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Cumar F. A., Maggio B., Caputto R. Ganglioside-cholera toxin interactions: a binding and lipid monolayer study. Mol Cell Biochem. 1982 Aug 6;46(3):155–160. doi: 10.1007/BF00239664. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Moss J., Richards R. L., Brady R. O., Alving C. R. Liposomes as model membranes for ligand-receptor interactions: studies with choleragen and glycolipids. Biochemistry. 1979 Jun 12;18(12):2562–2567. doi: 10.1021/bi00579a020. [DOI] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hanai N., Dohi T., Nores G. A., Hakomori S. A novel ganglioside, de-N-acetyl-GM3 (II3NeuNH2LacCer), acting as a strong promoter for epidermal growth factor receptor kinase and as a stimulator for cell growth. J Biol Chem. 1988 May 5;263(13):6296–6301. [PubMed] [Google Scholar]
- Higashi H., Fukui Y., Ueda S., Kato S., Hirabayashi Y., Matsumoto M., Naiki M. Sensitive enzyme-immunostaining and densitometric determination on thin-layer chromatography of N-glycolylneuraminic acid-containing glycosphingolipids, Hanganutziu-Deicher antigens. J Biochem. 1984 May;95(5):1517–1520. doi: 10.1093/oxfordjournals.jbchem.a134760. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y., Hyogo A., Nakao T., Tsuchiya K., Suzuki Y., Matsumoto M., Kon K., Ando S. Isolation and characterization of extremely minor gangliosides, GM1b and GD1 alpha, in adult bovine brains as developmentally regulated antigens. J Biol Chem. 1990 May 15;265(14):8144–8151. [PubMed] [Google Scholar]
- Hirabayashi Y., Nakao T., Irie F., Whittaker V. P., Kon K., Ando S. Structural characterization of a novel cholinergic neuron-specific ganglioside in bovine brain. J Biol Chem. 1992 Jun 25;267(18):12973–12978. [PubMed] [Google Scholar]
- Hirabayashi Y., Nakao T., Matsumoto M., Obata K., Ando S. Improved method for large-scale purification of brain gangliosides by Q-sepharose column chromatography. Immunochemical detection of C-series polysialogangliosides in adult bovine brains. J Chromatogr. 1988 Jul 22;445(2):377–384. doi: 10.1016/s0021-9673(01)84550-7. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Elwing H., Fredman P., Strannegård O., Svennerholm L. Gangliosides as receptors for bacterial toxins and Sendai virus. Adv Exp Med Biol. 1980;125:453–470. doi: 10.1007/978-1-4684-7844-0_40. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Elwing H., Fredman P., Svennerholm L. Polystyrene-adsorbed gangliosides for investigation of the structure of the tetanus-toxin receptor. Eur J Biochem. 1980 May;106(2):371–379. doi: 10.1111/j.1432-1033.1980.tb04583.x. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner T. A., Jr, Prestegard J. H., Demou P. C., Yu R. K. High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear two-dimensional spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry. 1983 May 24;22(11):2676–2687. doi: 10.1021/bi00280a014. [DOI] [PubMed] [Google Scholar]
- Livingston B. D., De Robertis E. M., Paulson J. C. Expression of beta-galactoside alpha 2,6 sialyltransferase blocks synthesis of polysialic acid in Xenopus embryos. Glycobiology. 1990 Sep;1(1):39–44. doi: 10.1093/glycob/1.1.39. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Smith D. F., Ginsburg V. Detection of gangliosides that bind cholera toxin: direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980 Dec;109(2):399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- Manzi A. E., Sjoberg E. R., Diaz S., Varki A. Biosynthesis and turnover of O-acetyl and N-acetyl groups in the gangliosides of human melanoma cells. J Biol Chem. 1990 Aug 5;265(22):13091–13103. [PubMed] [Google Scholar]
- McDonald O. B., Hannun Y. A., Reynolds C. H., Sahyoun N. Activation of casein kinase II by sphingosine. J Biol Chem. 1991 Nov 15;266(32):21773–21776. [PubMed] [Google Scholar]
- Nores G. A., Hanai N., Levery S. B., Eaton H. L., Salyan E. K., Hakomori S. Synthesis and characterization of lyso-GM3 (II3Neu5Ac Lactosyl sphingosine), de-N-acetyl-GM3 (II3NeuNH2 lactosyl Cer), and related compounds. Carbohydr Res. 1988 Aug 15;179:393–410. doi: 10.1016/0008-6215(88)84135-1. [DOI] [PubMed] [Google Scholar]
- Pushkareva MYu, Khan W. A., Alessenko A. V., Sahyoun N., Hannun Y. A. Sphingosine activation of protein kinases in Jurkat T cells. In vitro phosphorylation of endogenous protein substrates and specificity of action. J Biol Chem. 1992 Jul 25;267(21):15246–15251. [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., Cuatrecasas P. Mechanism of activation of adenylate cyclase by cholera toxin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3438–3442. doi: 10.1073/pnas.72.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariola H., Aufderheide E., Bernhard H., Henke-Fahle S., Dippold W., Ekblom P. Antibodies to cell surface ganglioside GD3 perturb inductive epithelial-mesenchymal interactions. Cell. 1988 Jul 15;54(2):235–245. doi: 10.1016/0092-8674(88)90556-9. [DOI] [PubMed] [Google Scholar]
- Song Y., Kitajima K., Inoue S., Inoue Y. Isolation and structural elucidation of a novel type of ganglioside, deaminated neuraminic acid (KDN)-containing glycosphingolipid, from rainbow trout sperm. The first example of the natural occurrence of KDN-ganglioside, (KDN)GM3. J Biol Chem. 1991 Nov 15;266(32):21929–21935. [PubMed] [Google Scholar]
- Staerk J., Ronneberger J., Wiegandt H., Ziegler W. Interaction of ganglioside G Gtet1 and its derivatives with choleragen. Eur J Biochem. 1974 Oct 1;48(1):103–110. doi: 10.1111/j.1432-1033.1974.tb03747.x. [DOI] [PubMed] [Google Scholar]
- Wu G. S., Ledeen R. Quantification of gangliotetraose gangliosides with cholera toxin. Anal Biochem. 1988 Sep;173(2):368–375. doi: 10.1016/0003-2697(88)90201-1. [DOI] [PubMed] [Google Scholar]